Abstract
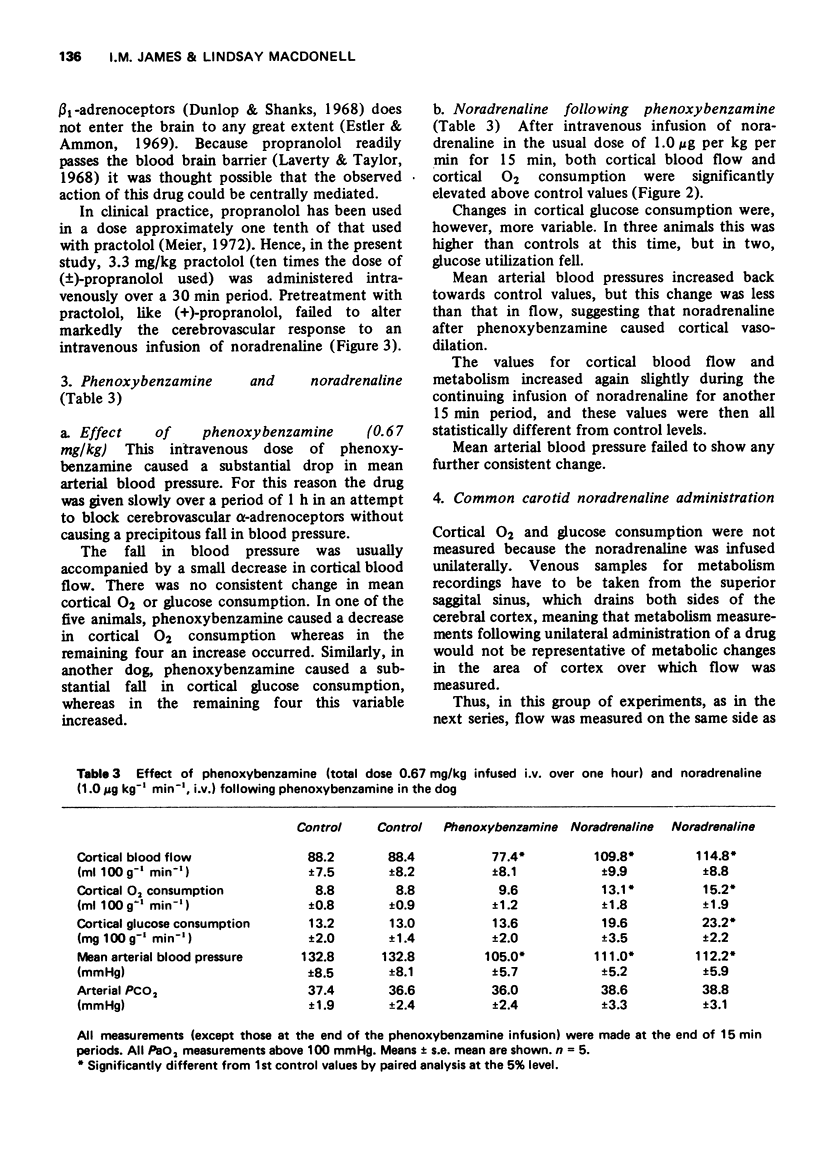
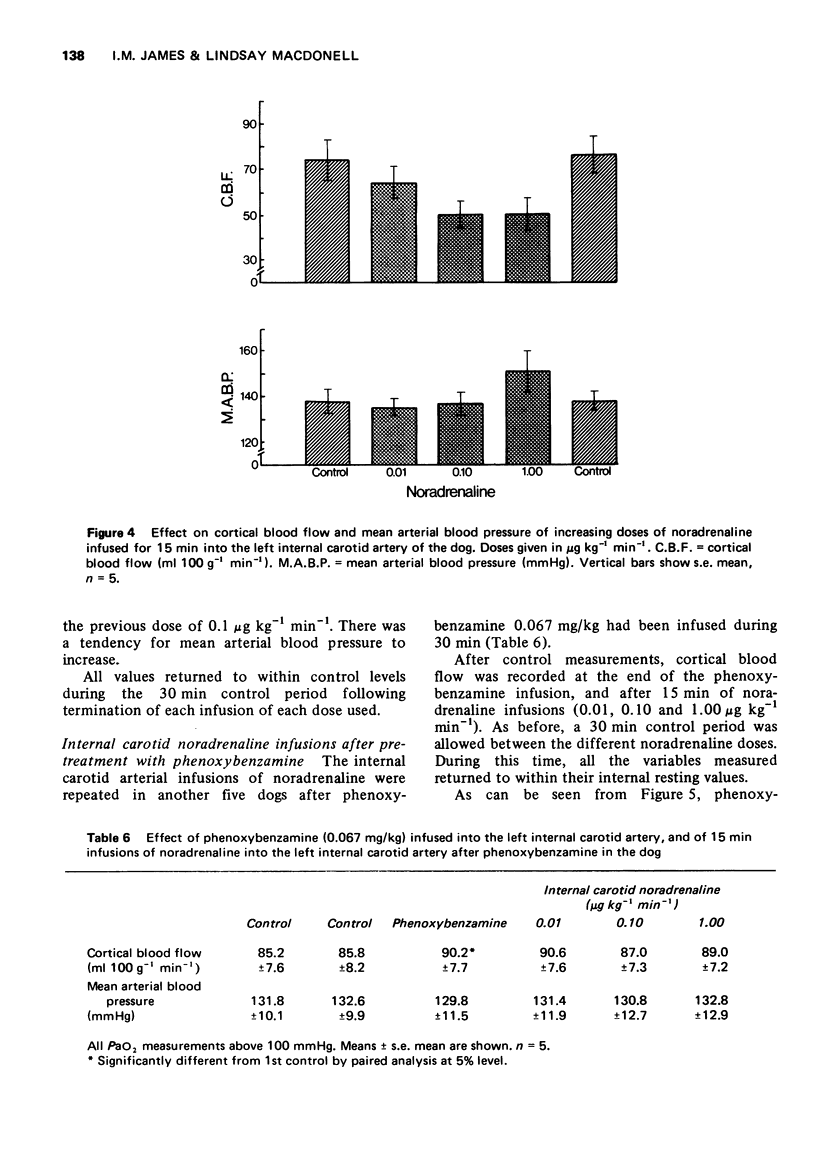
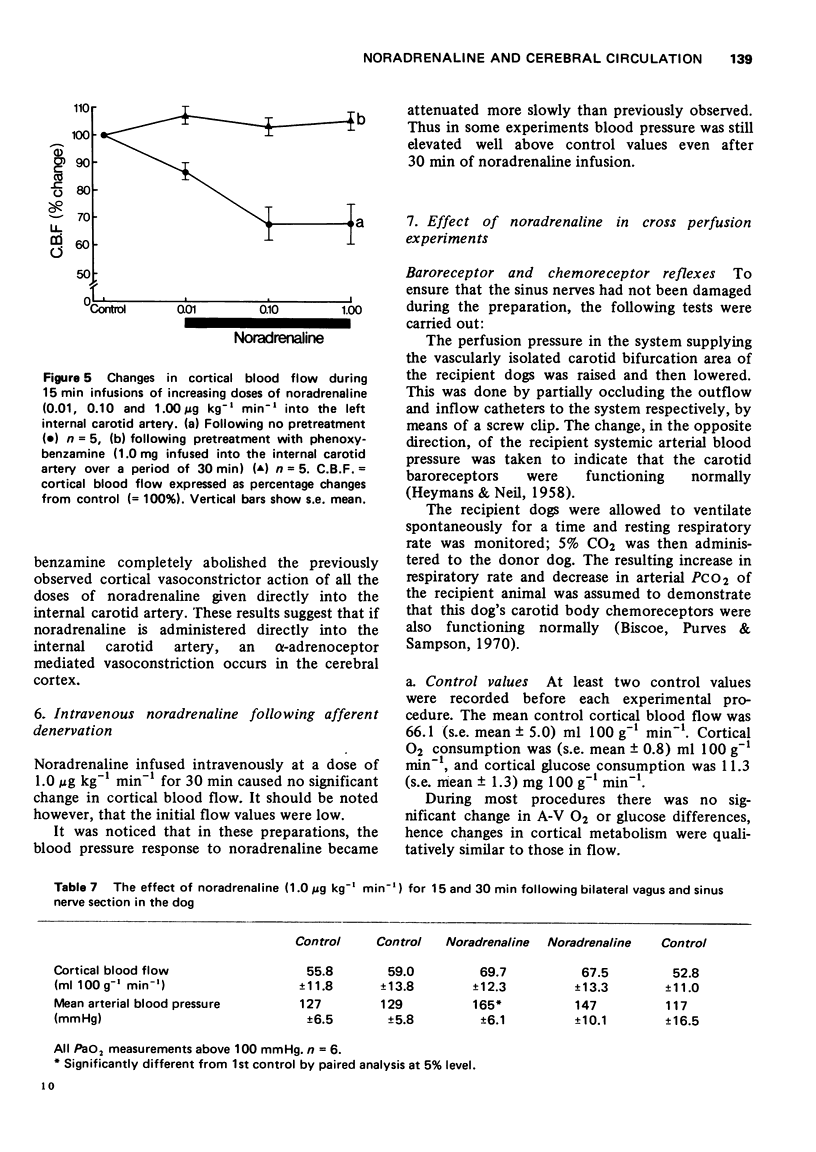
1 Noradrenaline infused into the internal carotid artery of the dog (0.01-1 