Abstract
Cysteine synthesis from sulfide and O-acetyl-l-serine (OAS) is a reaction interconnecting sulfate, nitrogen, and carbon assimilation. Using Lemna minor, we analyzed the effects of omission of CO2 from the atmosphere and simultaneous application of alternative carbon sources on adenosine 5′-phosphosulfate reductase (APR) and nitrate reductase (NR), the key enzymes of sulfate and nitrate assimilation, respectively. Incubation in air without CO2 led to severe decrease in APR and NR activities and mRNA levels, but ribulose-1,5-bisphosphate carboxylase/oxygenase was not considerably affected. Simultaneous addition of sucrose (Suc) prevented the reduction in enzyme activities, but not in mRNA levels. OAS, a known regulator of sulfate assimilation, could also attenuate the effect of missing CO2 on APR, but did not affect NR. When the plants were subjected to normal air after a 24-h pretreatment in air without CO2, APR and NR activities and mRNA levels recovered within the next 24 h. The addition of Suc and glucose in air without CO2 also recovered both enzyme activities, with OAS again influenced only APR. 35SO42− feeding showed that treatment in air without CO2 severely inhibited sulfate uptake and the flux through sulfate assimilation. After a resupply of normal air or the addition of Suc, incorporation of 35S into proteins and glutathione greatly increased. OAS treatment resulted in high labeling of cysteine; the incorporation of 35S in proteins and glutathione was much less increased compared with treatment with normal air or Suc. These results corroborate the tight interconnection of sulfate, nitrate, and carbon assimilation.
Plants, yeast, and most prokaryotes cover their demand for reduced sulfur, which is essential for function of proteins, oligopeptides, and many coenzymes, by reduction of inorganic sulfate. In the pathway of sulfate assimilation of plants, sulfate is first activated by ATP sulfurylase to adenosine 5′-phosphosulfate, which is reduced to sulfite by adenosine 5′-phosphosulfate reductase (APR) in a glutathione-dependent reaction. Sulfite is further reduced to sulfide by a ferredoxin-dependent sulfite reductase and sulfide incorporated into the amino acid skeleton of O-acetyl-l-Ser (OAS) by OAS (thiol) lyase, forming Cys (Brunold, 1990; Leustek et al., 2000). Cys can further be metabolized to Met or directly incorporated into proteins or glutathione, a tripeptide with important functions as storage and transport form of reduced sulfur, in oxidative stress defense, regulation of sulfur assimilation, etc. (Noctor et al., 1998). Thus, Cys synthesis from OAS and sulfide is a central point of cellular metabolism as this reaction interconnects sulfate, nitrate, and carbon assimilation.
Several studies have established regulatory interactions between sulfate and nitrate assimilation in plants (Brunold, 1993; Takahashi and Saito, 1996; Kim et al., 1999; Koprivova et al., 2000). The two assimilatory pathways are well coordinated so that deficiency for one element represses the other pathway. The activities of ATP sulfurylase, APR, and OAS (thiol) lyase decreased under nitrogen-deficient conditions in Lemna minor and cultured tobacco (Nicotiana tabacum) cells (Reuveny et al., 1980; Smith, 1980; Brunold and Suter, 1984). The addition of nitrate or ammonia to the N-deficient medium quickly restored the activity of these enzymes. Supplementing ammonia or amino acids (Arg, Asn, and Gln) to normal nutrient solution caused an 50% to 110% increase in extractable APR activity in L. minor and increased the flux through the sulfate assimilation measured as incorporation of 35S in proteins after feeding with [35S]sulfate (Brunold and Suter, 1984; Suter et al., 1986). In Arabidopsis, deprivation of a nitrogen source for 3 d led to 30% and 50% decrease of APR activity in leaves and roots, respectively, whereas the concentrations of Cys and glutathione were not affected (Koprivova et al., 2000). The decrease of APR activity correlated with decreased mRNA and enzyme levels. On the other hand, in plants, sulfur deficiency results in a reduction of nitrate reductase (NR) activity and an accumulation of amino acids (Reuveny et al., 1980; Migge et al., 2000; Prosser et al., 2001), whereas in cyanobacteria, NR is decreased and nitrite accumulates (Krämer and Schmidt, 1989). However, the reduction of NR activity and mRNA levels seems to be a relatively late process in plant adaptation to sulfur-limiting conditions (Prosser et al., 2001).
The molecular mechanisms of the coordination of sulfate and nitrate assimilation are not yet completely understood. OAS is considered to connect these pathways as it regulates sulfate uptake and assimilation. In the presence of excess sulfate, OAS seems to be limiting for Cys synthesis (Rennenberg, 1983). Overexpression of Ser acetyltransferase, the enzyme synthesizing OAS, led to increased Cys and glutathione (GSH) concentrations in transgenic potato (Solanum tuberosum) and tobacco (Blaszczyk et al., 1999; Harms et al., 2000). OAS accumulates during sulfur starvation and may thus act as a signal of the sulfur status (Kim et al., 1999). The addition of OAS increases sulfate uptake and APR activity and mRNA level also at normal sulfate levels (Neuenschwander et al., 1991; Smith et al., 1997; Koprivova et al., 2000). It is apparent that sulfate reduction is regulated by nitrogen nutrition on the transcriptional level, and OAS plays a major role in this regulation (Koprivova et al., 2000).
