Abstract
Severe quantitative and qualitative brown adipocyte defects are common in obesity. To investigate whether aberrant expression of tumor necrosis factor α (TNF-α) in obesity is involved in functional brown fat atrophy, we have studied genetically obese (ob/ob) mice with targeted null mutations in the genes encoding the two TNF receptors. The absence of both TNF receptors or p55 receptor alone resulted in a significant reduction in brown adipocyte apoptosis and an increase in β3-adrenoreceptor and uncoupling protein-1 expression in obese mice. Increased numbers of multilocular functionally active brown adipocytes, and improved thermoregulation was also observed in obese animals lacking TNF-α function. These results indicate that TNF-α plays an important role in multiple aspects of brown adipose tissue biology and mediates the abnormalities that occur at this site in obesity.
Obesity in experimental models is ubiquitously associated with abnormalities in brown adipose tissue (BAT) (1, 2). In adult obese animals, the amount of thermogenically active BAT as well as the expression of β3-adrenoceptors in brown adipocytes are substantially reduced, potentially resulting in alterations of metabolic function and thermoregulation (3–5). The molecular basis of these obesity-related changes is poorly understood. It has been demonstrated that expression of tumor necrosis factor α (TNF-α) in adipose tissue is elevated in a variety of experimental obesity models (6–9) and obese humans (10, 11). Owing to its ability to inhibit insulin receptor signaling (12–14), TNF-α represents a component of obesity-related insulin resistance (15). More recently, TNF-α has been shown to induce brown and white adipocyte apoptosis in vitro (16–18). Interestingly, in genetic models of obesity, the number of apoptotic cells in the brown fat is dramatically higher than that in control animals (16). This leads to reduction in the number of multilocular thermogenically active brown fat cells and therefore causes BAT functional atrophy, which is characterized by defective thermoregulation in both genetic and dietary obesity. In addition, because BAT is an important target for insulin action and other aspects of energy metabolism, its atrophy could further contribute to the abnormal metabolic profile in obesity. Here, we have tested whether abnormal TNF-α production in obesity contributes to any of these abnormalities in BAT.
Materials and Methods
Generation of ob/ob Mice Deficient in TNF Receptors (TNFR).
Obese (ob/ob) mice deficient in TNFR were generated as described (19–21). Mice with targeted null mutations at both TNFR 1 and 2 loci (p55−/− and p75−/−, respectively) were crossed with Ob/ob mice, to produce animals heterozygous at the TNFR1, TNFR2, and ob loci (p55+/−, p75+/−, and Ob/ob). The resulting triple heterozygotes were then crossbred with each other to produce OB/ob mice wild type at TNFR loci or with homozygous null mutations in each or both TNFR. The subsequent intercrossing of these animals produced control OB/OB and ob/ob mice and littermates with mutations at one or both TNFR (a detailed description of these animals is reported in refs. 19–21). All of the animal experiments were carried out in accordance with the highest standards of humane animal care.
Terminal Deoxynucleotidyltransferase-Mediated UTP End Labeling (TUNEL) Staining.
In situ fragmentation of DNA was investigated in BAT slices derived from 8-week-old lean control, ob/ob control, and TNFR-deficient female mice by means of a detection kit (Boehringer Mannheim) based on direct alkaline phosphatase detection of digoxigenin-labeled 3′-OH ends of genomic DNA fragments. The assay was performed according to the manufacturer's instructions and the enzymatic reaction was developed by using 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium as chromogenic substrates. Ten high-power fields (×100) in each representative section of TUNEL-stained interscapular depots for different experiments were randomly observed and brown adipocyte apoptotic and nonapoptotic nuclei were distinguished according to the level of staining and morphological parameters, such as chromatin condensation, aggregation along the nuclear periphery, and apoptotic bodies.
Brown Adipocyte Isolation, Cell Culture, and Treatment.
Brown fat precursor cells were enzymatically isolated from the interscapular BAT of male Sprague-Dawley rats (150–160 g body weight) (Harlan Nossan, Correzzana, Italy), which were kept in standard laboratory conditions (12 h light/dark cycle; food and water ad libitum), as described (16). Three million cells seeded in 24-well culture plates (Nunclon Delta, Milan, Italy) were cultured under a water-saturated atmosphere of 6% CO2 in air at 37°C in 2.0 ml of a culture medium, as described (16). In the experiments analyzing the effects of TNF-α, the cultured cells were exposed to the culture medium containing murine recombinant TNF-α (freshly diluted in buffer as requested by manufacturer; Genzyme) with specific activities of 5 × 107 units/mg for 4 h and then were harvested. The medium was discarded, the wells were washed twice with 2 ml of ice-cold PBS (Sigma), and the cells were scraped out with a disposable cell scraper (Nunc, Milan, Italy) for mRNA extraction and reverse transcription–PCR analysis.
cAMP Accumulation.
