Abstract
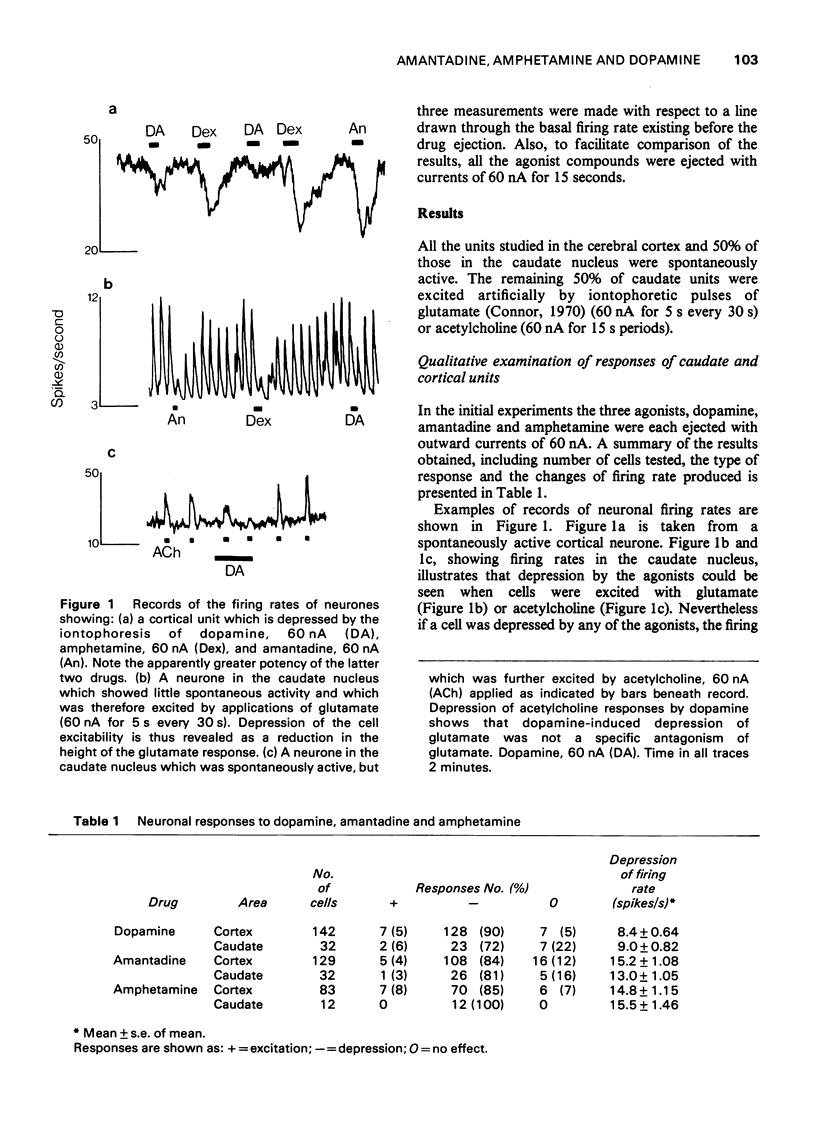
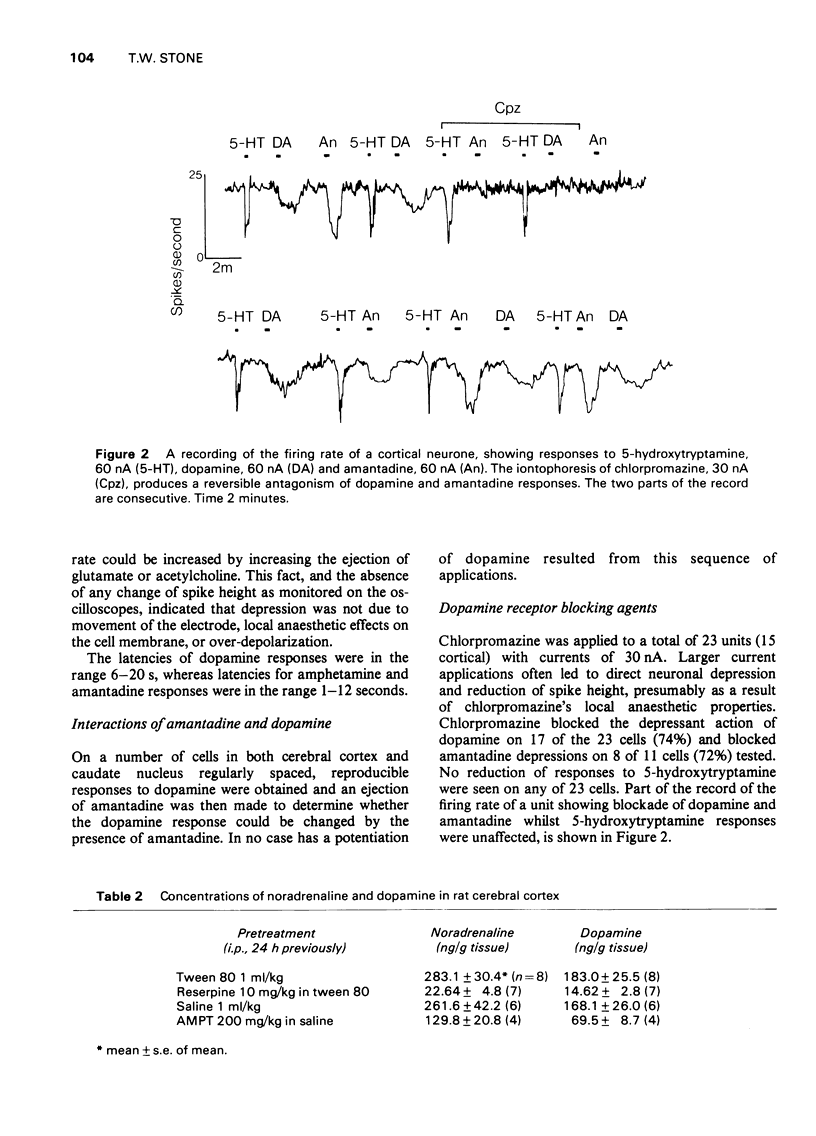
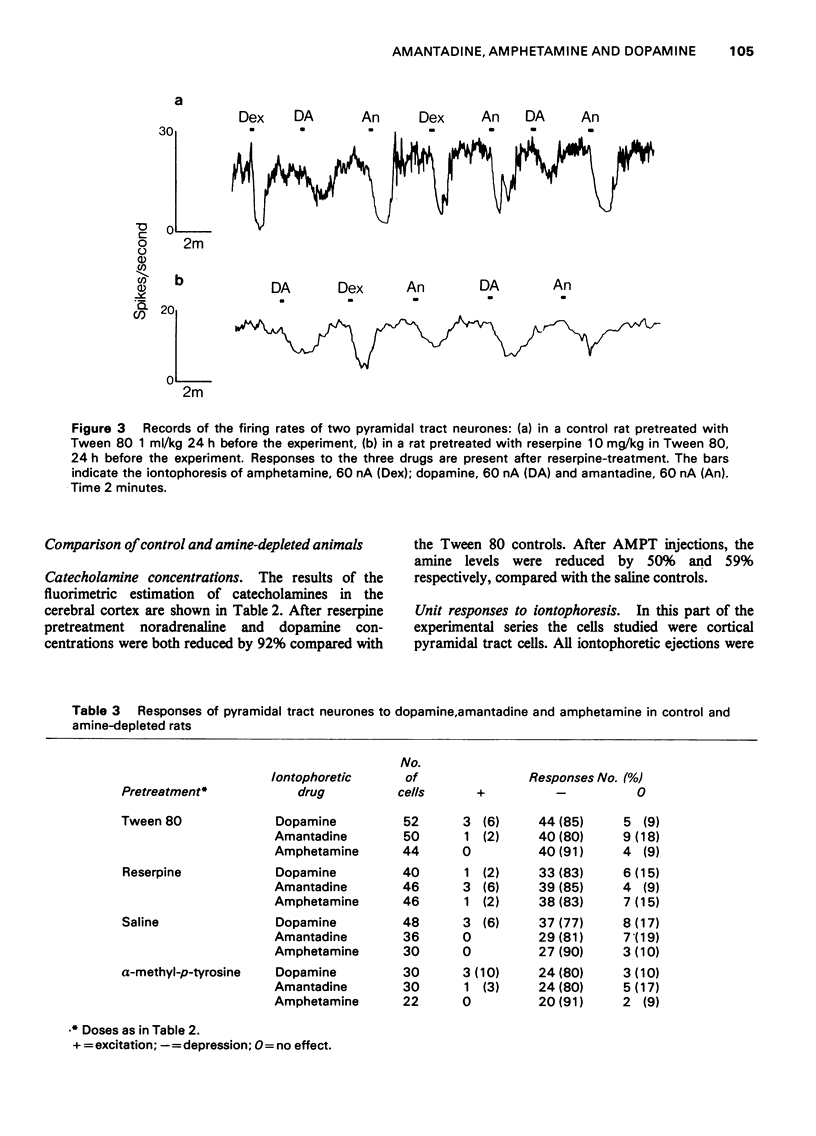
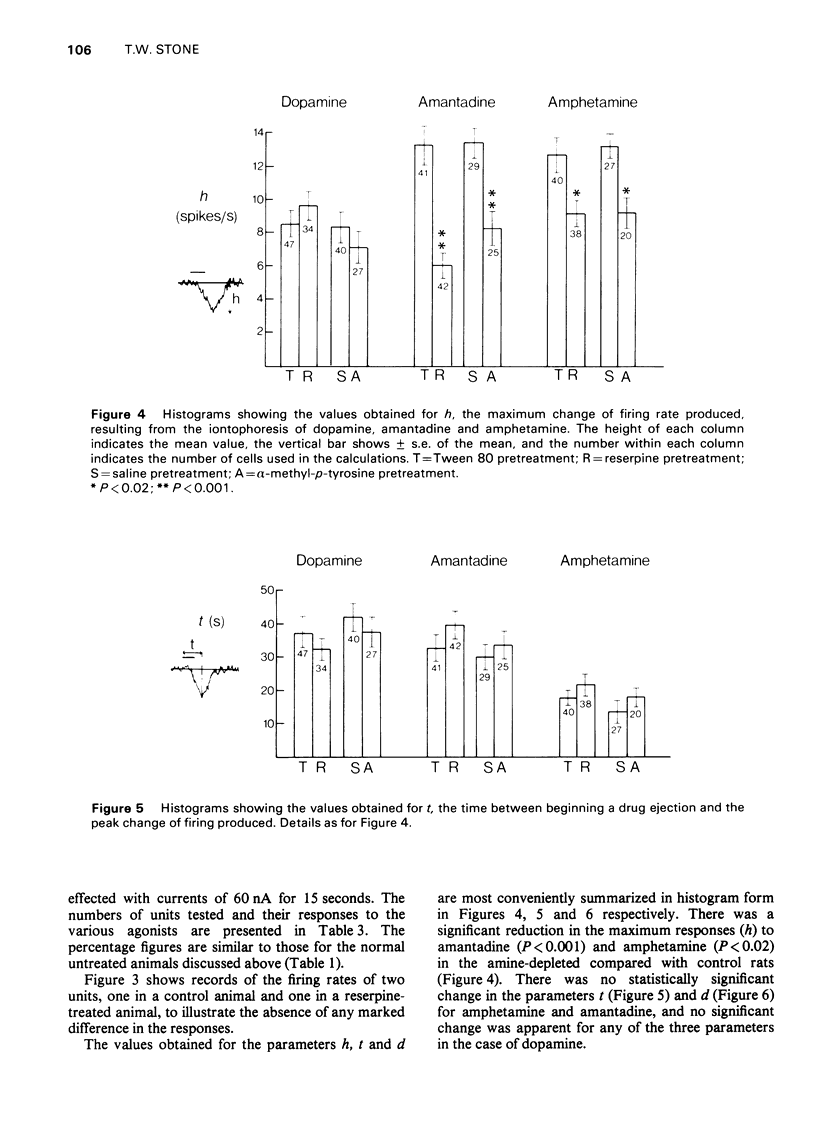
1. Dopamine amantadine and amphetamine have been applied directly by microiontophoresis to single neurones in the caudate nucleus and cerebral cortex of rats anaesthetized with urethane. 2. The predominant response to all three agents was a depression of neuronal firing rate. The responses to dopamine and amantadine could be antagonized by the dopamine receptor blocking agent, chlorpromazine. 3. Amantadine did not cause any potentiation of dopamine responses, suggesting that inhibition of amine uptake was not responsible for its effects. 4. The responses of pyramidal tract cells in the cerebral cortex to dopamine, amphetamine and amantadine were compared in control groups of rats and rats pretreated with reserpine (10 mg/kg i.p.) or alpha-methyl-p-tyrosine methyl ester (200 mg/kg i.p.). The reduction of cortical catecholamine concentrations was confirmed by a direct fluorimetric assay method. 5. Responses to dopamine were unaltered in the amine-depleted animals compared with controls. Responses to amantadine and amphetamine were reduced but not abolished. 6. It is concluded that amantadine acts partly by releasing catecholamines from neuronal stores. The residual responses to amantadine and amphetamine may be the result of a direct postsynaptic receptor stimulation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andén N. E., Dahlström A., Fuxe K., Larsson K. Functional role of the nigro-neostriatal dopamine neurons. Acta Pharmacol Toxicol (Copenh) 1966;24(2):263–274. doi: 10.1111/j.1600-0773.1966.tb00389.x. [DOI] [PubMed] [Google Scholar]