Very little is known about the interactions of sulfur and carbon assimilation. It is clear that sulfate assimilation is dependent on photosynthesis as a direct or indirect source of reduction equivalents, as demonstrated by the light dependency of sulfate reduction by broken chloroplasts (Schmidt and Trebst, 1969). The flux through sulfate assimilation is lower in the dark than in the light (Kopriva et al., 1999). On the other hand, sulfur limitation reduced growth and photosynthesis of the green alga Dunaliella salina (Giordano et al., 2000). The addition of Suc to Arabidopsis plants in the dark induced the accumulation of APR mRNA, protein, and enzyme activity, revealing that sulfate assimilation is directly regulated by carbohydrates (Kopriva et al., 1999).
Here, we report the effects of omission of CO2 from air and its substitution by different carbon sources on APR and NR, the key enzymes of sulfate and nitrate assimilation, respectively. To address the flux through the sulfate assimilation pathway under these conditions, we also determined the incorporation of [35S]sulfate into Cys, glutathione, and proteins.
RESULTS
Effect of CO2 Deficiency on APR and NR
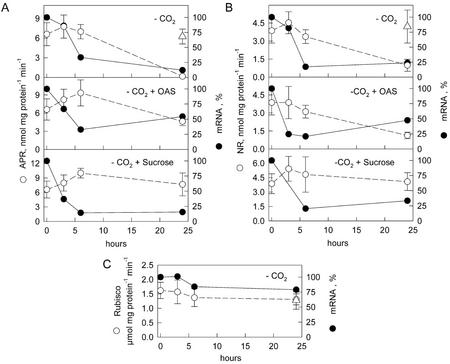
To investigate the interactions between sulfate and carbon assimilation, liquid cultures of L. minor plants were incubated in air without CO2. After 24 h, the activity of APR decreased to almost undetectable levels (Fig. 1). NR decreased to 25% of the control levels, but the activity of Rubisco was not affected. The reduction in APR and NR activity was accompanied by a rapid reduction of the corresponding mRNA levels. Already after 6 h of treatment in air without CO2, the mRNA levels dropped to under 25% of the control levels and remained low during further incubation. The small subunit of Rubisco mRNA levels were only slightly reduced during the 24-h treatment. When an alternative carbon source, 2 mm Suc, was present in the nutrient solution during the treatment, APR and NR activities were not affected by the CO2 absence. It is surprising that Suc addition did not influence the decrease in mRNA levels. Because OAS is considered to be the molecular signal coordinating nitrate and sulfate assimilation, we wanted to test whether this compound may also participate in the coordination of sulfate and carbon assimilation. The presence of 1 mm OAS in the nutrient solution during the treatment in air without CO2 reduced the effect on APR activity, which reached 75% of the control in normal air after 24 h. No influence of OAS on NR activity was observed. Although the reduction in APR mRNA level was attenuated by OAS, the mRNA level declined to 50% of the control levels (Fig. 1).
Figure 1.
Effect of incubation in air without CO2 on APR and NR in L. minor. Liquid cultures of L. minor were cultivated in air without CO2 (−CO2) and with the simultaneous addition of 1 mm OAS (−CO2 + OAS) or 2 mm Suc (−CO2 + Suc) in continuous light. APR (A), NR (B), and Rubisco (C) activities (o) and mRNA levels (●) were measured at the time points indicated, and Δ indicates enzyme activities in normal air. Mean values ± sd from four measurements of the enzyme activities and mean values of two measurements of mRNA levels are shown. The mRNA level at the beginning of incubation in air without CO2 was set to 100%.
Effect of Supply of Different Carbon Sources on APR and NR
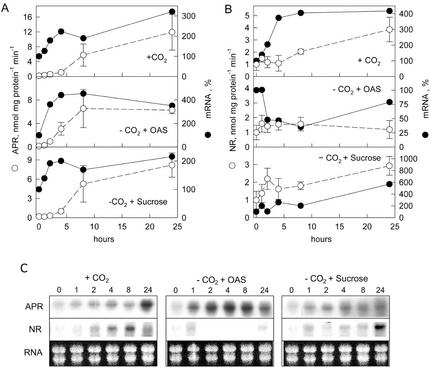
When the plants incubated in air without CO2 for 24 h were supplied with normal air, APR activity increased and reached 2-fold higher activity in 24 h than before the treatment (Fig. 2A, compare with Fig. 1). In contrast, when further cultivated without CO2, the activity remained almost undetectable (data not shown). The induction of APR activity by normal air was a relatively slow process; the activity was significantly increased only after 8 h. The addition of Suc instead of normal air led to a similar time course of induction of APR, but after 24 h, APR activity was lower than in plants supplied with normal air. OAS also induced APR activity in air without CO2; the increase was faster and at 24 h, the activity was comparable with that in plants supplied with Suc and was lower than in plants supplied with normal air (Fig. 2A). After the addition of OAS, the activity was significantly enhanced already after 4 h and reached maximum 8 h after the beginning of the experiment. The addition of another carbohydrate, Glc, affected APR activity in the same way as Suc (data not shown). The induction of APR activity was preceded in all cases by an increase in APR mRNA levels (Fig. 2, A and C). Incubation in normal air or the addition of Suc led to a rapid increase in APR mRNA levels, which after 2 h was enhanced 2-fold. OAS treatment resulted in even a higher increase in the APR mRNA level, reaching 400% of that at the beginning of the experiment within 2 h.
