Abstract
1 It is suggested that analgesia could be produced by drug action at the spinal level through (a) interference with neurotransmission at primary afferent terminals; (b) enhancement of the `gate control' of the sensory input to the spinal cord mediated through descending spinal tracts; or (c) increased presynaptic inhibition of primary afferents by a direct action.
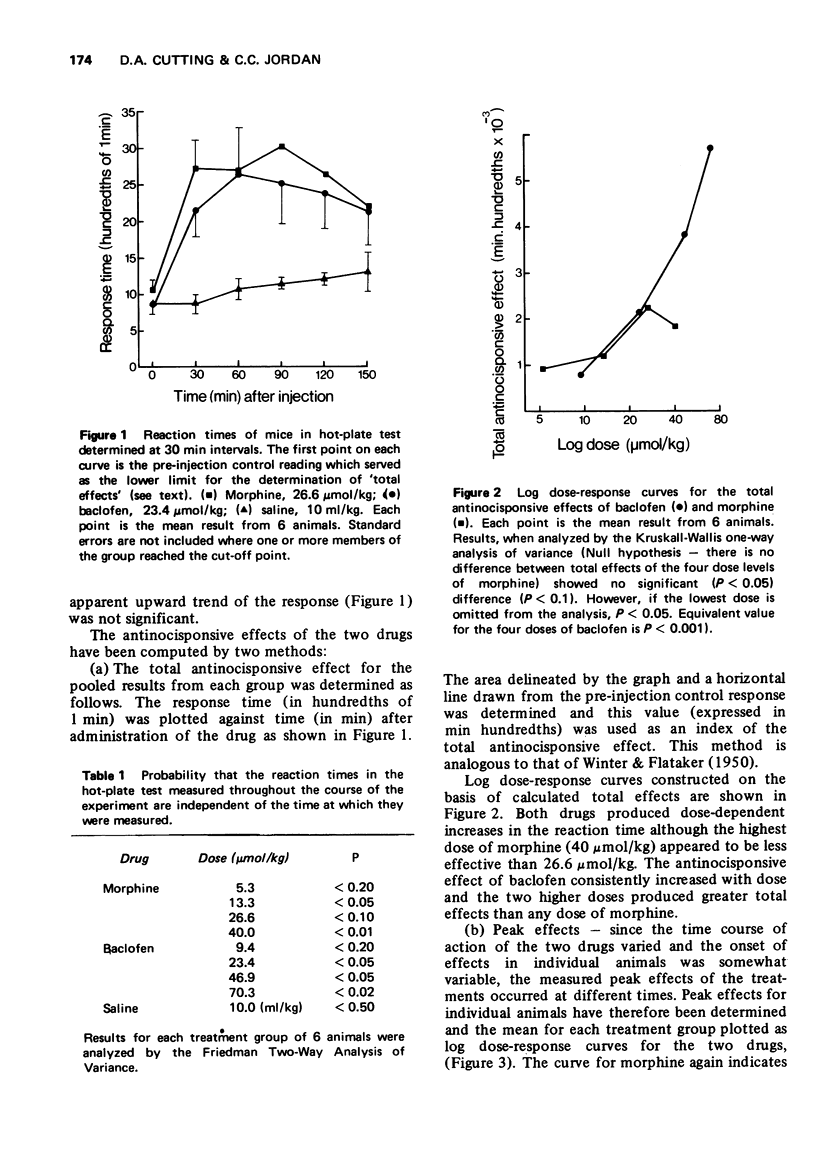
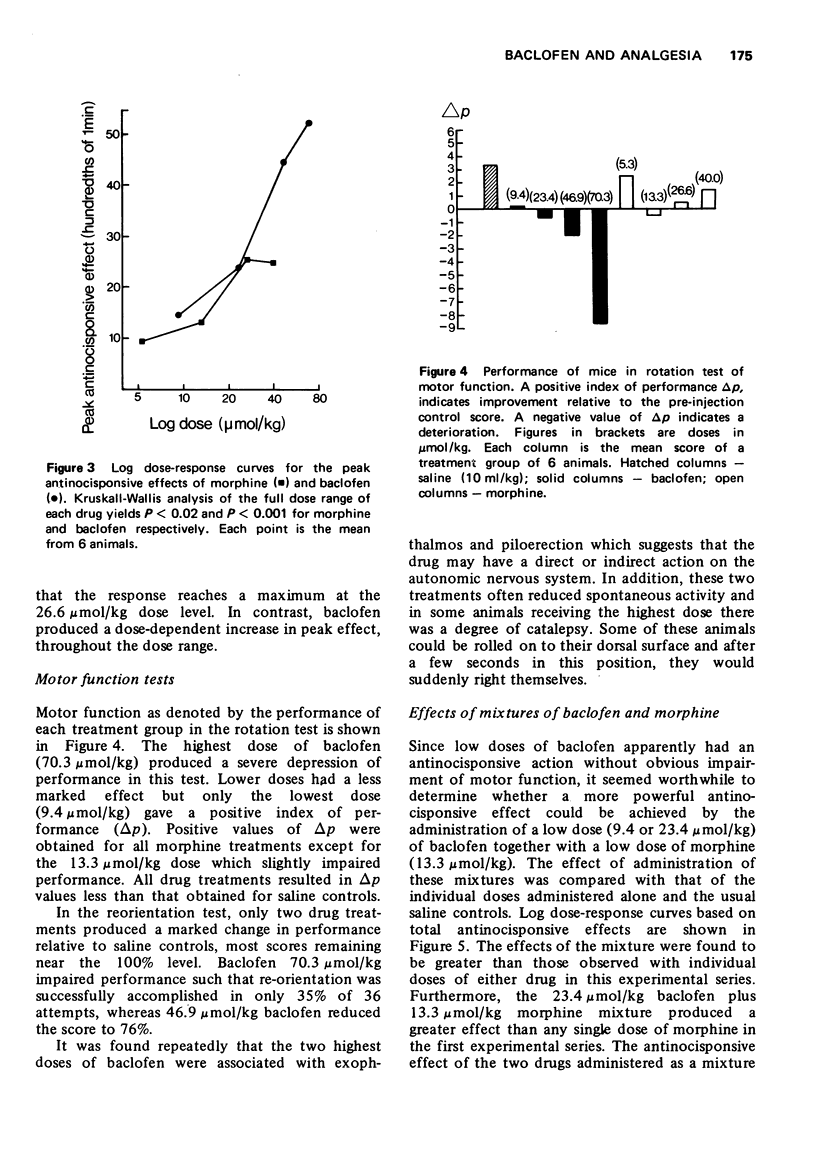
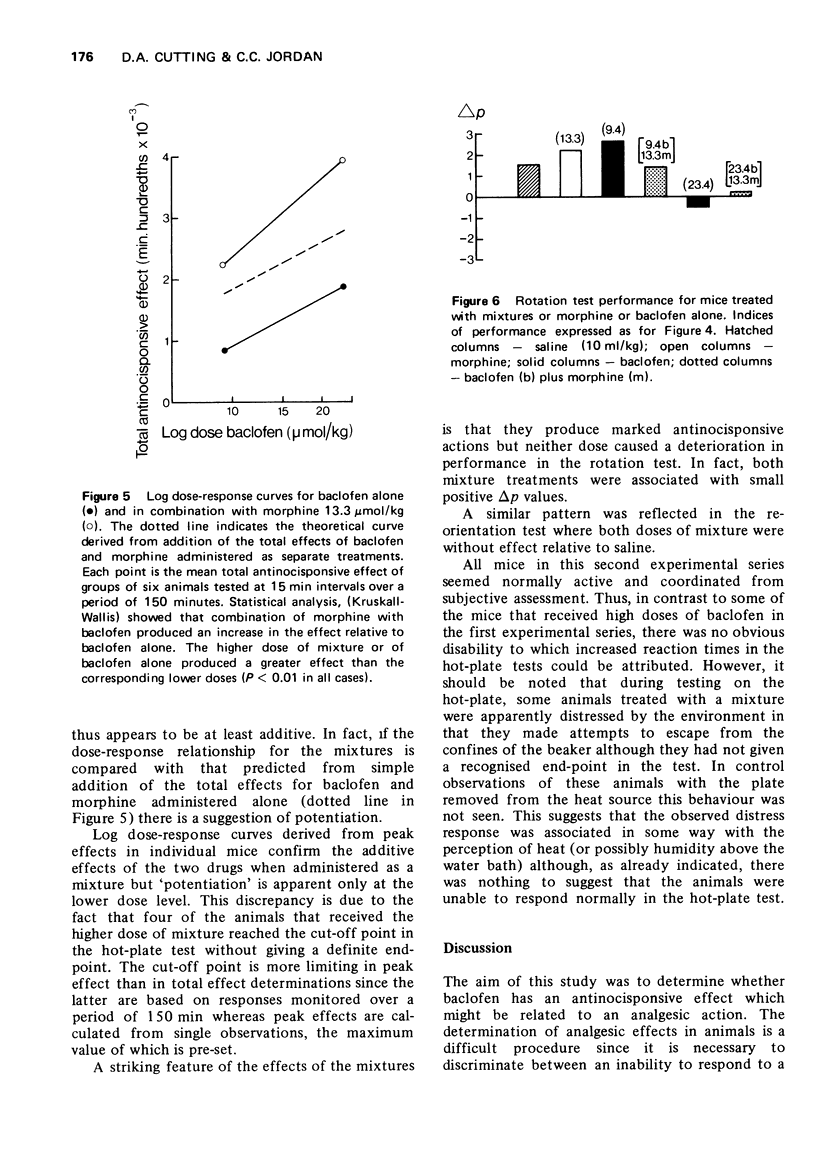
2 Baclofen (9.4-70.3 μmol/kg, i.p.), which may mimic spinal presynaptic inhibition, produced a dose-dependent increase in the response times of mice in a hot-plate test, but high doses also impaired motor function.