Cells were grown for 4 or 8 days (i.e., preconfluent or confluent cells). Two hours before each experiment, the medium was replaced by PBS containing 5 mM 3-isobutyl-1-methylxanthine. Cells were then incubated at 37°C for 30 min with or without CGP12177A [(−)-4-(3-t-butylamino-2-hydroxypropoxy) benzimidazol-2-one) from CIBA–Geigy]. The reaction was stopped in ice by removing the incubation medium. The cells were harvested with ice-cold PBS, containing 5 mM 3-isobutyl-1-methylxanthine, and after sonication (100 w for 10 s) were centrifuged for 5 min. The resulting infranatants (i.e., liquid between floating fat cake and pelleted cell debris) were stored until cAMP determination (RIA, DuPont/NEN). Protein content was measured by the BCA Protein Assay (Pierce).
Reverse Transcription–PCR.
Reverse transcription–PCR was performed and its products were quantitated as described (22). Total cytoplasmic RNA was isolated from cultured cells or BAT by using the RNazol method (TM Cinna Scientific, Friendswood, TX). A control experiment without reverse transcriptase was performed for each sample to verify that the amplification was not caused by any residual genomic DNA. The mRNA for β-actin was examined as the reference cellular transcript in each PCR reaction. The nucleotide sequences of the amplified products were determined, and were found to be identical to the mRNA products of the rat and mouse TNF-α, β3-adrenoreceptor, uncoupling protein 1 (UCP-1), and β-actin genes.
Morphological Evaluation of Brown Adipocytes.
Five serial BAT slices at 200-μm distance were prepared from three animals in each group and stained with hematoxylin-eosin. Morphological evaluation was performed in six optical fields randomly taken in each slice at ×25 magnification, and multilocularity of brown adipocytes were counted and scored by two expert independent observers.
Body Temperature Measurements.
The body temperature of each mouse was first measured at room temperature with a digital thermometer equipped with a rectal probe of 1-mm diameter. After insertion of the probe 30 mm into the rectum, core body temperature is recorded on stabilization of the temperature reading for 10 consecutive seconds. Mice were then transferred to a cold room and housed in prechilled individual cages at 4°C where the body temperature was measured every 2 h. To prevent heat conduction to the animals, all equipment used in the experiments was prechilled and the animals were handled by using cryogloves.
Statistical Methods.
Raw data from each experiment was normalized, combined, and analyzed by using either an analysis of variance with Newman-Keuls' multiple comparison post hoc test or t test.
Results
Apoptosis of Multilocular Brown Adipocytes Is Decreased in Obese Animals with TNF-α Signaling Deficiency.
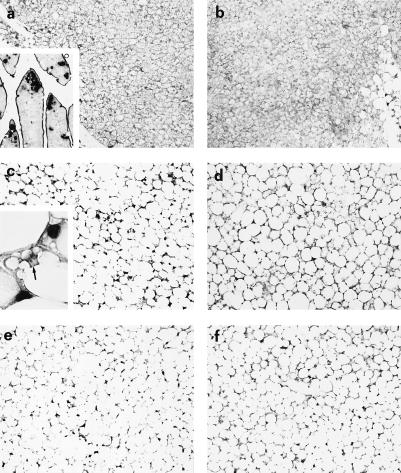
To investigate the role of TNF-α in obesity-induced functional atrophy of BAT in vivo, we first analyzed the apoptosis of brown adipocytes in genetically obese (ob/ob) mice with targeted mutations either in p55, p75, or both TNFR. In these obese animals, signaling and function of TNF-α is partially or completely abolished (19–21). TUNEL staining was used for the in situ measurements of apoptotic cell number in BAT sections obtained from these animals (23). Fig. 1 (a and c) shows that there was significantly higher number of positive nuclei for DNA fragmentation in BAT of ob/ob animals (wild type at TNFR loci) than in that of the lean control mice (65 ± 4 vs. 1 ± 0.5% positive nuclei over total cell nuclei; P < 0.005). Apoptosis in TUNEL-positive cells of BAT from genetically obese animals was further validated by several other techniques, including agarose gel electrophoresis and transmission electron microscopy (16). In contrast to the ob/ob animals, the number of apoptotic nuclei in the ob/ob mice lacking p55 (ob/ob-p55−/−) or both TNFR (ob/ob-p55−/−p75−/−) were significantly lower and approached the ratios observed in the control lean mice (2 ± 0.3 and 4 ± 1% positive nuclei, respectively; P < 0.005) (Fig. 1 d and f). In ob/ob mice lacking p75 TNFR (ob/ob-p75−/−), the number of apoptotic cells (25 ± 3% positive nuclei, Fig. 1e) was significantly higher than those seen in control lean animals as well as ob/ob-p55−/−p75−/− mice, but lower than those seen in obese controls (ob/ob). It is known that in obese animals, the typical multilocular brown adipocytes (i.e., thermogenically active small fat cells with numerous small lipid droplets dispersed in the cytoplasmic space around a central nucleus) are substituted by unilocular adipocytes morphologically reminiscent of white adipocytes (i.e., large fat cells filled by a single lipid droplet that flattens the nucleus to the cell membrane, and likely thermogenically inactive) (Fig. 1 a and c). Interestingly, the multilocular brown adipocytes in ob/ob mice lacking one or both TNFR were more abundant than in ob/ob mice (55.2, 70.2, and 86.1% higher than ob/ob, in ob/ob-p75−/−, ob/ob-p55−/−, and ob/ob-p55−/−p75−/−, respectively). This pronounced multilocularity of adipocytes is likely to reflect active thermogenic metabolism of the cells (24). These findings demonstrate that the activity of TNF-α is the primary determinant of the BAT apoptosis and altered morphology of multilocular adipocytes seen in obesity because complete absence of TNF signaling confers essentially total protection from obesity-induced BAT apoptosis and the decline in the number of typical brown fat cells. Although the p55 receptor appears to be the predominant mediator of this activity, the results obtained in ob/ob-p75−/− mice indicate the involvement of p75 TNFR, albeit to a lesser extent, in obesity-induced apoptosis of the brown adipocytes.