Figure 2.
Effect of the addition of OAS and Suc to plants preincubated in air without CO2. Liquid cultures of L. minor were cultivated in air without CO2 for 24 h in continuous light. After reaeration with normal air (+CO2) or addition of 1 mm OAS (−CO2 + OAS) and 2 mm Suc (−CO2 + Suc) and further cultivation in air without CO2, APR (A) and NR (B) activities (o) and mRNA levels (●) were measured at the time points indicated. Mean values ± sd from four measurements of the enzyme activities and mean values of two measurements of mRNA levels are shown. The mRNA level after 24 h of incubation in air without CO2 was set to 100%. C, Representative northern blots of APR and NR. Ethidium bromide-stained RNA (RNA) is shown as a control of RNA intactness and loading.
In addition, NR activity recovered to the levels before the treatment after 24 h of growth in air without CO2 and a further 24 h in normal air (Fig. 2B, compare with Fig. 1). The induction of NR by normal air followed the same time course as APR; a significant increase could be detected only after 8 h (Fig. 2B). The addition of Suc led to a quicker response: NR was increased already after 2 h. Similar to APR, Glc induced NR activity in the same manner as Suc (data not shown). In contrast to APR, Suc or Glc treatment increased NR activity to the same level as normal air. OAS did not affect NR activity. Similar to APR, the induction of NR was preceded by an increase in mRNA level. Suc was more effective than a resupply of normal air because the mRNA level increased 6-fold compared with 4-fold after reaeration. OAS treatment resulted in a further decrease of NR mRNA.
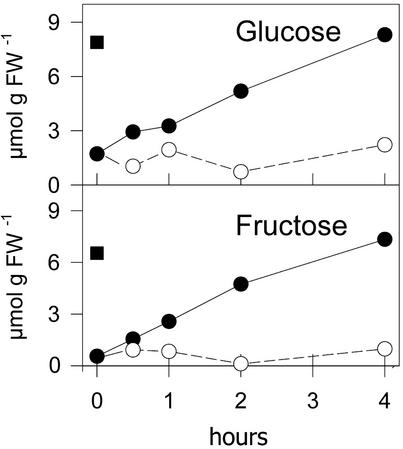
As demonstrated in Figure 3, incubation in air without CO2 for 24 h resulted in a depletion of cellular pools of the monosaccharides Glc and Fru. After a resupply of normal air, the carbohydrate pools recovered within 4 h. OAS, on the other hand, was not able to supply the carbon necessary for recovery of Glc and Fru pools, and the contents of the two monosaccharides remained low.
Figure 3.
Changes in monosaccharides after reaeration with normal air or the addition of OAS to plants preincubated in air without CO2. Liquid cultures of L. minor were cultivated in air without CO2 for 24 h in continuous light. The concentration of Glc and Fru was measured after reaeration with normal air (●) or the addition of 1 mm OAS and further cultivation in air without CO2 (○) at the time points indicated. █, Indicates control concentrations in normal air. Mean values from two measurements are indicated.
In Vivo Flux through the Sulfate Assimilation Pathway
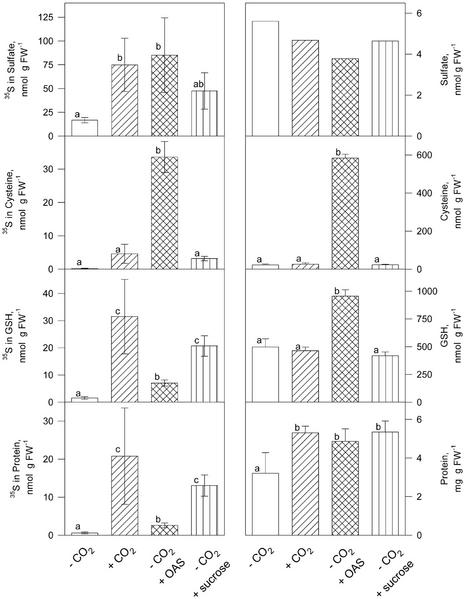
For analyzing in vivo sulfate assimilation, plants that were cultivated in air without CO2 for 24 h and were resupplied with normal air or different carbon sources for 24 h were fed with 35SO42− in the nutrient solution during the last 4 h of the treatment. The amount of radioactivity incorporated into thiols and proteins was measured. Incubation in air without CO2 almost completely abolished the flux through sulfate assimilation, and accumulation of labeled sulfate was largely inhibited. However, the treatment had no effect on the sulfate pool or on the concentrations of Cys and glutathione (Fig. 4). When the plants were resupplied with normal air or different carbon sources, the 35SO42− accumulation was 3- to 5-fold induced. All compounds supported protein synthesis, as demonstrated by increased soluble protein concentrations. Cys and GSH concentrations were only affected by treatment with OAS, which resulted in a 25-fold increase in Cys and a 2-fold increased GSH level. The flux through sulfate assimilation, measured as incorporation of 35S in thiols and proteins, was increased by all three carbon sources. Labeling of Cys was 100-fold enhanced due to the addition of OAS, whereas normal air and Suc increased the labeling 15- and 12-fold, respectively. Incorporation of 35S into glutathione and proteins increased most by a resupply of normal air; the effect of Suc was approximately 30% smaller, and OAS increased the labeling of GSH and proteins only 3- to 4-fold. Related to the sulfate uptake, addition of Suc was as effective as incubation in normal air in enhancing the flux through sulfate assimilation; in both treatments, 43% of 35S was found in the reduced form, compared with 33% and 11% for OAS supplement and the control in air without CO2, respectively.