μg kg-1 min-1) constricts the blood vessels of the cortex. This constriction is mediated by the action of noradrenaline on α-adrenoceptors of the cerebral arteries.
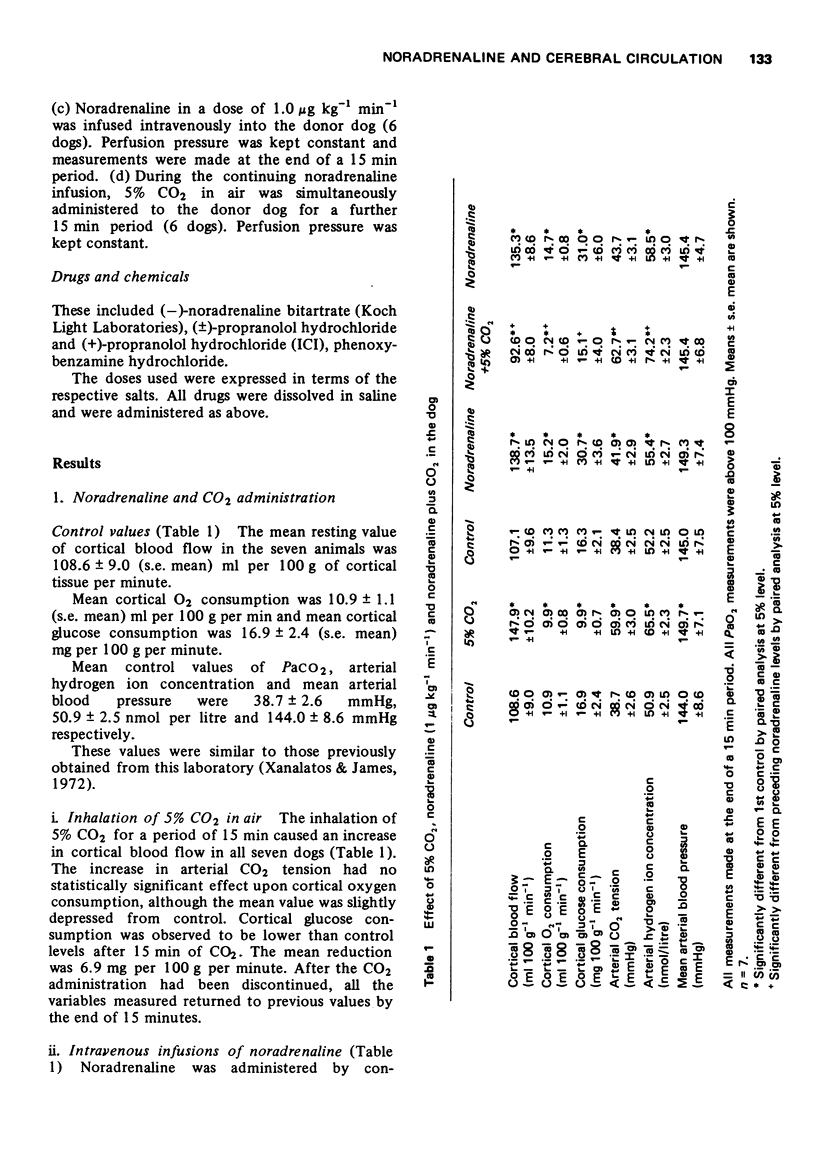
2 Intravenous (1 μg kg-1 min-1) or intra common carotid arterial (0.01-1 μg kg-1 min-1) infusions of noradrenaline cause an increase in cortical blood flow that can be dissociated from changes in blood pressure.