Figure 1.
TUNEL staining of BAT. (a) Lean control mice acclimated to 28°C, the thermoneutral temperature. (Inset) Intestinal villi showing apoptotic apical epithelial cells as positive controls. (b) Lean control mice acclimated to cold temperature (10°C for 10 days). (c) ob/ob mice (wild type at TNFR loci). (Inset) A negative adipose cell nucleus (arrow) between two apoptotic nuclei. (d) ob/ob p55−/−. (e) ob/ob p75−/−. (f) ob/ob p55−/−p75−/− mice. [a–f and Inset a, ×125; Inset c, ×790.]
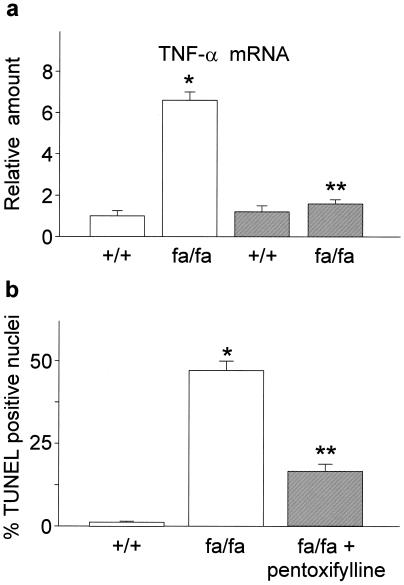
To further confirm these observations, we also investigated the effects of TNF-α inhibition on BAT survival in an additional model of genetic obesity (fa/fa rat) by chronic administration of pentoxifylline. Pentoxifylline is a methylxanthine, which represses TNF-α synthesis (25, 26). In obese Zucker fa/fa rats, two months of treatment with pentoxifylline (20 mg/kg body weight/day s.c.) dramatically decreased TNF-α production (Fig. 2a). Parallel to loss of TNF-α expression, a significant reduction was also evident in the number of apoptotic nuclei in brown fat, which results in increased multilocular brown adipocyte survival (Fig. 2b). These data clearly demonstrated that two independent means of blocking TNF-α function results in significant reversal of BAT defects in two different models of experimental obesity.
Figure 2.
Effect of pentoxifylline treatment on BAT TNF-α expression and apoptosis in 4-week-old obese Zucker fa/fa rats. (a) TNF-α mRNA levels were quantitated as described in Materials and Methods and expressed relative to untreated control (+/+) rats (open bars). (b) Percentage TUNEL-positive nuclei over total cell nuclei. Data reported as the means ± SEM. Closed bars, pentoxifylline-treated rats. *, P < 0.005 vs. lean controls; **, P < 0.005 vs. untreated animals; n = 6.