- Arbuthnott G. W., Crow T. J. Relation of contraversive turning to unilateral release of dopamine from the nigrostriatal pathway in rats. Exp Neurol. 1971 Mar;30(3):484–491. doi: 10.1016/0014-4886(71)90149-x. [DOI] [PubMed] [Google Scholar]
- BERTLER A., ROSENGREN E. Occurrence and distribution of catechol amines in brain. Acta Physiol Scand. 1959 Dec 12;47:350–361. [PubMed] [Google Scholar]
- Baldessarini R. J., Lipinski J. F., Chace K. V. Effects of amantadine hydrochloride on catecholamine metabolism in the brain of the rat. Biochem Pharmacol. 1972 Jan;21(1):77–87. doi: 10.1016/0006-2952(72)90252-3. [DOI] [PubMed] [Google Scholar]
- Bianchi C., Tomasi L. Central nervous system and autonomic nervous system effects of amantadine and of some standard anti-Parkinson drugs. Pharmacology. 1973;10(4):226–237. doi: 10.1159/000136443. [DOI] [PubMed] [Google Scholar]
- Boakes R. J., Bradley P. B., Candy J. M. A neuronal basis for the alerting action of (+)-amphetamine. Br J Pharmacol. 1972 Jul;45(3):391–403. doi: 10.1111/j.1476-5381.1972.tb08096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw C. M., Roberts M. H., Szabadi E. Effect of tricyclic antidepressants on monoamine responses of single cortical neurones. Br J Pharmacol. 1971 Feb;41(2):394P–395P. [PMC free article] [PubMed] [Google Scholar]
- Carlsson A., Lindqvist M., Dahlström A., Fuxe K., Masuoka D. Effects of the amphetamine group on intraneuronal brain amines in vivo and in vitro. J Pharm Pharmacol. 1965 Aug;17(8):521–523. doi: 10.1111/j.2042-7158.1965.tb07717.x. [DOI] [PubMed] [Google Scholar]