3 Morphine hydrochloride (5.3-40 μmol/kg, i.p.) increased the response time of mice in the hot-plate test and had little effect on motor function.
4 Combination of baclofen (9.4 or 23.4 μmol/kg) with morphine (13.3 μmol/kg) produced greater increases in response time than either drug administered alone but with little concurrent effect on motor function.
5 The possibility that baclofen may have some analgesic action and a potentiating effect on other analgesics is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akil H., Mayer D. J., Liebeskind J. C. Comparison chez le rat entre l'analgésie induite par stimulation de la substance grise péri-aqueducale et l'analgésie morphinique. C R Acad Sci Hebd Seances Acad Sci D. 1972 Jun 26;274(26):3603–3605. [PubMed] [Google Scholar]
- Barker J. L., Nicoll R. A. Gamma-aminobutyric acid: role in primary afferent depolarization. Science. 1972 Jun 2;176(4038):1043–1045. doi: 10.1126/science.176.4038.1043. [DOI] [PubMed] [Google Scholar]
- Benoist J. M., Besson J. M., Boissier J. R. Modifications of presynaptic inhibition of various origins by local application of convulsant drugs on cat's spinal cord. Brain Res. 1974 May 10;71(1):172–177. doi: 10.1016/0006-8993(74)90203-0. [DOI] [PubMed] [Google Scholar]
- Benoist J. M., Besson J. M., Conseiller C., Le Bars D. Action of bicuculline on presynaptic inhibition of various origins in the cat's spinal cord. Brain Res. 1972 Aug 25;43(2):672–676. doi: 10.1016/0006-8993(72)90428-3. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A. Bicuculline, an antagonist of GABA and synaptic inhibition in the spinal cord of the cat. Brain Res. 1971 Sep 10;32(1):69–96. doi: 10.1016/0006-8993(71)90156-9. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Game C. J., Johnston G. A., McCulloch R. M. Central effects of beta-(para-chlorophenyl)-gamma-aminobutyric acid. Brain Res. 1974 Apr 26;70(3):493–499. doi: 10.1016/0006-8993(74)90257-1. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., SCHMIDT R. F., WILLIS W. D. Presynaptic inhibition of the spinal monosynaptic reflex pathway. J Physiol. 1962 May;161:282–297. doi: 10.1113/jphysiol.1962.sp006886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., SCHMIDT R., WILLIS W. D. PHARMACOLOGICAL STUDIES ON PRESYNAPTIC INHIBITION. J Physiol. 1963 Oct;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham L. T., Jr, Shank R. P., Werman R., Aprison M. H. Distribution of some synaptic transmitter suspects in cat spinal cord: glutamic acid, aspartic acid, gamma-aminobutyric acid, glycine and glutamine. J Neurochem. 1967 Apr;14(4):465–472. doi: 10.1111/j.1471-4159.1967.tb09545.x. [DOI] [PubMed] [Google Scholar]
- Haldeman S., Huffman R. D., Marshall K. C., McLennan H. The antagonism of the glutamate-induced and synaptic excitations of thalamic neurones. Brain Res. 1972 Apr 28;39(2):419–425. doi: 10.1016/0006-8993(72)90445-3. [DOI] [PubMed] [Google Scholar]
- Haldeman S., McLennan H. The antagonistic action of glutamic acid diethylester towards amino acid-induced and synaptic excitations of central neurones. Brain Res. 1972 Oct 27;45(2):393–400. doi: 10.1016/0006-8993(72)90470-2. [DOI] [PubMed] [Google Scholar]
- Hammerschlag R., Weinreich D. Glutamic acid and primary afferent transmission. Adv Biochem Psychopharmacol. 1972;6:165–180. [PubMed] [Google Scholar]
