Abstract
1 The formation of [14C]-3,4-dihydroxyphenylalanine (DOPA) from [14C]-tyrosine, in the presence of the amino acid decarboxylase inhibitor, brocresine (3-hydroxy-4-bromobenzyloxyamine dihydrogen phosphate), was greatly enhanced in rat vasa deferentia depolarized by a KCl-enriched Krebs-Henseleit solution (52 mM KCl) compared with tissues maintained in unmodified Krebs-Henseleit solution. 2 When the conversion of tyrosine was allowed to proceed as far as catecholamine (brocresine absent) no significant difference was observed between the accumulation of [14C]-catecholamines (CA) in depolarized rat vasa deferentia and the accumulation in control (non-depolarized) tissues. 3 Endogenous CA levels in the depolarized rat vasa deferentia fell to 67% of the controls after a 1 h incubation period and to 53% at the end of 2 hours. 4 Chromatographic separation on Amberlite CG-120 columns of the newly synthesized CA and catechol metabolites from the rat vas deferens revealed that a very high proportion was present as dopamine. The percentage distribution after 1 h incubation in control Krebs-Henseleit was: noradrenaline (NA): 30.6 +/- 5.2; dopamine 56.9 +/- 5.9; acid metabolites: 12.8 +/- 1.1; and in KCl-rich Krebs-Henseleit, NA: 32; dopamine: 44.7 and acid metabolites 23.3. In contrast to the newly synthesized (14C-labelled) CA, endogenous dopamine comprises only 10% of the endogenous CA stores in rat vas deferens. 5 The distribution of newly synthesized NA and dopamine in rat vas deferens is strikingly different from that of guinea-pig vas deferens where more than 80% of newly formed amine is present as NA. In the latter tissue depolarization with K+ causes a striking increase in CA biosynthesis.
Full text
PDF

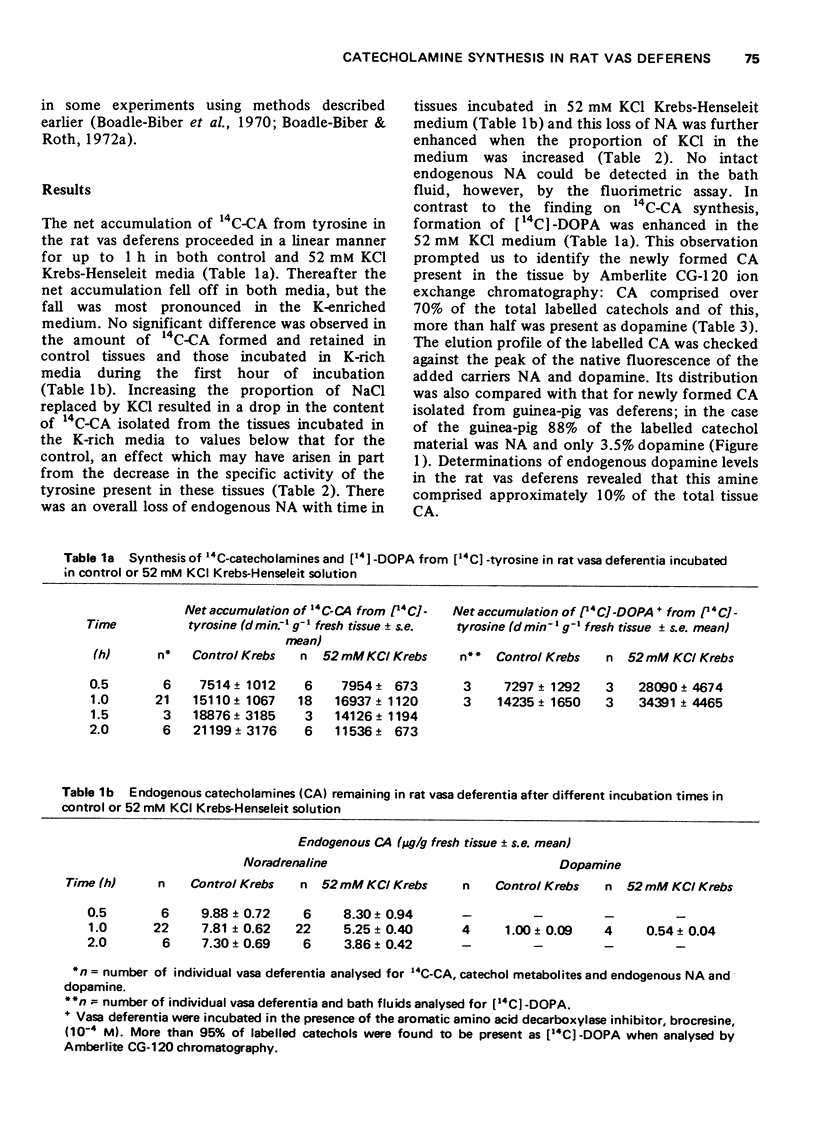


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alousi A., Weiner N. The regulation of norepinephrine synthesis in sympathetic nerves: effect of nerve stimulation, cocaine, and catecholamine-releasing agents. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1491–1496. doi: 10.1073/pnas.56.5.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage A. K., Dean A. C. The effects of pressure and pharmacologically active substances on gastric peristalsis in a transmurally stimulated rat soomach-duodenum preparation. J Physiol. 1966 Jan;182(1):42–56. doi: 10.1113/jphysiol.1966.sp007807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisby M. A., Fillenz M. Effect of perfusion with K-rich solutions on the noradrenaline content of the rat vas deferens. J Physiol. 1969 Sep;204(1):22P–23P. [PubMed] [Google Scholar]