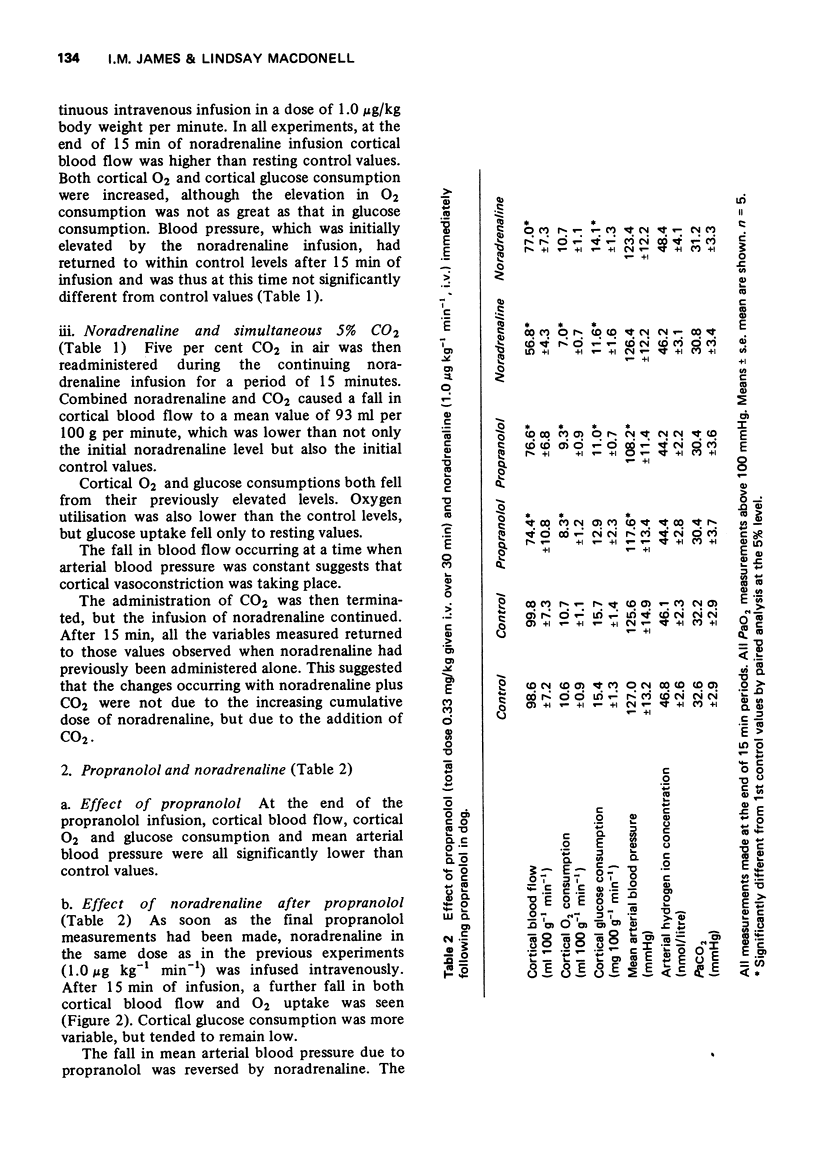
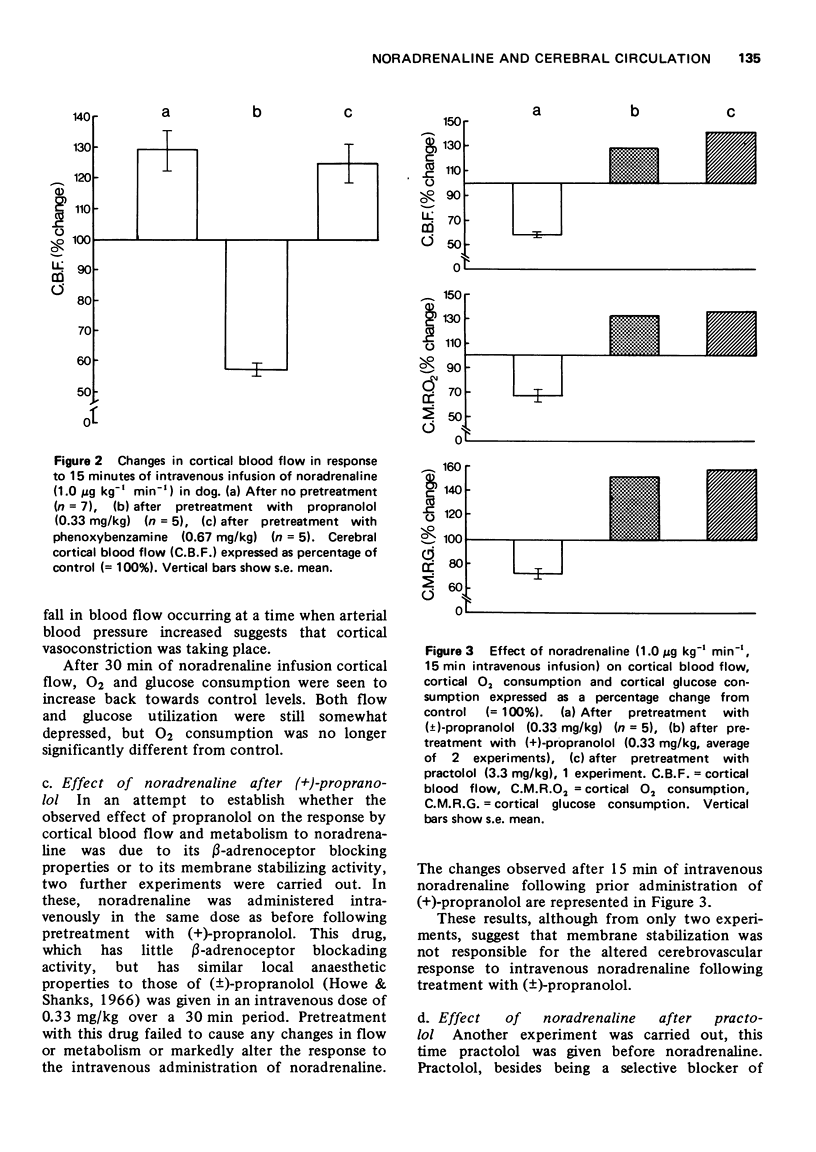
3 The effect of intravenous noradrenaline on the cortical blood vessels and metabolism is blocked by high PaCO2 levels, or by the prior administration of (±)-propranolol. (+)-Propranolol is without such effect.
4 Following section of both vagi and both sinus nerves, intravenous noradrenaline fails to cause an increase in cortical blood flow.