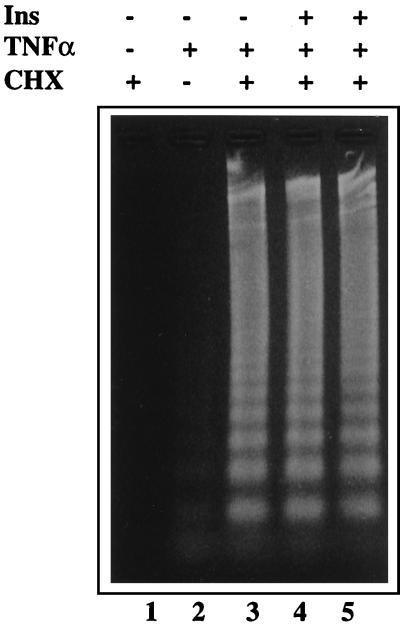
It is possible that insulin action may regulate the apoptotic response under certain circumstances. In this regard, the lack of p55 or both p55 and p75 TNFR results in increased insulin sensitivity in the ob/ob mice as evidenced by lower steady-state glucose and insulin levels and improved insulin and glucose tolerance tests (19–21). Similarly, TNF-α ligand-deficiency results in improved insulin sensitivity in several models of obesity (20, 27). Thus, blocking TNF-α action and improvement of insulin sensitivity seems to parallel the decrease in the number of apoptotic brown fat cells in a variety of experimental paradigms. These observations raise the possibility that alterations in insulin sensitivity might play an important role in obesity-induced BAT apoptosis if insulin acts to prevent apoptosis of brown adipocytes. To test this possibility, we asked whether insulin interferes with the apoptotic effects of TNF-α in brown adipocytes. As shown in Fig. 3, acute (4 h) or chronic (24–72 h) treatment with 0.1–100 nM insulin did not counteract the TNF-α-induced apoptosis of cultured brown adipocytes. Taken together with the results obtained from animals with defective TNF-α function, our findings suggest that lack of BAT apoptosis in these obese animals is likely to be the direct result of loss of TNF-α function rather than an indirect action through increased insulin sensitivity. However, the possibility remains that alterations in insulin sensitivity in vivo might still influence the extent of apoptosis in BAT.
Figure 3.
TNF-α-induced fragmentation of the genomic DNA in cultured brown adipocytes in the presence and absence of insulin. The cells were pretreated with 100 nM insulin for 4 h (lane 4) or 48 h (lane 5), and incubated with TNF-α (10 pM) plus cycloheximide (10 μg/ml) for 4 h. (Lane 1) cycloheximide alone; (lane 2) untreated cells; (lane 3) TNF-α plus cycloheximide. The gel shown is representative of data obtained in six independent experiments.
TNF-α Decreases the Expression of Functionally Active β3-Adrenoceptors in Brown Adipocytes and BAT.
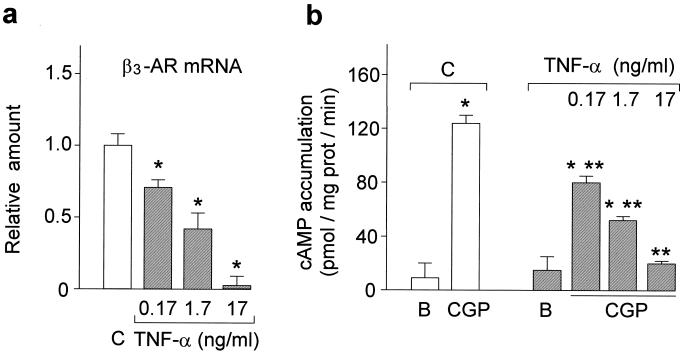
We next started to investigate the effects of TNF-α on potential molecular targets that are critical for BAT function. BAT is a heavily innervated tissue. Noradrenaline, which is released from the sympathetic nerve endings, strongly affects BAT functions, including glucose and lipid metabolism (28), heat production (29), and cell proliferation and survival (16, 30). The latter could be readily illustrated by the virtual absence of apoptosis in BAT on stimulation of sympathetic activity (16) (i.e., cold exposure, Fig. 1b). These actions are mediated primarily through β3-adrenoceptors, which are also highly expressed in brown adipocytes (31–33). Selective activation of β3-adrenoceptors by synthetic agonists stimulates thermogenesis and improves glycemic control in obese and insulin-resistant animals (31). Hence, in obesity, markedly decreased β3-adrenoreceptor expression and activity is believed to contribute to the pathophysiology of the disease (4, 5). However, the underlying molecular mechanisms for the β3-adrenoreceptor deficiency are not known. Here, we demonstrate that TNF-α plays an important role in this β3-adrenoreceptor deficiency associated with obesity by regulating its expression and activity in brown adipocytes and BAT. As shown in Fig. 4a, exposure to 0.17–17 ng/ml recombinant murine TNF-α (concentrations comparable with the 0.082 ± 0.004 ng/ml present in the plasma of obese rodents) (6), results in dose-dependent inhibition of β3-adrenoreceptor gene expression in cultured rat brown adipocytes. This reduction in β3-adrenoreceptor gene expression also leads to significant functional deficiency in these cells. In TNF-α-treated brown adipocytes, the β3-adrenoreceptor-specific partial agonist CGP12177A was markedly less effective in stimulating cAMP accumulation compared with control brown adipocytes (Fig. 4b).
Figure 4.
Effect of TNF-α treatment on β3-adrenoceptors in brown adipocytes. (a) β3-adrenoreceptor (β3-AR) mRNA levels were quantitated as described in Materials and Methods and expressed relative to untreated cells (C, open bars) (*, P < 0.005 vs. untreated cells; n = 4) and reported as the means ± SEM. (b) The cAMP accumulations induced by 10 μM CGP12177A are expressed as pmol/mg protein/min and are the means ± SEM of three different experiments, each performed in triplicate. (*, P < 0.005 vs. baseline; **, P < 0.005 vs. untreated cells). B, baseline value.