Figure 4.
Incorporation of 35S from [35S]sulfate into thiols and proteins. The plants were cultivated in air without CO2 for 24 h, for 24 h in air without CO2, for 24 h with normal air, in air without CO2 for 48 h, and with 1 mm OAS during the last 24 h, and in air without CO2 for 48 h and with 2 mm Suc during the last 24 h. 35SO42− was added to the nutrient solution for the last 4 h of the treatments. Radioactive sulfur (left) and total content (right) of SO42−, Cys, GSH, and protein was measured. Mean values ± sd of four measurements are presented. Values indicated by different letters represent significant differences at P ≤ 0.01.
DISCUSSION
Until now, little attention has been paid to the interactions between sulfate and carbon assimilation. In contrast, nitrate assimilation was shown to be dependent on the supply of carbon skeletons produced during CO2 assimilation (Oaks, 1994). NR, as the key enzyme of this pathway, is inactivated upon exposure of plants to air without CO2 (Kaiser and Förster, 1989). NR activity and expression is induced by light, but also by sugars in the dark (Cheng et al., 1992; Campbell, 1999). The level of NR transcript is modulated by CO2 availability; it decreases when CO2 assimilation is diminished, e.g. due to water stress (Foyer et al., 1998), and increases upon exposure of plants to elevated CO2 (Fonseca et al., 1997). The present measurements with L. minor fit exactly to the expected pattern. In air without CO2, NR activity and mRNA levels decreased and this decrease could be attenuated by feeding Suc (Fig. 1B). Corresponding to this, the activity and mRNA level were recovered after incubation in normal air or by the addition of Glc or Suc in the air without CO2 (Fig. 2B). It seems that the carbon sources effective in reactivation of NR must enter the carbohydrate metabolism and be able to replenish the hexose pools (Fig. 3) or to increase the content of sugar phosphates, which are known to protect NR against dark inactivation (De Cires et al., 1993) most probably due to the inactivation of the NR protein kinase (Kaiser et al., 1999). Not all carbon sources were able to restore NR activity after the treatment in air without CO2, as demonstrated by lack of effect after addition of OAS (Figs. 1B and 2B) most probably due to the presence of an amino group in this compound. OAS, as a source of reduced nitrogen, was shown to repress NR activity (Neuenschwander et al., 1991). Whether OAS is the compound signaling the sulfur status of the plant toward nitrate assimilation is a matter of debate because the regulation of nitrate uptake and reduction can also be triggered by changes in concentrations of free amino acids, which were shown to accumulate upon sulfur deficiency (Klapheck et al., 1982; Migge et al., 2000; Prosser et al., 2001).
For Cys synthesis, sulfate assimilation requires the carbon skeletons of OAS as acceptors of reduced sulfide. APR activity was induced by feeding OAS in the light and in the dark (Neuenschwander et al., 1991). Increased synthesis of OAS due to overexpression of Ser acetyltransferase affected sulfate assimilation and resulted in increased Cys and glutathione synthesis (Blaszczyk et al., 1999; Harms et al., 2000). Apart from the regulation by OAS, to the best of our knowledge, the interactions of sulfate assimilation with assimilation of CO2 or carbohydrates were described only by Kopriva et al. (1999). In that report, it was shown that APR activity and flux through sulfate assimilation are higher in the light than in the dark and that Suc feeding in the dark can induce APR activity and mRNA accumulation and thus mimic the effect of illumination. However, light increases not only the carbon assimilates, but also provides reduction power. In accordance with this, Suc may serve as a source of carbon skeletons or may enter the oxidative pentose phosphate cycle and thus provide reduction equivalents for reduction of sulfate. The direct connection between carbon assimilation and sulfate reduction is clearly confirmed in the present study by the reduction of APR activity during the incubation in air without CO2 (Fig. 1A). The mechanism of this regulation is independent from the process of CO2 fixation because addition of Suc prevents the decrease in APR activity (Fig. 1A). The inactivation of APR is most probably transcriptionally regulated because the APR mRNA level decreased simultaneously with the enzyme activity, indicating that APR is affected in similar way as NR (Kaiser and Huber, 2001). It is noteworthy that although in Arabidopsis, three isoforms of APR exist and are differentially regulated by light and nitrogen (Kopriva et al., 1999, 2000), in L. minor, with most probably two APR genes present in the genome, the isoform analyzed in this report contributes at least 85% to the total APR mRNA pool (Suter et al., 2000).
