Abstract
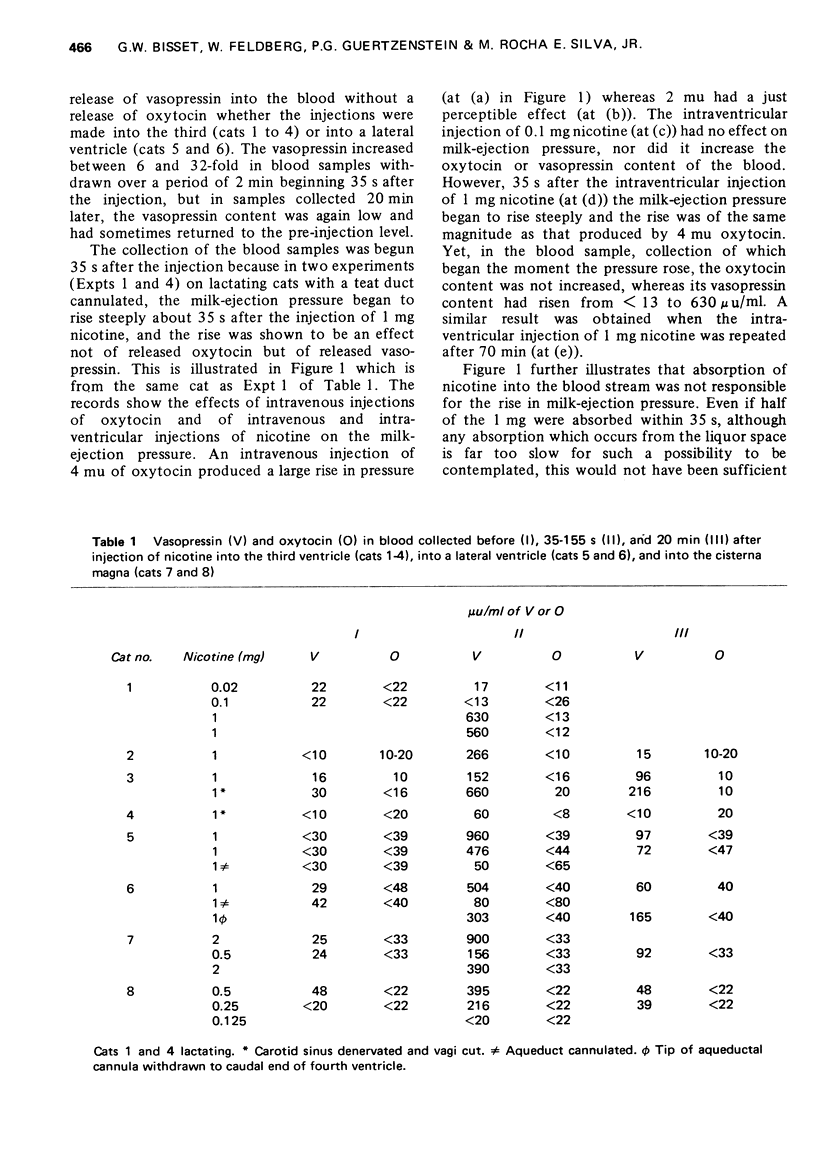
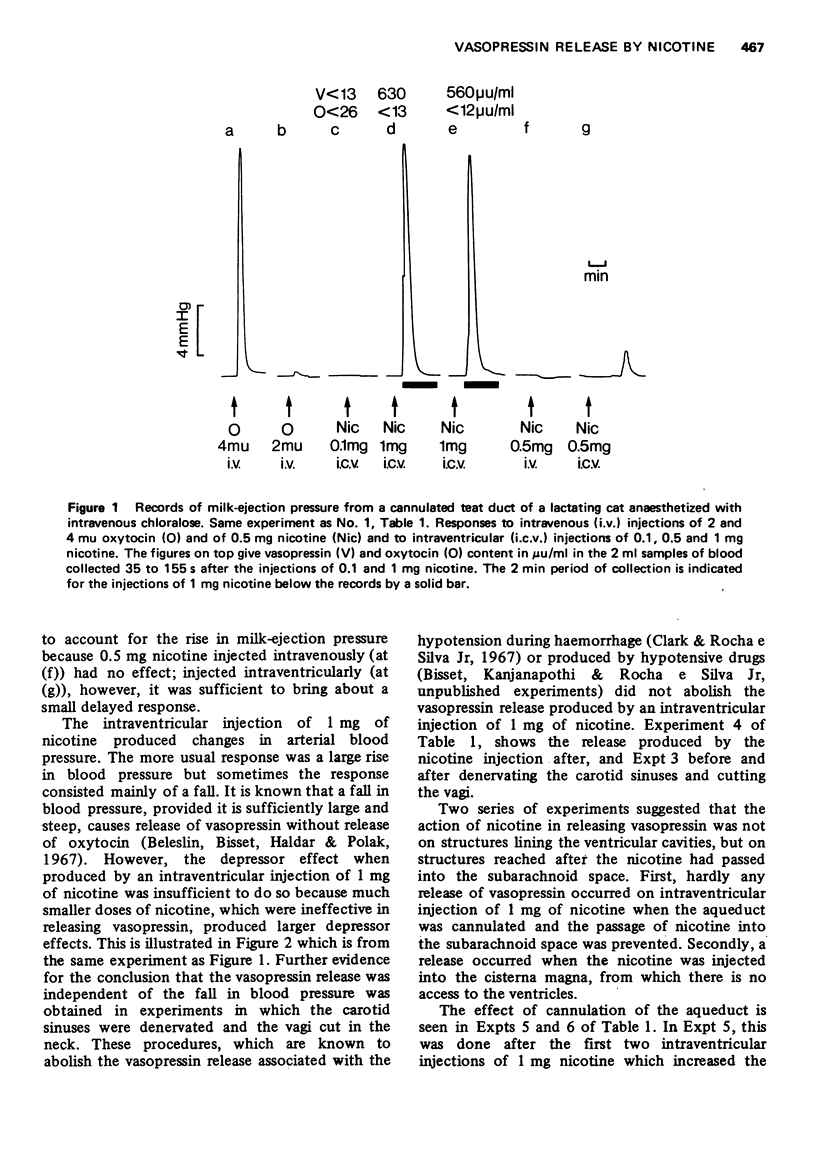
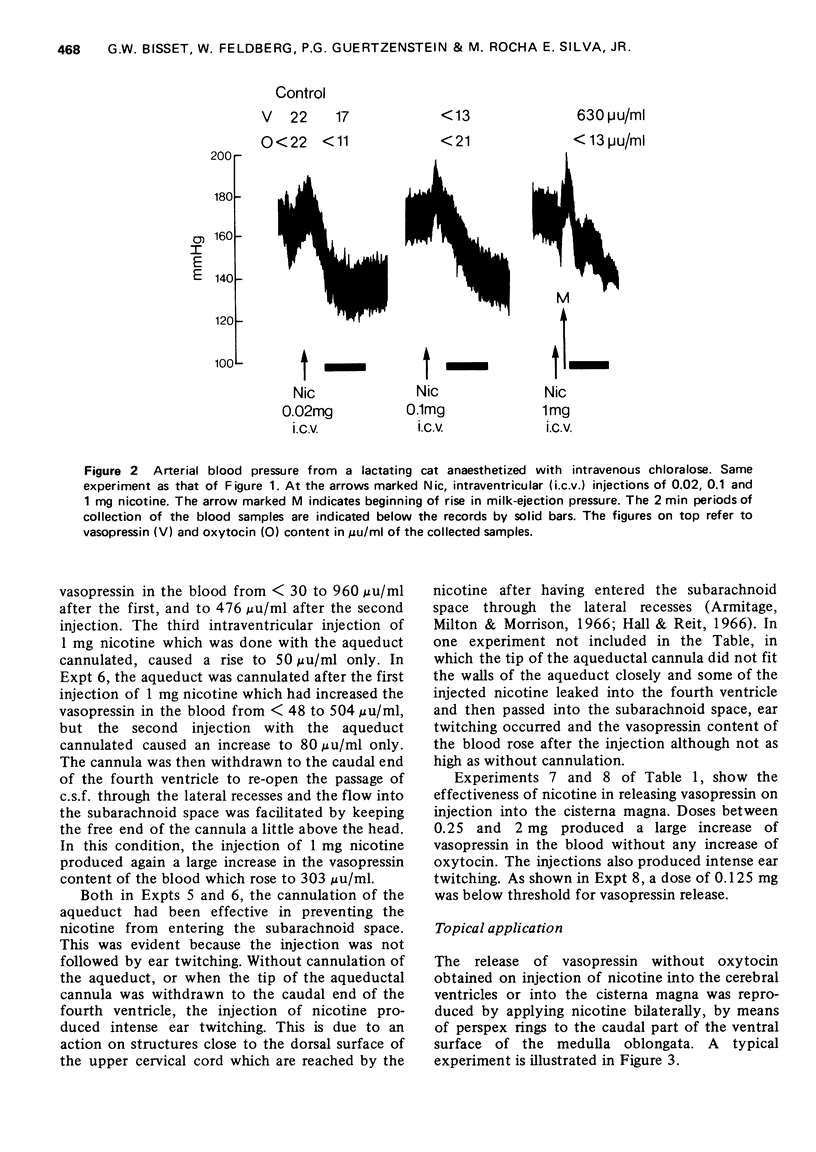
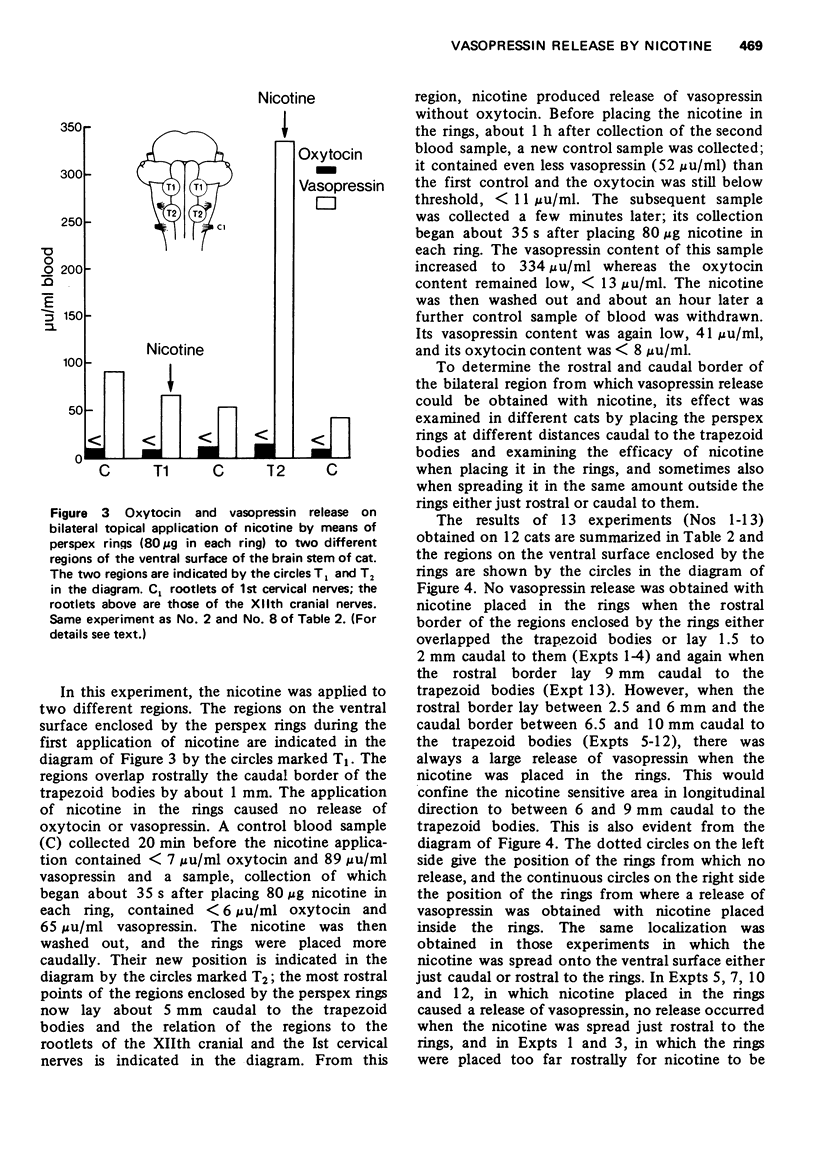
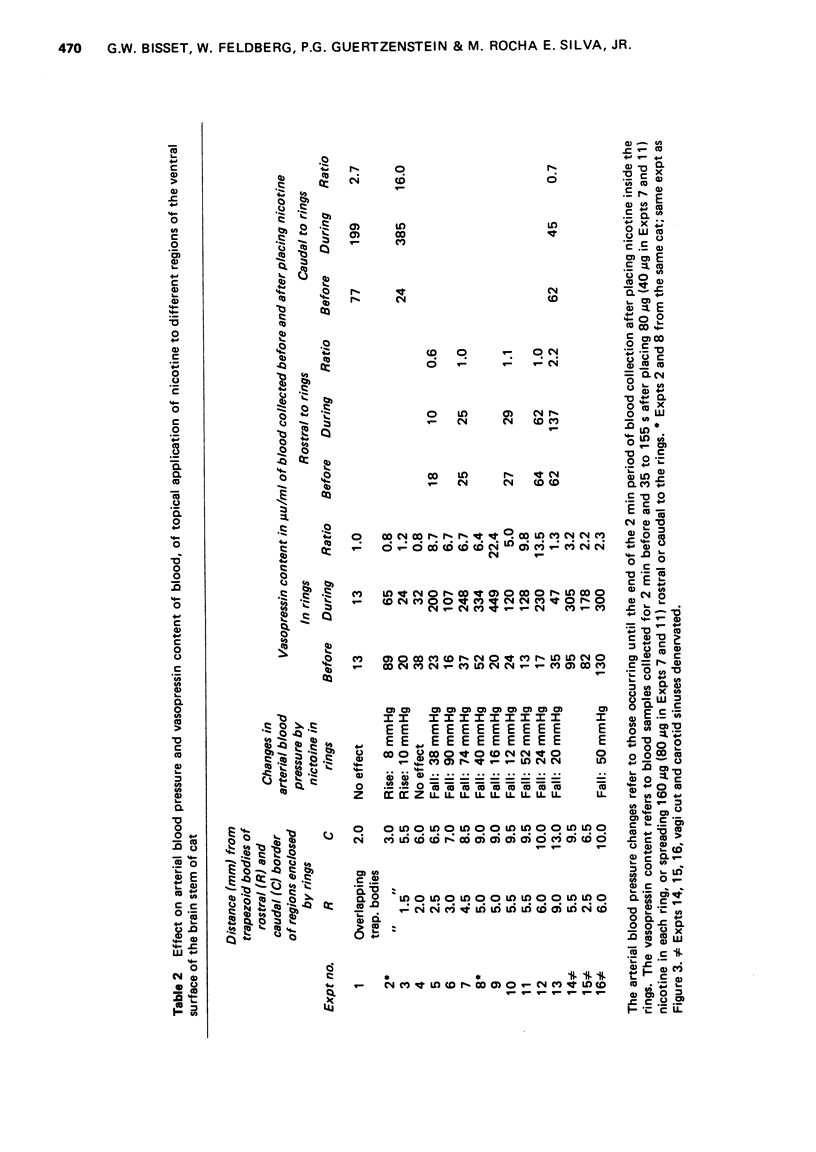
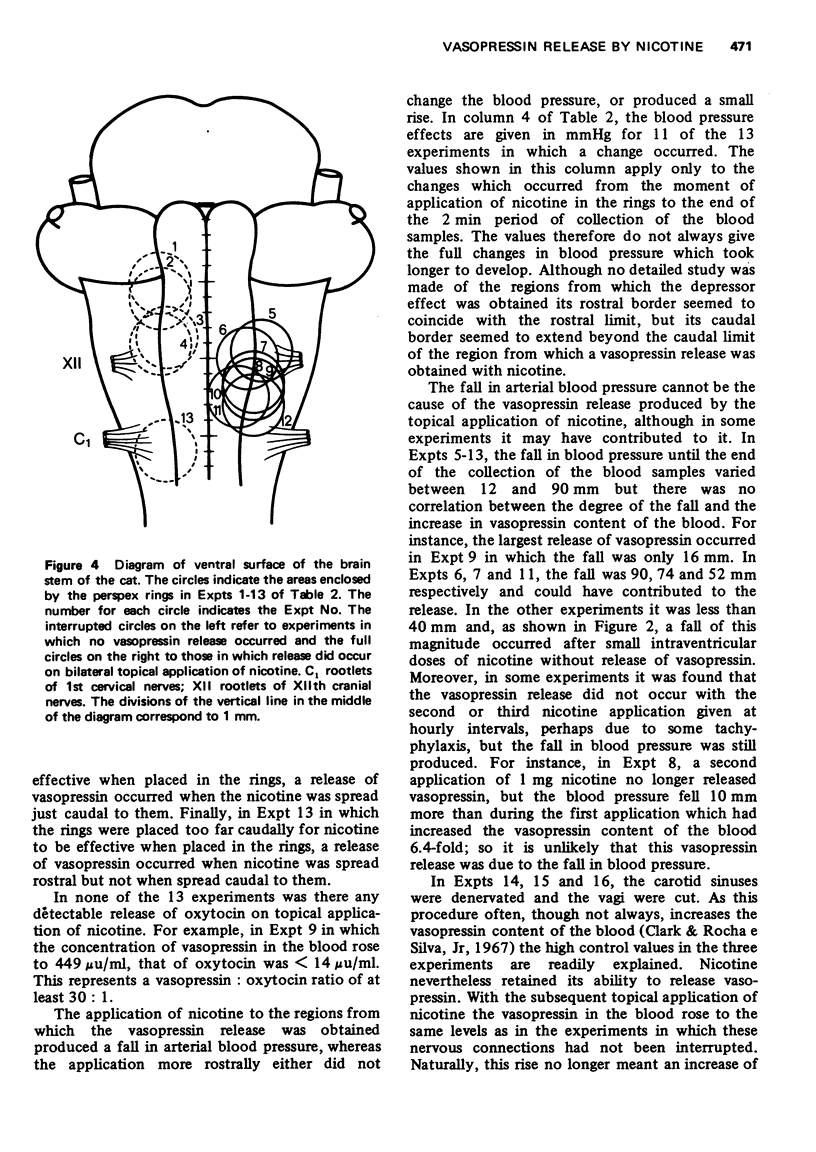
1. In cats anaesthetized with chloralose the release of neurohypophysial hormones was examined after injection of nicotine into the cerebral ventricles or cisterna magna or its topical application through perspex rings to the ventral surface of the brain stem. The release was measured by assaying the hormones in samples of venous blood. 2. Injected into a lateral or the third cerebral ventricle, nicotine (0.5 to 1 mg) produced release of vasopressin without oxytocin. When the aqueduct was cannulated, preventing access to the fourth ventricle and to the subarachnoid space, this release did not occur. 3. Vasopressin was also released without oxytocin when nicotine (0.25 to 2 mg) was injected into the subarachnoid space through the cisterna magna. With this route of administration the nicotine did not enter any part of the ventricular system. 