β3-Adrenoreceptor and UCP-1 Expression Is Increased in BAT of Obese Animals with TNF-α Signaling Deficiency.
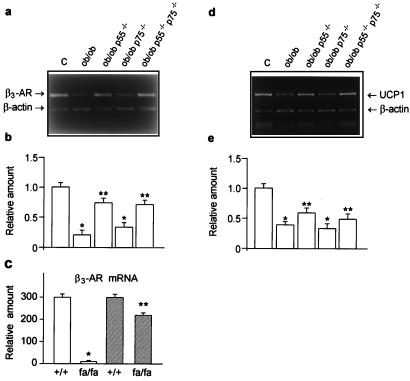
Because decreased β3-adrenoreceptor expression and action in brown adipocytes are well-documented features of obesity (4, 5), it is feasible to hypothesize that TNF-α might play a role in this β3-adrenoreceptor deficiency in vivo. To test this possibility, we examined the expression of β3-adrenoceptors in BAT of obese mice lacking TNF receptors and obese rats and mice treated with the TNF-α inhibitor, pentoxifylline. As shown in Fig. 5a, β3-adrenoreceptor mRNA levels in ob/ob mice were significantly lower than the lean controls. Strikingly, in BAT of ob/ob-p55−/−p75−/− and ob/ob-p55−/− mice, the β3-adrenoreceptor mRNA levels were much higher than those of ob/ob animals and comparable to those of lean mice (Fig. 5 a and b). In contrast, the β3-adrenoreceptor mRNA levels in BAT of ob/ob-p75−/− mice were low and comparable to those of ob/ob mice (Fig. 5 a and b). Finally, the β3-adrenoreceptor expression and function, as measured by both mRNA levels and CGP12177A-induced cAMP production were also normalized in BAT of obese fa/fa rats after two months treatment with pentoxifylline (Fig. 5c and data not shown). Taken together, these results demonstrate that the obesity-related β3-adrenoreceptor deficiency in BAT is induced by TNF-α predominantly acting through the p55 TNF receptor.
Figure 5.
Effects of TNF-α signaling blockade on β3-adrenoreceptor and UCP-1 expression in obesity. (a and d) Reverse transcription–PCR analysis of the β3-adrenoreceptor (β3-AR) and UCP-1 mRNA content in lean control (C), ob/ob, ob/ob p55−/−, ob/ob p75−/−, ob/ob p55−/−p75−/− mice. (b and e) β3-adrenoreceptor and UCP-1 mRNA levels were quantitated as described in Materials and Methods and expressed relative to lean control mice (*, P < 0.01 vs. lean controls; **, P < 0.05 vs. ob/ob and ob/ob p75−/−, n = 4). (c) Effect of pentoxifylline treatment on β3-adrenoreceptor expression in the brown fat of obese Zucker fa/fa rats. β3-adrenoreceptor mRNA levels are expressed relative to untreated control (+/+) rats (open bars) (*, P < 0.01 vs. lean controls; **, P < 0.005 vs. untreated animals; n = 6) and reported as the means ± SEM. Closed bars, pentoxifylline-treated rats.
Noradrenaline-stimulation of β3-adrenoceptors induces BAT thermogenesis by activating an uncoupling mechanism to dissipate protons across the mitochondrial membrane (34). The mitochondrial UCPs, particularly UCP-1, in the mitochondrial inner membrane of mammalian brown fat generates heat by uncoupling oxidative phosphorylation (35). This process protects against cold (36) and regulates energy balance (37). Given the importance of UCP-1 in the regulation of energy expenditure in rodents, we examined UCP-1 expression in the BAT of obese mice lacking TNF receptors. Whereas the lack of p75 receptor alone did not change the UCP-1 gene expression, the absence of p55 or both TNF receptors resulted in increased UCP-1 mRNA levels compared with ob/ob mice (Fig. 5 d and e). The expression of other UCP isoforms, UCP-2 and UCP-3, was not altered in animals lacking TNFR (data not shown). These data further support the evidence that the absence of TNFR increases the number of functionally active brown adipocytes.
Lack of TNFR Leads to Significant Improvement in Thermoadaptive Capacity of Obese Animals.