Thus, the regulation by CO2 is another example of a common regulation of APR and NR, the key enzymes of sulfate and nitrate assimilation, respectively, which contributes to the coordination of the two pathways. Both enzymes undergo diurnal rhythms with maximum activity at daytime and are inactivated by darkness (Galangau et al., 1988; Huber et al., 1992; Koczy et al., 1997; Kopriva et al., 1999), both can be induced by Suc in the dark (Cheng et al., 1992; Kopriva et al., 1999), and both are reduced when the other element is limiting (Reuveny et al., 1980; Koprivova et al., 2000). However, the mechanisms of regulation by carbohydrates seem not to be identical. Whereas APR is regulated prevalently at the level of mRNA (Kopriva et al., 1999, 2000), posttranslational regulation plays an important role in modulation of NR activity. NR can be phosphorylated and in this form, can be inactivated by interaction with 14-3-3 proteins. The phosphorylation of NR and the degradation of NR-14-3-3 protein complex are inhibited by sugar-phosphates (Kaiser and Huber, 2001). Also, APR seems to be regulated posttranslationally by oxidative stress (Bick et al., 2001). However, nothing is known about possible regulation of APR by phosphorylation or protein-protein interactions and thus about even further reaching similarity of the two enzymes.
The addition of Suc prevented the deactivation of APR and NR by CO2 deficiency but was not as effective as normal air in restoring the enzyme activities after preincubation in air without CO2 (Fig. 2). Suc, in the absence of CO2, induced APR mRNA accumulation with the same time course as normal air; however, the levels of APR mRNA after 24 h were about 50% higher in plants supplied with air. This difference in mRNA level correlates perfectly with the results of enzyme activity measurements. Thus, Suc, although being the final product of carbon assimilation, is most probably not the molecular signal connecting carbon and sulfate assimilation. Also, OAS was less effective as CO2 in reactivating APR and did not affect NR activity at all. Thus, OAS is not a good candidate for signaling the carbon status. Because APR and NR are regulated by CO2 and carbohydrates in very similar manner, it can be expected that the same signal is regulating both pathways. This compound is most probably an intermediate in carbohydrate synthesis produced by CO2 fixation and Suc degradation.
The 35SO4 feeding experiment revealed that under CO2-deficient conditions, sulfate uptake and reduction were severely reduced. The decrease in APR activity and flux through sulfate assimilation was similar to that caused by prolonged cultivation of L. minor in the dark (Neuenschwander et al., 1991). The incubation in air without CO2 resulted in decreased protein concentrations. The concentration of thiols, Cys, and glutathione was not affected by this treatment or by substitution of CO2 with Suc. This result shows that, similar to nitrogen deficiency (Koprivova et al., 2000), the thiol concentrations in plants are very tightly regulated and even if sulfate uptake and APR activity are reduced, the concentrations of Cys and GSH remain stable (Fig. 4). A supply of OAS resulted in an enormous increase of Cys synthesis and content and, consequently, in enhanced GSH concentration. On the other hand, the incorporation of 35S in GSH and proteins were significantly lower in plants supplied with OAS than in those in normal air or after the addition of Suc. It seems that OAS can be rapidly metabolized to Cys but is not an efficient source of carbon skeletons for synthesis of amino acids and sugars. Thus, the low concentration of amino acids would prevent synthesis of GSH and proteins even if Cys concentration is high. GSH synthesis, although normally limited by availability of Cys (Strohm et al., 1995), is limited by supply of Gly in the dark (Noctor et al., 1997).
The described regulation of sulfate assimilation by carbohydrates is an important mechanism for coordinating the reduction of sulfate with the demand for reduced sulfur. When the carbohydrate production is low, during dark (Kopriva et al., 1999) or incubation in air without CO2, APR is reduced to prevent formation and accumulation of toxic amounts of Cys or intermediates of sulfate reduction, such as sulfite and sulfide. On the other hand, when energy is provided to plant tissues in the form of carbohydrates, sulfate assimilation is activated to supply the sulfur-containing amino acids for increased protein synthesis. The rapid increase of APR mRNA already 1 h after the addition of Suc or normal air points out a direct regulation of APR by photosynthates. The mechanism of the APR regulation by sugars will be subject of a further study.
In conclusion, in this report, we documented the dependence of sulfate assimilation upon the assimilation of carbon. In addition, the present results indicate once more the coordinated regulation of the sulfate and nitrate assimilation pathways. Therefore, the assimilatory pathways should not be viewed individually because they are tightly interconnected and very much influence each other.
MATERIALS AND METHODS
Plant Material
Lemna minor was cultivated in E-NO3 medium containing 1.67 mm NO3− and 0.88 mm SO42− as described, under constant conditions: continuous light (100 μE m−2 s−1), 25°C, 80% relative humidity, and 340 μL L−1 CO2 in air contained in a pressure flask (Brunold and Suter, 1984). The CO2-deficient conditions were established by aerating with air without CO2 from a pressure flask.
Enzyme Measurements
Plants were washed for 1 min with H2O at 4°C, and extracts were prepared by grinding 1:10 (w/v) in 0.1 m Tris-HCl, pH 8.0, containing 100 mm KCl, 20 mm MgCl2, and 10 mm dithioerythritol in a glass homogenizer. The homogenate was centrifuged for 10 min at 10,000g and the supernatant was used for the enzyme assays. APR activity in extracts was measured as the production of [35S]sulfite, assayed as acid volatile radioactivity, formed from [35S]APS in the presence of dithioerythritol (Brunold and Suter, 1990). NR activity was determined by measuring the NO2− formed from NO3− (Hageman and Reed, 1980). The protein concentrations of the extracts were determined according to Bradford (1976) with bovine serum albumin as standard (Bio-Rad Protein Assay; Bio-Rad Laboratories, Munich).