4. Applied through paired perspex rings placed across the ventral surface of the brain stem, nicotine again produced release of vasopressin without ocytocin. The amount of nicotine placed in each ring was usually 80 mug, but a release was obtained with 10 mug and in one experiment with as little as 5 mug. 5. The bilateral region on the ventral surface of the brain stem where nicotine acts when producing release of vasopressin lies lateral to the pyramids and in a longitudinal direction, 6 to 9 mm caudal to the trapezoid bodies. 6. The vasopressin release by nicotine injected intraventricularly or intracisternally, or applied topically to the ventral surface of the brain stem was not due to absorption of nicotine into the blood stream, nor to blood pressure effects. 7. It is concluded that nicotine acts on the ventral surface of the brain stem probably by activating the central projection to the supra-optic and possibly also the paraventricular nuclei of afferent pathways in the sinus and vagus nerves which control the release of vasopressin in response to changes in blood volume or distribution.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armitage A. K., Milton A. S., Morrison C. F. Effects of nicotine and some nicotine-like compounds injected into the cerebral ventricles of the cat. Br J Pharmacol Chemother. 1966 May;27(1):33–45. doi: 10.1111/j.1476-5381.1966.tb01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulsebrook L. H., Holland R. C. Central regulation of oxytocin release with and without vasopressin release. Am J Physiol. 1969 Apr;216(4):818–829. doi: 10.1152/ajplegacy.1969.216.4.818. [DOI] [PubMed] [Google Scholar]
- BANGHAM D. R., MUSSETT M. V. Third international standard for posterior pituitary; re-named third international standard for oxytocic, vasopressor and antidiuretic substances in 1956. Bull World Health Organ. 1958;19(2):325–340. [PMC free article] [PubMed] [Google Scholar]
- Bisset G. W., Clark B. J., Errington M. L. The hypothalamic neurosecretory pathways for the release of oxytocin and vasopressin in the cat. J Physiol. 1971 Aug;217(1):111–131. doi: 10.1113/jphysiol.1971.sp009562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset G. W., Clark B. J., Lewis G. P. The mechanism of the inhibitory action of adrenaline on the mammary gland. Br J Pharmacol Chemother. 1967 Nov;31(3):550–559. doi: 10.1111/j.1476-5381.1967.tb00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset G. W., Errington M. L., Richards C. D. The distribution of vasopressin and oxytocin in the hypothalamoneurohypophysial system of the guinea-pig. Br J Pharmacol. 