- Hillman P., Wall P. D. Inhibitory and excitatory factors influencing the receptive fields of lamina 5 spinal cord cells. Exp Brain Res. 1969;9(4):284–306. doi: 10.1007/BF00235240. [DOI] [PubMed] [Google Scholar]
- Johnson J. L. Glutamic acid as a synaptic transmitter in the nervous system. A review. Brain Res. 1972 Feb 11;37(1):1–19. doi: 10.1016/0006-8993(72)90343-5. [DOI] [PubMed] [Google Scholar]
- Johnston G. A. The intraspinal distribution of some depressant amino acids. J Neurochem. 1968 Sep;15(9):1013–1017. doi: 10.1111/j.1471-4159.1968.tb11644.x. [DOI] [PubMed] [Google Scholar]
- Jones I. M., Jordan C. C., Morton I. K., Stagg C. J., Webster R. A. The effect of chronic dorsal root section on the concentration of free amino acids in the rabbit spinal cord. J Neurochem. 1974 Dec;23(6):1239–1244. doi: 10.1111/j.1471-4159.1974.tb12223.x. [DOI] [PubMed] [Google Scholar]
- Koella W. P. Pharmacological aspects of spasticity with special reference to Lioresal. Postgrad Med J. 1972 Oct;48(Suppl):13–18. [PubMed] [Google Scholar]
- Konishi S., Otsuka M. The effects of substance P and other peptides on spinal neurons of the frog. Brain Res. 1974 Jan 18;65(3):397–410. doi: 10.1016/0006-8993(74)90231-5. [DOI] [PubMed] [Google Scholar]
- Levy R. A., Anderson E. G. The role of gamma-aminobutyric acid as a mediator of positive dorsal root potentials. Brain Res. 1974 Aug 9;76(1):71–82. doi: 10.1016/0006-8993(74)90514-9. [DOI] [PubMed] [Google Scholar]
- Levy R. A. GABA: a direct depolarizing action at the mammalian primary afferent terminal. Brain Res. 1974 Aug 9;76(1):155–160. doi: 10.1016/0006-8993(74)90522-8. [DOI] [PubMed] [Google Scholar]
- Levy R. A., Repkin A. H., Anderson E. G. The effect of bicuculline on primary afferent terminal excitability. Brain Res. 1971 Sep 10;32(1):261–265. doi: 10.1016/0006-8993(71)90178-8. [DOI] [PubMed] [Google Scholar]
- Liebeskind J. C., Guilbaud G., Besson J. M., Oliveras J. L. Analgesia from electrical stimulation of the periaqueductal gray matter in the cat: behavioral observations and inhibitory effects on spinal cord interneurons. Brain Res. 1973 Feb 28;50(2):441–446. doi: 10.1016/0006-8993(73)90748-8. [DOI] [PubMed] [Google Scholar]
- Melzack R., Wall P. D. Pain mechanisms: a new theory. Science. 1965 Nov 19;150(3699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Nishi S., Minota S., Karczmar A. G. Primary afferent neurones: the ionic mechanism of GABA-mediated depolarization. Neuropharmacology. 1974 Mar;13(3):215–219. doi: 10.1016/0028-3908(74)90110-5. [DOI] [PubMed] [Google Scholar]
- Pierau F. K., Zimmermann P. Action of a GABA-derivative on postsynaptic potentials and membrane properties of cats' spinal motoneurones. Brain Res. 1973 May 17;54:376–380. doi: 10.1016/0006-8993(73)90064-4. [DOI] [PubMed] [Google Scholar]
- REXED B. The cytoarchitectonic organization of the spinal cord in the cat. J Comp Neurol. 1952 Jun;96(3):414–495. doi: 10.1002/cne.900960303. [DOI] [PubMed] [Google Scholar]
- WINTER C. A., FLATAKER L. Studies on heptazone (6-morpholino-4,4-diphenyl-3-heptanone hydrochloride) in comparison with other analgesic drugs. J Pharmacol Exp Ther. 1950 Mar;98(3):305–317. [PubMed] [Google Scholar]