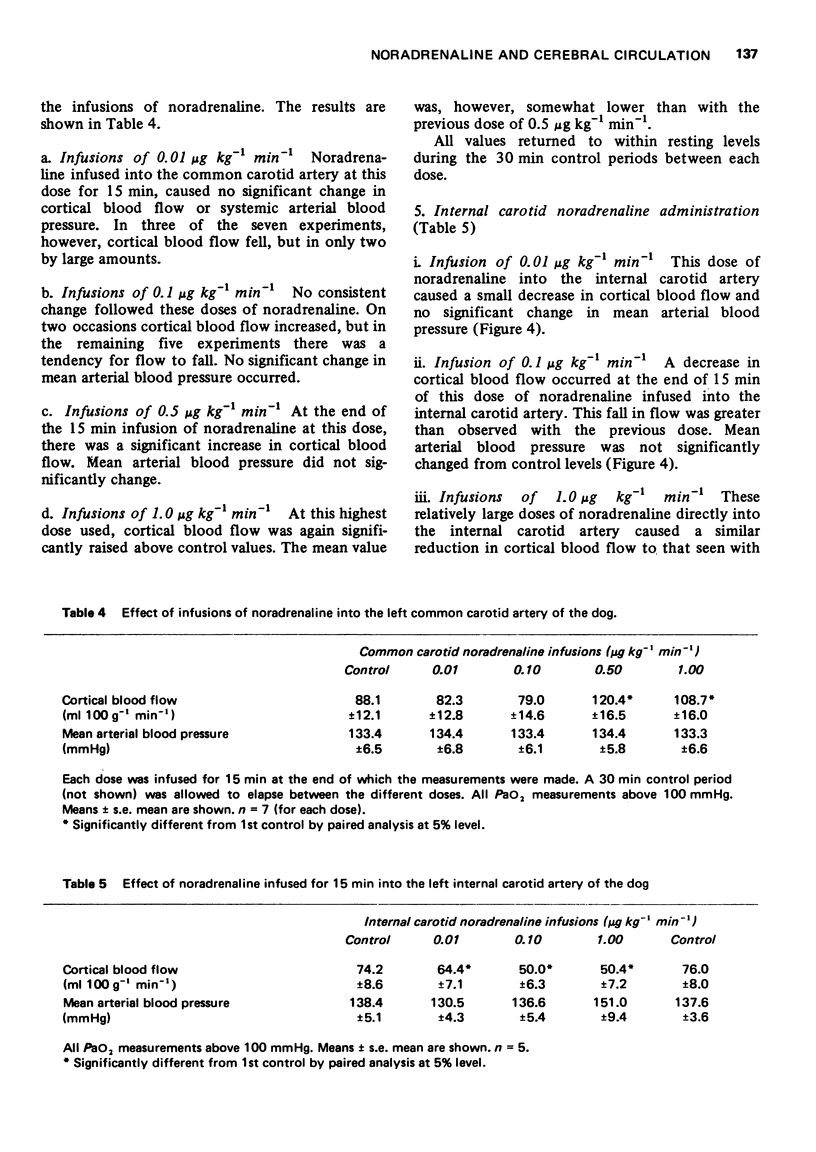
5 In another series of animals the area of the carotid bifurcation was vascularly isolated and perfused with blood from a second dog. Chemoreceptor and baroreceptor activity was shown to be intact.
6 Administration of 5% CO2 to the donor dog caused an increase in cerebral blood flow in the recipient dog.
7 Administration of intravenous noradrenaline (1.0 μg kg-1 min-1) to the donor animal caused an increase in cerebral blood flow, cerebral O2 and glucose utilization of the recipient.
8 Administration of 5% CO2 and intravenous (-)-noradrenaline (1.0 μg kg-1 min-1) caused a further increase in flow and metabolism.
9 This evidence suggests that the cerebrovasodilatation observed following intravenous noradrenaline is reflex and is triggered by chemoreceptor activity.