- Boadle-Biber M. C., Hughes J., Roth R. H. Acceleration of noradrenaline biosynthesis in the guinea-pig vas deferens by potassium. Br J Pharmacol. 1970 Dec;40(4):702–720. doi: 10.1111/j.1476-5381.1970.tb10648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boadle-Biber M. C., Roth R. H. Effect of drugs on the synthesis of noradrenaline in guinea-pig vas deferens. Br J Pharmacol. 1972 Dec;46(4):696–707. doi: 10.1111/j.1476-5381.1972.tb06894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. C., Chang J. C. A change in the subcellular distribution of noradrenaline in the rat isolated vas deferens effected by nerve stimulation. Br J Pharmacol Chemother. 1965 Dec;25(3):758–762. doi: 10.1111/j.1476-5381.1965.tb01798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz G. P., Kopin I. J. Release of dopamine-beta-hydroxylase with norepinephrine during cat splenic nerve stimulation. Nature. 1970 Jul 25;227(5256):406–407. doi: 10.1038/227406a0. [DOI] [PubMed] [Google Scholar]
- Hughes J. Evaluation of mechanisms controlling the release and inactivation of the adrenergic transmitter in the rabbit portal vein and vas deferens. Br J Pharmacol. 1972 Mar;44(3):472–491. doi: 10.1111/j.1476-5381.1972.tb07285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörtnagl H., Winkler H. Bovine splenic nerve: characterization of noradrenaline-containing vesicles and other cell organelles by density gradient centrifugation. J Physiol. 1969 Nov;205(1):103–114. doi: 10.1113/jphysiol.1969.sp008954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverty R., Taylor K. M. The fluorometric assay of catecholamines and related compounds: improvements and extensions to the hydroxyindole technique. Anal Biochem. 1968 Feb;22(2):269–279. doi: 10.1016/0003-2697(68)90316-3. [DOI] [PubMed] [Google Scholar]
- Roth R. H., Stjärne L., von Euler U. S. Acceleration of noradrenaline biosynthesis by nerve stimulation. Life Sci. 1966 Jun;5(12):1071–1075. doi: 10.1016/0024-3205(66)90089-0. [DOI] [PubMed] [Google Scholar]
- Roth R. H., Stjärne L., von Euler U. S. Factors influencing the rate of norepinephrine biosynthesis in nerve tissue. J Pharmacol Exp Ther. 1967 Dec;158(3):373–377. [PubMed] [Google Scholar]
- Stjärne L., Lishajko F. Localization of different steps in noradrenaline synthesis to different fractions of a bovine splenic nerve homogenate. Biochem Pharmacol. 1967 Sep 9;16(9):1719–1728. doi: 10.1016/0006-2952(67)90247-x. [DOI] [PubMed] [Google Scholar]
- Weiner N. Regulation of norepinephrine biosynthesis. Annu Rev Pharmacol. 1970;10:273–290. doi: 10.1146/annurev.pa.10.040170.001421. [DOI] [PubMed] [Google Scholar]
- Weinshilboum R. M., Thoa N. B., Johnson D. G., Kopin I. J., Axelrod J. Proportional release of norepinephrine and dopamine- -hydroxylase from sympathetic nerves. Science. 1971 Dec 24;174(4016):1349–1351. doi: 10.1126/science.174.4016.1349. [DOI] [PubMed] [Google Scholar]