- Carlsson A., Lindqvist M., Fuxe K., Hamberger B. The effect of (+)-amphetamine on various central and peripheral catecholamine-containing neurones. J Pharm Pharmacol. 1966 Feb;18(2):128–130. doi: 10.1111/j.2042-7158.1966.tb07835.x. [DOI] [PubMed] [Google Scholar]
- Cashin C. H., Sutton S. The effect of anti-Parkinson drugs on catalepsy induced by -methyl-p-tyrosine in rats pretreated with intraventricular 6-hydroxydopamine. Br J Pharmacol. 1973 Mar;47(3):658P–659P. [PMC free article] [PubMed] [Google Scholar]
- Christie J. E., Crow T. J. Turning behaviour as an index of the action of amphetamines and ephedrines on central dopamine-containing neurones. Br J Pharmacol. 1971 Nov;43(3):658–667. doi: 10.1111/j.1476-5381.1971.tb07195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. D. Caudate nucleus neurones: correlation of the effects of substantia nigra stimulaton with iontophoretic dopamine. J Physiol. 1970 Jul;208(3):691–703. doi: 10.1113/jphysiol.1970.sp009143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox B., Tha S. J. Effects of amantadine and L-dopa on apomorphine- and d-amphetamine-induced stereotyped behaviour in rats. Eur J Pharmacol. 1973 Oct;24(1):96–100. doi: 10.1016/0014-2999(73)90118-0. [DOI] [PubMed] [Google Scholar]
- Cox R. H., Jr, Perhach J. L., Jr A sensitive, rapid and simple method for the stimultaneous spectrophotofluorometric determinations of norepinephrine, dopamine, 5-hydroxytryptamine and 5-hydroxy-indoleacetic acid in discrete areas of brain. J Neurochem. 1973 Jun;20(6):1777–1780. doi: 10.1111/j.1471-4159.1973.tb00294.x. [DOI] [PubMed] [Google Scholar]