10 The evidence also suggests that the antagonism of the cortical dilatory effects of intravenous noradrenaline by raised PaCO2 in the intact animal must be at a site different from the peripheral chemoreceptors.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENFEY B. G., VARMA D. R. INHIBITION OF SYMPATHOMIMETIC EFFECTS ON THE HEART. Br J Pharmacol Chemother. 1964 Oct;23:399–404. doi: 10.1111/j.1476-5381.1964.tb01596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADLEY A. F., SEVERINGHAUS J. W., STUPFEL M. Variations of serum carbonic acid pK with pH and temperature. J Appl Physiol. 1956 Sep;9(2):197–200. doi: 10.1152/jappl.1956.9.2.197. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Purves M. J., Sampson S. R. The frequency of nerve impulses in single carotid body chemoreceptor afferent fibres recorded in vivo with intact circulation. J Physiol. 1970 May;208(1):121–131. doi: 10.1113/jphysiol.1970.sp009109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black A. M., Comroe J. H., Jr, Jacobs L. Species difference in carotid body response of cat and dog to dopamine and serotonin. Am J Physiol. 1972 Nov;223(5):1097–1102. doi: 10.1152/ajplegacy.1972.223.5.1097. [DOI] [PubMed] [Google Scholar]
- Carr P. W., Cooper T., Daggett W. M., Lish P. M., Nugent G. G., Powers P. C. Effects of compound MJ-1988 on myocardial contractility, oxygen consumption, coronary blood flow and vascular resistance. Br J Pharmacol Chemother. 1967 Sep;31(1):56–65. doi: 10.1111/j.1476-5381.1967.tb01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alecy L. G., Feigl E. O. Sympathetic control of cerebral blood flow in dogs. Circ Res. 1972 Aug;31(2):267–283. doi: 10.1161/01.res.31.2.267. [DOI] [PubMed] [Google Scholar]
- DAHL E., NELSON E. ELECTRON MICROSCOPIC OBSERVATIONS ON HUMAN INTRACRANIAL ARTERIES. II. INNERVATION. Arch Neurol. 1964 Feb;10:158–164. doi: 10.1001/archneur.1964.00460140044007. [DOI] [PubMed] [Google Scholar]
- Dunlop D., Shanks R. G. Selective blockade of adrenoceptive beta receptors in the heart. Br J Pharmacol Chemother. 1968 Jan;32(1):201–218. doi: 10.1111/j.1476-5381.1968.tb00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estler C. J., Ammon H. P. Absence of central nervous system effects of practalol [ICI 50,172; 4-(2-hydroxy-3-isopropylaminopropoxy)-acetanilide], a new adrenergic beta-receptor blocking drug. J Pharm Pharmacol. 1969 Aug;21(8):554–555. doi: 10.1111/j.2042-7158.1969.tb08315.x. [DOI] [PubMed] [Google Scholar]
- GLASS H. I., HARPER A. M., GLOVER M. M. The measurement of local cortical blood flow in the brain by the analysis of the clearance curve of krypton-85. Phys Med Biol. 1961 Jul;6:65–71. doi: 10.1088/0031-9155/6/1/305. [DOI] [PubMed] [Google Scholar]
- Gilboe D. D., Betz A. L. Oxygen uptake in the isolated canine brain. Am J Physiol. 1973 Mar;224(3):588–595. doi: 10.1152/ajplegacy.1973.224.3.588. [DOI] [PubMed] [Google Scholar]
- HEGEDUS S. A., SHACKELFORD R. T. A COMPARATIVE-ANATOMICAL STUDY OF THE CRANIO-CERVICAL VENOUS SYSTEMS IN MAMMALS, WITH SPECIAL REFERENCE TO THE DOG: RELATIONSHIP OF ANATOMY TO MEASUREMENTS OF CEREBRAL BLOOD FLOW. Am J Anat. 1965 Mar;116:375–386. doi: 10.1002/aja.1001160204. [DOI] [PubMed] [Google Scholar]
- Harper A. M., Deshmukh V. D., Rowan J. O., Jennett W. B. The influence of sympathetic nervous activity on cerebral blood flow. Arch Neurol. 1972 Jul;27(1):1–6. doi: 10.1001/archneur.1972.00490130003001. [DOI] [PubMed] [Google Scholar]
- Howe R., Shanks R. G. Optical isomers of propranolol. Nature. 1966 Jun 25;210(5043):1336–1338. doi: 10.1038/2101336a0. [DOI] [PubMed] [Google Scholar]