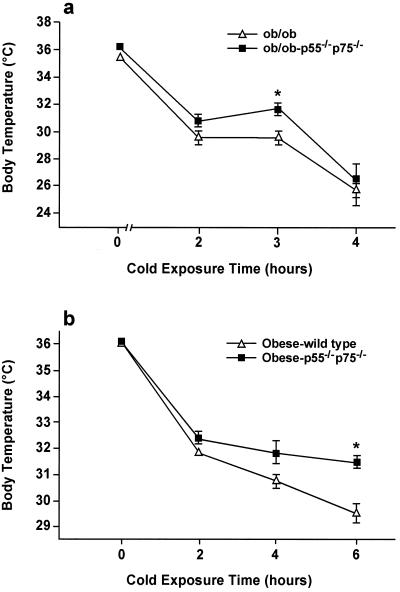
It is important to determine whether these changes observed in BAT in TNFR-deficient obese mice lead to improved BAT function in vivo. To address this, we assessed the effect of complete absence of TNF-α function on thermoregulation in control and TNFR-deficient obese mice. The mice were exposed to cold (4°C) for different periods of time and the rate and time of decline in core body temperature was used as a measure of sensitivity to cold and the thermoadaptive capacity. These experiments demonstrated improved thermoadaptation in ob/ob p55−/−p75−/− mice compared with that of ob/ob mice with intact TNF-α function. In this model, differences in core body temperature were most significant after 3 h cold exposure (Fig. 6a) and diminished after prolonged cold exposure. We also tested thermoregulation in an additional model of obesity induced by high fat diet. In this model, the decrease in body temperature was also significantly higher in obese wild type compared with obese p55−/−p75−/− mice. Furthermore, the magnitude of the effects was greater in this model compared with the ob/ob mice and evident even after 6 h of cold exposure (Fig. 6b). These results suggest that TNF-α is at least partially responsible for altered BAT function in obese mice and lack of TNFRs leads to significant improvement in thermoadaptive capacity.
Figure 6.
Thermoregulation in p55−/−p75−/− ob/ob and diet-induced obese mice. Body temperature in mice (10 in each group) housed at 4°C was measured every 2 h. *P < 0.05 obese TNFR deficient vs. obese wild type.
Discussion
Recent studies on transgenic models indicated the importance of brown fat in metabolic regulation and energy balance. For example, partial ablation of BAT has resulted in severe obesity, insulin resistance, and decreased O2 consumption, illustrating a potential role for BAT mass and function for energy metabolism (8, 38). On the other hand, even more significant reduction in BAT mass through alternative transgenic means has not resulted in obesity but caused defects in thermogenesis (39). In line with this, genetic deletion of critical individual functional components of BAT such as UCP-1 has resulted in cold sensitivity but not obesity (40), whereas β3-adrenoreceptor-deficient mice have minimal obesity and relatively normal thermoregulation (41).
In our experiments, despite significant obesity in both groups, the total body weights of the female TNFR-deficient ob/ob mice were less than wild-type animals (65.93 ± 13.59 g vs. 72.92 ± 4.72 g, P < 0.05). In previous work, no statistically significant differences were evident in the total body composition between these two groups of different age (20). On the surface, our findings would appear to support the role of BAT mainly in thermoregulation rather than in body weight regulation under the experimental conditions used in this study. On the other hand, the small change in body weight in TNFR-deficient mice might be the result of the combined effects of improved insulin sensitivity and enhanced survival of white adipocytes, which will stimulate weight gain (42) and increased BAT function or mass, which will tend to decrease body weight (3). Indeed, TNF-α can induce apoptosis of human white preadipocytes and adipocytes in vitro (18). Most interestingly, insulinopenia in ob/ob mice has been recently demonstrated to result in massive white adipocyte apoptosis (43). Thus, it is possible to speculate that the lack of function of TNF-α may result in growth of white fat mass, both directly (absence of apoptosis of white adipocytes) and indirectly (improvement of insulin resistance) and counteract the potential effects of improved BAT function. Consequently, in the absence of any further stimulation, the net change in body weight has remained small (19). However, most forms of obesity are also associated with deficiency in the signals activating BAT. For example, all models of animal obesity exhibit leptin deficiency or resistance (44). Similarly, defective adrenergic input is a well-established component of obesity. Defective action of these important effectors might also limit the complete expression of the BAT functions (45) despite significant increase in cellular viability. In fact, this might explain the better thermoadaptive response in dietary obesity compared with the ob/ob mice deficient in TNF function.
Nevertheless, these observations indicate that BAT physiology is quite complex and its atrophy in obesity is likely to be the combined result of defects at multiple sites rather than any one functional component alone (40, 41). In this study, we provided clear evidence that TNF-α is involved in generating defects at several functionally significant loci in obesity including apoptosis of multilocular brown adipocytes, expression of β3-adrenoceptors and UCP-1, and decreased thermoadaptive response. These significant alterations are likely to contribute to the metabolic and thermogenic changes seen in obesity and insulin-resistance syndromes.
The fact that mice lacking TNF-α function, despite decreased insulin resistance, maintain a lower body weight supports the relevance of improved thermoregulation in body energy balance. Because improvement of insulin resistance by most therapeutic approaches consistently results in body weight gain (42, 46), and consequent risk factors (46), our findings could be of significant therapeutic relevance. Indeed, inhibitors of TNF-α function or production, alone or in combination with other modalities, could improve insulin resistance in type 2 diabetes mellitus without a concomitant increase in body weight caused by their ability to counteract functional BAT atrophy. Finally, because several therapeutic approaches such as leptin replacement and β3-adrenoreceptor agonists involve activation of brown adipocytes, the effectiveness of these modalities could be significantly enhanced when combined with TNF-α antagonists because of the presence of a larger and healthier brown adipocyte pool.