1973 Jun;48(2):263–272. doi: 10.1111/j.1476-5381.1973.tb06912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset G. W., Feldberg W. Release of vasopressin without oxytocin by nicotine injected into the cerebral ventricles of anaesthetized cats. J Physiol. 1973 May;231(1):36P–37P. [PubMed] [Google Scholar]
- Bousquet P., Guertzenstein P. G. Localization of the central cardiovascular action of clonidine. Br J Pharmacol. 1973 Dec;49(4):573–579. doi: 10.1111/j.1476-5381.1973.tb08532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn J. H., Truelove L. H., Burn I. Antidiuretic Action of Nicotine. Br Med J. 1945 Mar 24;1(4394):403–406. doi: 10.1136/bmj.1.4394.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B. J., Silva MR Jr E. An afferent pathway for the selective release of vasopressin in response to carotid occlusion and haemorrhage in the cat. J Physiol. 1967 Aug;191(3):529–542. doi: 10.1113/jphysiol.1967.sp008266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey P. K., Feldberg W., Wendlandt S. Comparison of the hyperglycaemic effect of adrenaline and morphine introduced into the liquor space. J Physiol. 1975 Mar;246(1):213–228. doi: 10.1113/jphysiol.1975.sp010887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edery H., Guertzenstein P. G. A central vasodepressor effect of Dyflos. Br J Pharmacol. 1974 Apr;50(4):481–487. doi: 10.1111/j.1476-5381.1974.tb08581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W., Guertzenstein P. G. A vasodepressor effect of pentobarbitone sodium. J Physiol. 1972 Jul;224(1):83–103. doi: 10.1113/jphysiol.1972.sp009882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W., Gupta K. P. Morphine hyperglycaemia. J Physiol. 1974 May;238(3):487–502. doi: 10.1113/jphysiol.1974.sp010539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W., Shaligram S. V. The hyperglycaemic effect of morphine. Br J Pharmacol. 1972 Dec;46(4):602–618. doi: 10.1111/j.1476-5381.1972.tb06887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertzenstein P. G., Silver A. Fall in blood pressure produced from discrete regions of the ventral surface of the medulla by glycine and lesions. J Physiol. 