- Iwayama T., Furness J. B., Burnstock G. Dual adrenergic and cholinergic innervation of the cerebral arteries of the rat. An ultrastructural study. Circ Res. 1970 May;26(5):635–646. doi: 10.1161/01.res.26.5.635. [DOI] [PubMed] [Google Scholar]
- Iwayama T. Ultrastructural changes in the nerves innervating the cerebral artery after sympathectomy. Z Zellforsch Mikrosk Anat. 1970;109(4):465–480. doi: 10.1007/BF00343962. [DOI] [PubMed] [Google Scholar]
- James I. M., Millar R. A., Purves M. J. Observations on the extrinsic neural control of cerebral blood flow in the baboon. Circ Res. 1969 Jul;25(1):77–93. doi: 10.1161/01.res.25.1.77. [DOI] [PubMed] [Google Scholar]
- Joels N., White H. The contribution of the arterial chemoreceptors to the stimulation of respiration by adrenaline and noradrenaline in the cat. J Physiol. 1968 Jul;197(1):1–23. doi: 10.1113/jphysiol.1968.sp008541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajikawa H. Mode of sympathetic innervation of the cerebral vessels demonstrated by the fluorescent histochemical technique in rats and cats. Nihon Geka Hokan. 1969 Mar 1;38(2):227–235. [PubMed] [Google Scholar]
- LASSEN N. A. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959 Apr;39(2):183–238. doi: 10.1152/physrev.1959.39.2.183. [DOI] [PubMed] [Google Scholar]
- LINDEN R. J., LEDSOME J. R., NORMAN J. SIMPLE METHODS FOR THE DETERMINATION OF THE CONCENTRATIONS OF CARBON DIOXIDE AND OXYGEN IN BLOOD. Br J Anaesth. 1965 Feb;37:77–88. doi: 10.1093/bja/37.2.77. [DOI] [PubMed] [Google Scholar]
- Laverty R., Taylor K. M. Propranolol uptake into the central nervous system and the effect on rat behaviour and amine metabolism. J Pharm Pharmacol. 1968 Aug;20(8):605–609. doi: 10.1111/j.2042-7158.1968.tb09821.x. [DOI] [PubMed] [Google Scholar]
- Nielsen K. C., Owman C. Contractile response and amine receptor mechanisms in isolated middle cerebral artery of the cat. Brain Res. 1971 Mar 19;27(1):33–42. doi: 10.1016/0006-8993(71)90370-2. [DOI] [PubMed] [Google Scholar]
- Oberdörster G., Lang R., Zimmer R. Direct effects of - and -sympathomimetic amines on the cerebral circulation of the dog. Pflugers Arch. 1973 May 18;340(2):145–160. doi: 10.1007/BF00588173. [DOI] [PubMed] [Google Scholar]
- Olivares G. J., Smith N. T., Aronow L. Effect of propranolol on alpha-adrenergic blockade in the dog and isolated rabbit aortic strip. Br J Pharmacol Chemother. 1967 Jun;30(2):240–250. doi: 10.1111/j.1476-5381.1967.tb02130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte J., Purves M. J. The role of the carotid body chemoreceptors and carotid sinus baroreceptors in the control of cerebral blood vessels. J Physiol. 1974 Mar;237(2):315–340. doi: 10.1113/jphysiol.1974.sp010484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves M. J. Respiratory and circulatory effects of breathing 100 per cent oxygen in the new-born lamb before and after denervation of the carotid chemoreceptors. J Physiol. 1966 Jul;185(1):42–59. doi: 10.1113/jphysiol.1966.sp007971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. S., Robison S. C., Miller H. A., Harrison D. C. Effects of respiratory acidosis on circulatory response to isoproterenol. Am J Physiol. 1970 Feb;218(2):448–452. doi: 10.1152/ajplegacy.1970.218.2.448. [DOI] [PubMed] [Google Scholar]
- Xanalatos C., James I. M. Effect of arterial CO 2 pressure on the response of cerebral and hind-limb blood flow and metabolism to isoprenaline infusion in the dog. Clin Sci. 1972 Jan;42(1):63–68. doi: 10.1042/cs0420063. [DOI] [PubMed] [Google Scholar]