Acknowledgments
We thank P. Mantegazza and A. Valerio for critical support and comments on the manuscript. This work was supported in part by grants from the University of Milan (M.O.C.), European Community (S.C.), National Institutes of Health, and American Diabetes Association (G.S.H.).
Abbreviations
- TNF
tumor necrosis factor
- TNFR
tumor necrosis factor receptor
- UCP
uncoupling protein
- BAT
brown adipose tissue
- TUNEL
terminal deoxynucleotidyltransferase-mediated UTP end labeling
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Trayhurn P. In: Brown Adipose Tissue. Trayhurn P, Nicholls D G, editors. London: Edward Arnold; 1986. pp. 299–338. [Google Scholar]
- 2.Himms-Hagen J. Prog Lipid Res. 1989;28:67–115. doi: 10.1016/0163-7827(89)90009-x. [DOI] [PubMed] [Google Scholar]
- 3.Himms-Hagen J, Desautels M. Biochem Biophys Res Commun. 1978;83:628–635. doi: 10.1016/0006-291x(78)91036-7. [DOI] [PubMed] [Google Scholar]
- 4.Muzzin P, Revelli J-P, Kuhne F, Gocayne J D, McCombie W R, Venter J C, Giacobino J-P, Fraser C M. J Biol Chem. 1991;266:24053–24058. [PubMed] [Google Scholar]
- 5.Collins S, Daniel K W, Rohlfs E M, Ramkumar V, Taylor I L, Gettys T W. Mol Endocrinol. 1994;8:518–527. doi: 10.1210/mend.8.4.7914350. [DOI] [PubMed] [Google Scholar]
- 6.Hotamisligil G S, Shargill N S, Spiegelman B M. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann C, Lorenz K, Braithwaite S S, Colca J R, Palazuk B J, Hotamisligil G S, Spiegelman B M. Endocrinology. 1994;134:264–270. doi: 10.1210/endo.134.1.8275942. [DOI] [PubMed] [Google Scholar]
- 8.Hamann A, Benecke H, Le Marchand-Brustel Y, Susulic V S, Lowell B B, Flier J S. Diabetes. 1995;44:1266–1273. doi: 10.2337/diab.44.11.1266. [DOI] [PubMed] [Google Scholar]
- 9.Kern P A, Saghizadeh M, Ong J M, Bosch R J, Deem R, Simsolo R B. J Clin Invest. 1995;95:2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hotamisligil G S, Arner P, Caro I E, Atkinson R L, Spiegelman B M. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saghizadeh M, Ong J M, Garvey W T, Henry R R, Kern P A. J Clin Invest. 1996;97:1111–1116. doi: 10.1172/JCI118504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feinstein R, Kanety A, Papa M Z, Lunenfeld B, Karasik A. J Biol Chem. 1993;268:26055–26058. [PubMed] [Google Scholar]
- 13.Hotamisligil G S, Murray D L, Choy L N, Spiegelman B M. Proc Natl Acad Sci USA. 1994;91:4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotamisligil G S, Peraldi P, Budavari A, Ellis R, White M F, Spiegelman B M. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 15.Hotamisligil G S. Exp Clin Endocrinol Diabetes. 1999;107:119–125. doi: 10.1055/s-0029-1212086. [DOI] [PubMed] [Google Scholar]
- 16.Nisoli E, Briscini L, Tonello C, De Giuli-Morghen C, Carruba M O. Cell Death Differ. 1997;4:771–778. doi: 10.1038/sj.cdd.4400292. [DOI] [PubMed] [Google Scholar]
- 17.Porras A, Alvarez A M, Valladores A, Benito M. FEBS Lett. 1997;416:324–328. doi: 10.1016/s0014-5793(97)01204-0. [DOI] [PubMed] [Google Scholar]
- 18.Prins J B, Niesler C U, Winterford C M, Bright N A, Siddle K, O'Rahilly S, Walker N I, Cameron D P. Diabetes. 1997;46:1939–1944. doi: 10.2337/diab.46.12.1939. [DOI] [PubMed] [Google Scholar]
- 19.Uysal K T, Weisbrock S M, Hotamisligil G S. Endocrinology. 1998;139:4832–4838. doi: 10.1210/endo.139.12.6337. [DOI] [PubMed] [Google Scholar]
- 20.Uysal K T, Wiesbrock S M, Marino M W, Hotamisligil G S. Nature (London) 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 21.Samad F, Uysal K T, Wiesbrock S M, Pandey M, Hotamisligil G S, Loskutoff D J. Proc Natl Acad Sci USA. 1999;96:6902–6907. doi: 10.1073/pnas.96.12.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nisoli E, Tonello C, Briscini L, Carruba M O. Endocrinology. 1997;138:676–682. doi: 10.1210/endo.138.2.4956. [DOI] [PubMed] [Google Scholar]