1974 Oct;242(2):489–503. doi: 10.1113/jphysiol.1974.sp010719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G. H., Reit E. Analysis of some central actions of nicotine injected into the cerebral ventricles of cats. J Physiol. 1966 Jul;185(2):400–417. doi: 10.1113/jphysiol.1966.sp007992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovický P. Uber die Glia marginalis und oberglächliche Nervenzellen im Hirmstamm der Katze. Z Anat Entwicklungsgesch. 1968 Nov 4;127(3):221–231. [PubMed] [Google Scholar]
- Tindal J. S., Knaggs G. S., Turvey A. Preferential release of oxytocin from the neurohypophysis after electrical stimulation of the afferent path of the milk-ejection reflex in the brain of the guinea-pig. J Endocrinol. 1968 Feb;40(2):205–214. doi: 10.1677/joe.0.0400205. [DOI] [PubMed] [Google Scholar]
- Trouth C. O., Loeschcke H. H., Berndt J. A superficial substrate on the ventral surface of the medulla oblongata influencing respiration. Pflugers Arch. 1973 Mar 21;339(2):135–152. doi: 10.1007/BF00587180. [DOI] [PubMed] [Google Scholar]
- Trouth C. O., Loeschcke H. H., Berndt J. Histological structures in the chemosensitive regions on the ventral surface of the cat's medulla oblongata. Pflugers Arch. 1973 Mar 30;339(3):171–183. doi: 10.1007/BF00587370. [DOI] [PubMed] [Google Scholar]