Isolation of Total RNA and Northern Blotting
The plants were pulverized with mortar and pestle in liquid nitrogen, and the RNA was isolated by phenol extraction and selective precipitation with LiCl. Total RNA was separated on an agarose-formaldehyde gel. The RNA was transferred onto Hybond-N nylon membranes (Amersham Biosciences, Freiburg, Germany) and was hybridized with 32P-labeled cDNA probes for APR, NR, and Rubisco small subunit that were isolated from L. minor total RNA by reverse transcriptase-PCR with degenerated primers against conserved domains (Suter et al., 2000). The membranes were washed four times at different concentrations of SSC in 0.1% (w/v) SDS for 20 min, the final washing step being 0.5× SSC and 0.1% (w/v) SDS at 65°C, and they were exposed to a phosphorimager (Bio-Rad, Reinach, Switzerland) for 24 h. The images were quantified using the software Molecular analyst (Bio-Rad, Reinach, Switzerland). Ethidium bromide-stained ribosomal RNA was used as standard for equal loading and RNA intactness. Average values from quantification of two blots with independent RNA preparations are shown.
Determination of Carbohydrates
For determination of carbohydrates, 50 mg of powdered plant material was extracted with 1 mL of distilled water and 100 mg of polyvinylpyrrolidone (Sigma, Munich). The extracts were further diluted with water as required for HPLC analysis. Samples of 100 μL were injected into an HPLC system, separated on a CarboPac 1 separation column, and measured by means of a pulsed amperometric detector as previously described (Heizmann et al., 2001). Individual carbohydrates were identified and quantified with internal and external standards.
Feeding of 35SO42− and Determination of 35S in Thiols and Proteins
Four 30-mL liquid cultures containing approximately 100 fronds of L. minor were preincubated in air without CO2 for 24 h in E-NO3 nutrient solution containing 0.88 mm SO42− and were resupplied with normal air or cultivated further in air without CO2 and addition of 1 mm OAS, 2 mm Suc, or without any additional carbon source for additional 24 h. In the last 4 h of the treatment, 1 mCi 35SO42− (Hartmann, Braunschweig, Germany) was added to each culture. The L. minor plants were washed twice for 15 min in ice water, extracted 1:10 (w/v) in glass potters in 0.1 m HCl containing 1 mm Na2 EDTA, and the extracts were centrifuged for 20 min at 4°C. The samples were analyzed as described in Kopriva et al. (1999) and Koprivova et al. (2000). The thiols in the supernatant were reduced with bis-(2-mercaptoethylsulfone) and were labeled by monobromobimane as described by Kranner and Grill (1996). A 100-μL aliquot of each sample was separated by reverse-phase HPLC as previously described (Rüegsegger and Brunold, 1992), and fractions of 0.75 mL were collected in scintillation vials. The 35S radioactivity was determined in a Betamatic V liquid scintillation counter (Kontron, Zurich). The radioactivity in the first five fractions of the eluate corresponded to 35SO42−. Total sulfate was quantified using a NaOH gradient on an ion chromatographic system (DX-500; Dionex, Sunnyvale, CA). Total Cys, γ-EC, and GSH were analyzed by the same HPLC system as described by Rüegsegger and Brunold (1992). For measurement of 35S incorporation into proteins, these were precipitated from 200 μL of extract with 10% (w/v) trichloroacetic acid, washed twice with 1% (w/v) trichloroacetic acid and once with 96% (w/v) ethanol, and redissolved in 400 μL of 0.2 m NaOH. Radioactivity in an aliquot of the protein solution was determined using a liquid scintillation counter.
Statistical Analysis
The Student Newmann Keuls method (SigmaStat for Windows, version 1.0, 1992–1994; Jandel Corporation, Costa Madre, CA) was used to determine significant differences in the enzyme activities and the contents of labeled thiols.
ACKNOWLEDGMENTS
We thank Monika Eiblmeier (Department of Tree Physiology, Freiburg) for sugar measurements and Prof. Karl Erismann (Bern) for providing Lemna stock cultures.
Footnotes
This work was supported by the Swiss National Foundation (grant no. 3149246–96 to C.B.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.007773.