- 23.Mesner P W, Kaufmann S H. In: Apoptosis: Pharmacological Implications and Therapeutic Opportunities. Kaufmann S H, editor. New York: Academic; 1997. pp. 57–87. [Google Scholar]
- 24.Klaus S. BioEssays. 1997;19:215–223. doi: 10.1002/bies.950190307. [DOI] [PubMed] [Google Scholar]
- 25.Strieter R M, Remick D G, Ward P A, Spengler R N, Lynch J P, III, Larrick J, Kunkel S L. Biochem Biophys Res Commun. 1988;155:1230–1236. doi: 10.1016/s0006-291x(88)81271-3. .J. P. [DOI] [PubMed] [Google Scholar]
- 26.Soupison T, Yang S, Bernard C, Moreau R, Kirstetter P, D'Almeida M, Cailmail S, Tedgui A, Lebrec D. Clin Sci. 1996;91:29–33. doi: 10.1042/cs0910029. [DOI] [PubMed] [Google Scholar]
- 27.Ventre J, Doebber T, Wu M, MacNaul K, Stevens K, Pasparakis M, Kollias G, Moller D E. Diabetes. 1997;46:1526–1531. doi: 10.2337/diab.46.9.1526. [DOI] [PubMed] [Google Scholar]
- 28.Girardier L, Seydoux J. In: Brown Adipose Tissue. Trayhurn P, Nicholls D G, editors. London: Edward Arnold; 1986. pp. 122–151. [Google Scholar]
- 29.Barnard T, Mory G, Néchad M. Biogenic Amines in Development. Amsterdam: Elsevier/North–Holland; 1980. [Google Scholar]
- 30.Bronnikov G, Houstek J, Nedergaard J. J Biol Chem. 1992;267:2006–2013. [PubMed] [Google Scholar]
- 31.Arch J R S, Kaumann A J. Med Res Rev. 1993;13:663–729. doi: 10.1002/med.2610130604. [DOI] [PubMed] [Google Scholar]
- 32.Nisoli E, Tonello C, Carruba M O. Arch Int Pharmacodyn Ther. 1995;329:436–453. [PubMed] [Google Scholar]
- 33.Zhao J, Cannon B, Nedergaard J. Am J Physiol. 1998;275:R2002–R2011. doi: 10.1152/ajpregu.1998.275.6.R2002. [DOI] [PubMed] [Google Scholar]
- 34.Ricquier D, Bouillaud F, Toumelin P, Mory G, Bazin R, Arch J, Penicaud L. J Biol Chem. 1986;261:13905–13911. [PubMed] [Google Scholar]
- 35.Nicholls D G, Locke R M. Physiol Rev. 1984;64:1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Foster D O, Frydman M L. Can J Physiol Pharmacol. 1979;57:257–270. doi: 10.1139/y79-039. [DOI] [PubMed] [Google Scholar]
- 37.Rothwell N J, Stock M J. Nature (London) 1979;281:31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- 38.Lowell B B, Susulic V, Hamann A, Lawitts J A, Himms-Hagen J, Boyer B B, Kozak L P, Flier J S. Nature (London) 1993;366:740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- 39.Stefl B, Janovska A, Hodny Z, Rossmeisl M, Horakov M, Syrovy I, Bemova J, Bendlova B, Kopecki J. Am J Physiol. 1998;274:E527–E533. doi: 10.1152/ajpendo.1998.274.3.E527. [DOI] [PubMed] [Google Scholar]
- 40.Enerback S, Jacobsson A, Simpson E M, Guerra C, Yamashita H, Harper M-E, Kozak L P. Nature (London) 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- 41.Susulic V S, Frederich R C, Lawitts J, Tozzo E, Kahn B B, Harper M E, Himms-Hagen J, Flier J S, Lowell B B. J Biol Chem. 1995;270:29483–29492. doi: 10.1074/jbc.270.49.29483. [DOI] [PubMed] [Google Scholar]
- 42.Odeleye O E, de Courten M, Pettitt D J, Ravussin E. Diabetes. 1997;46:1341–1346. doi: 10.2337/diab.46.8.1341. [DOI] [PubMed] [Google Scholar]
- 43.Loftus T M, Kuhajda F P, Lane M D. Proc Natl Acad Sci USA. 1998;95:14168–14172. doi: 10.1073/pnas.95.24.14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedman J M, Halaas J L. Nature (London) 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 45.Mantzoros C S, Frederich R C, Qu D, Lowell B B, Maratos-Flier E, Flier J S. Diabetes. 1998;47:230–238. doi: 10.2337/diab.47.2.230. [DOI] [PubMed] [Google Scholar]
- 46.U.K. Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:312–318. [PubMed] [Google Scholar]