LITERATURE CITED
- Bick JA, Setterdahl AT, Knaff DB, Chen Y, Pitcher LH, Zilinskas BA, Leustek T. Regulation of the plant-type 5′-adenylyl sulfate reductase by oxidative stress. Biochemistry. 2001;40:9040–9048. doi: 10.1021/bi010518v. [DOI] [PubMed] [Google Scholar]
- Blaszczyk A, Brodzik R, Sirko A. Increased resistance to oxidative stress in transgenic tobacco plants overexpressing bacterial serine acetyltransferase. Plant J. 1999;20:237–243. doi: 10.1046/j.1365-313x.1999.00596.x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein dye-binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brunold C. Reduction of sulfate to sulfide. In: Rennenberg H, Brunold C, De Kok LJ, Stulen I, editors. Sulfur Nutrition and Sulfur Assimilation in Higher Plants. The Hague, The Netherlands: SPB Academic Publishing; 1990. pp. 13–31. [Google Scholar]
- Brunold C. Regulatory interactions between sulfate and nitrate assimilation. In: Rennenberg H, Brunold C, De Kok LJ, Stulen I, editors. Sulfur Nutrition and Sulfur Assimilation in Higher Plants. The Hague, The Netherlands: SPB Academic Publishing; 1993. pp. 61–75. [Google Scholar]
- Brunold C, Suter M. Regulation of sulfate assimilation by nitrogen nutrition in the duckweed Lemna minor L. Plant Physiol. 1984;76:579–583. doi: 10.1104/pp.76.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunold C, Suter M. Adenosine 5′-phosphosulfate sulfotransferase. In: Lea P, editor. Methods in Plant Biochemistry. Vol. 3. London: Academic Press; 1990. pp. 339–343. [Google Scholar]
- Campbell WH. Nitrate reductase structure, function and regulation: bridging the gap between biochemistry and physiology. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:277–303. doi: 10.1146/annurev.arplant.50.1.277. [DOI] [PubMed] [Google Scholar]
- Cheng CL, Acedo GN, Cristinsin M, Conkling MA. Sucrose mimics the light induction of Arabidopsis nitrate reductase gene transcription. Proc Natl Acad Sci USA. 1992;89:1861–1864. doi: 10.1073/pnas.89.5.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cires A, de la Torre A, Lara C. Involvement of CO2 fixation products in the light-dark modulation of nitrate reductase activity in barley leaves. Physiol Plant. 1993;89:577–581. [Google Scholar]
- Fonseca F, Bowsher CG, Stulen I. Impact of elevated atmospheric CO2 on nitrate reductase transcription and activity in leaves and roots of Plantago major. Physiol Plant. 1997;100:940–948. [Google Scholar]
- Foyer CH, Valadier MH, Migge A, Becker TW. Drought-induced effects on nitrate reductase activity and mRNA and on the coordination of nitrogen and carbon metabolism in maize leaves. Plant Physiol. 1998;117:283–292. doi: 10.1104/pp.117.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galangau F, Daniel-Vedel F, Moureaux T, Dorbe MF, Leydecker MT, Caboche M. Expression of leaf nitrate reductase genes from tomato and tobacco in relation to light-dark regimes and nitrate supply. Plant Physiol. 1988;88:383–388. doi: 10.1104/pp.88.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano M, Pezzoni V, Hell R. Strategies for the allocation of resources under sulfur limitation in the green alga Dunaliella salina. Plant Physiol. 2000;124:857–864. doi: 10.1104/pp.124.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman RH, Reed AJ. Nitrate reductase from higher plants. Methods Enzymol. 1980;69:270–280. [Google Scholar]
- Harms K, von Ballmoos P, Brunold C, Hofgen R, Hesse H. Expression of a bacterial serine acetyltransferase in transgenic potato plants leads to increased levels of cysteine and glutathione. Plant J. 2000;22:335–343. doi: 10.1046/j.1365-313x.2000.00743.x. [DOI] [PubMed] [Google Scholar]
- Heizmann U, Kreuzwieser J, Schnitzler J-P, Brüggemann N, Rennenberg H. Assimilate transport in the xylem sap of young oak (Quercus robur) trees. Plant Biol. 2001;3:132–138. [Google Scholar]
- Huber JL, Huber SC, Campbell WH, Redinbaugh MG. Reversible light/dark modulation of spinach leaf nitrate reductase activity involves protein phosphorylation. Arch Biochem Biophys. 1992;296:58–65. doi: 10.1016/0003-9861(92)90544-7. [DOI] [PubMed] [Google Scholar]
- Kaiser WM, Förster J. Low CO2 prevents nitrate reduction in leaves. Plant Physiol. 1989;91:970–974. doi: 10.1104/pp.91.3.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WM, Huber SC. Post-translational regulation of nitrate reductase: mechanism, physiological relevance and environmental triggers. J Exp Bot. 2001;52:1981–1989. doi: 10.1093/jexbot/52.363.1981. [DOI] [PubMed] [Google Scholar]
- Kaiser WM, Weiner H, Huber SC. Nitrate reductase in higher plants: a case study for transduction of environmental stimuli into control of catalytic activity. Physiol Plant. 1999;105:385–390. [Google Scholar]
- Kim H, Hirai MY, Hayashi H, Chino M, Naito S, Fujiwara T. Role of O-acetyl-l-serine in the coordinated regulation of the expression of a soybean seed storage-protein gene by sulfur and nitrogen nutrition. Planta. 1999;209:282–289. doi: 10.1007/s004250050634. [DOI] [PubMed] [Google Scholar]
- Klapheck S, Grosse W, Bergmann L. Effect of sulfur-deficiency on protein synthesis and amino acid accumulation in cell suspension cultures of Nicotiana tabacum. Z Pflanzenphysiol. 1982;108:235–245. [Google Scholar]
- Koczy G, Owttrim G, Brander K, Brunold C. Effect of chilling on the diurnal rhythm of enzymes involved in protection against oxidative stress in a chilling-tolerant and a chilling-sensitive maize genotype. Physiol Plant. 1997;99:249–254. [Google Scholar]
- Kopriva S, Muheim R, Koprivova A, Trachsel N, Catalano C, Suter M, Brunold C. Light regulation of assimilatory sulfate reduction in Arabidopsis thaliana. Plant J. 1999;20:37–44. doi: 10.1046/j.1365-313x.1999.00573.x. [DOI] [PubMed] [Google Scholar]
- Koprivova A, Suter M, Op den Camp R, Brunold C, Kopriva S. Regulation of sulfate assimilation by nitrogen in Arabidopsis. Plant Physiol. 2000;122:737–746. doi: 10.1104/pp.122.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer E, Schmidt A. Nitrite accumulation by Synechococcus 6301 as a consequence of carbon or sulfur deficiency. FEMS Microbiol Lett. 1989;59:191–196. [Google Scholar]
- Kranner I, Grill D. Determination of glutathione and glutathione disulfide in lichens: a comparison of frequently used methods. Phytochem Anal. 1996;7:24–28. [Google Scholar]
- Leustek T, Martin MN, Bick JA, Davies JP. Pathways and regulation of sulfur metabolism revealed through molecular and genetic studies. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:141–165. doi: 10.1146/annurev.arplant.51.1.141. [DOI] [PubMed] [Google Scholar]
- Migge A, Bork C, Hell R, Becker TW. Negative regulation of nitrate reductase gene expression by glutamine or asparagine accumulating in leaves of sulfur-deprived tobacco. Planta. 2000;211:587–595. doi: 10.1007/s004250000322. [DOI] [PubMed] [Google Scholar]
- Neuenschwander U, Suter M, Brunold C. Regulation of sulfate assimilation by light and O-acetyl-l-serine in Lemna minor L. Plant Physiol. 1991;97:253–258. doi: 10.1104/pp.97.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Arisi A-CM, Jouanin L, Kunert KJ, Rennenberg H, Foyer CH. Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot. 1998;49:623–647. [Google Scholar]
- Noctor G, Arisi A-CM, Jouanin L, Valadier M-H, Roux Y, Foyer CH. The role of glycine in determining the rate of glutathione synthesis in poplar: possible implications for glutathione production during stress. Physiol Plant. 1997;100:255–263. [Google Scholar]
- Oaks A. Efficiency of nitrogen utilization in C3 and C4 cereals. Plant Physiol. 1994;106:407–414. doi: 10.1104/pp.106.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser IM, Purves JV, Saker LR, Clarkson DT. Rapid disruption of nitrogen metabolism and nitrate transport in spinach plants deprived of sulphate. J Exp Bot. 2001;52:113–121. [PubMed] [Google Scholar]
- Rennenberg H. Role of O-acetylserine in hydrogen sulfide emission from pumpkin leaves in response to sulfate. Plant Physiol. 1983;73:560–565. doi: 10.1104/pp.73.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveny Z, Dougall DK, Trinity PM. Regulatory coupling of nitrate and sulfate assimilation pathways in cultured tobacco cells. Proc Natl Acad Sci USA. 1980;77:6670–6672. doi: 10.1073/pnas.77.11.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüegsegger A, Brunold C. Effect of cadmium on γ-glutamylcysteine synthesis in maize seedlings. Plant Physiol. 1992;99:428–433. doi: 10.1104/pp.99.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Trebst A. The mechanism of photosynthetic sulfate reduction by isolated chloroplasts. Biochim Biophys Acta. 1969;180:529–535. doi: 10.1016/0005-2728(69)90031-0. [DOI] [PubMed] [Google Scholar]
- Smith FW, Hawkesford MJ, Ealing PM, Clarkson DT, van den Berg PJ, Belcher AR, Warrilow AG. Regulation of expression of a cDNA from barley roots encoding a high affinity sulphate transporter. Plant J. 1997;12:875–884. doi: 10.1046/j.1365-313x.1997.12040875.x. [DOI] [PubMed] [Google Scholar]
- Smith IK. Regulation of sulfate assimilation in tobacco cells: effect of nitrogen and sulfur nutrition on sulfate permease and O-acetylserine sulfhydrylase. Plant Physiol. 1980;66:877–883. doi: 10.1104/pp.66.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohm M, Jouanin L, Kunert KJ, Pruvost C, Polle A, Foyer CH, Rennenberg H. Regulation of glutathione synthesis in leaves of transgenic poplar (Populus tremula × P. alba) overexpressing glutathione synthetase. Plant J. 1995;7:141–145. [Google Scholar]
- Suter M, Lavanchy P, von Arb C, Brunold C. Regulation of sulfate assimilation by amino acids in Lemna minor L. Plant Sci. 1986;44:125–132. [Google Scholar]
- Suter M, von Ballmoos P, Kopriva S, Op den Camp R, Schaller J, Kuhlemeier C, Schürmann P, Brunold C. Adenosine 5′-phosphosulfate sulfotransferase and adenosine 5′-phosphosulfate reductase are identical enzymes. J Biol Chem. 2000;275:930–936. doi: 10.1074/jbc.275.2.930. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Saito K. Subcellular localization of spinach cysteine synthase isoforms and regulation of their gene expression by nitrogen and sulfur. Plant Physiol. 1996;112:273–280. doi: 10.1104/pp.112.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]