- Crow T. J. The relationship between lesion site, dopamine neurons, and turning behavior in the rat. Exp Neurol. 1971 Aug;32(2):247–255. doi: 10.1016/0014-4886(71)90068-9. [DOI] [PubMed] [Google Scholar]
- Farnebo L. O., Fuxe K., Goldstein M., Hamberger B., Ungerstedt U. Dopamine and noradrenaline releasing action of amantadine in the central and peripheral nervous system: a possible mode of action in Parkinson's disease. Eur J Pharmacol. 1971 Sep;16(1):27–38. doi: 10.1016/0014-2999(71)90053-7. [DOI] [PubMed] [Google Scholar]
- Feltz P., De Champlain J. Enhanced sensitivity of caudate neurones to microiontophoretic injections of dopamine in 6-hydroxydopamine treated cats. Brain Res. 1972 Aug 25;43(2):601–605. doi: 10.1016/0006-8993(72)90414-3. [DOI] [PubMed] [Google Scholar]
- Feltz P., De Champlain J. Persistence of caudate unitary responses to nigral stimulation after destruction and functional impairment of the striatal dopaminergic terminals. Brain Res. 1972 Aug 25;43(2):595–600. doi: 10.1016/0006-8993(72)90413-1. [DOI] [PubMed] [Google Scholar]
- Feltz P. Sensitivity to haloperidol of caudate neurones excited by nigral stimulation. Eur J Pharmacol. 1971 May;14(4):360–364. doi: 10.1016/0014-2999(71)90190-7. [DOI] [PubMed] [Google Scholar]
- Fletcher E. A., Redfern P. H. The effect of amantadine on the uptake of dopamine and noradrenaline by rat brain homogenates. J Pharm Pharmacol. 1970 Dec;22(12):957–959. doi: 10.1111/j.2042-7158.1970.tb08486.x. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Hamberger B., Hökfelt T. Distribution of noradrenaline nerve terminals in cortical areas of the rat. Brain Res. 1968 Apr;8(1):125–131. doi: 10.1016/0006-8993(68)90175-3. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Ungerstedt U. Histochemical studies on the effect of (positive)-amphetamine, drugs of the imipramine group and tryptamine on central catecholamine and 5-hydroxytryptamine neurons after intraventricular injection of catecholamines and 5-hydroxytryptamine. Eur J Pharmacol. 1968 Sep;4(2):135–144. doi: 10.1016/0014-2999(68)90169-6. [DOI] [PubMed] [Google Scholar]
- Grelak R. P., Clark R., Stump J. M., Vernier V. G. Amantadine-dopamine interaction: possible mode of action in Parkinsonism. Science. 1970 Jul 10;169(3941):203–204. doi: 10.1126/science.169.3941.203. [DOI] [PubMed] [Google Scholar]
- Heikkila R. E., Cohen G. Evaluation of amantadine as a releasing agent or uptake blocker for H 3 -dopamine in rat brain slices. Eur J Pharmacol. 1972 Nov;20(2):156–160. doi: 10.1016/0014-2999(72)90144-6. [DOI] [PubMed] [Google Scholar]
- Heimans R. L., Rand M. J., Fennessy M. R. Effects of amantadine on uptake and release of dopamine by a particulate fraction of rat basal ganglia. J Pharm Pharmacol. 1972 Nov;24(11):875–879. doi: 10.1111/j.2042-7158.1972.tb08906.x. [DOI] [PubMed] [Google Scholar]
- Hoffer B. J., Siggins G. R., Bloom F. E. Studies on norepinephrine-containing afferents to Purkinje cells of rat cerebellum. II. Sensitivity of Purkinje cells to norepinephrine and related substances administered by microiontophoresis. Brain Res. 1971 Feb 5;25(3):523–534. doi: 10.1016/0006-8993(71)90458-6. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev. 1966 Jun;18(2):925–964. [PubMed] [Google Scholar]
- KRNJEVIC K., PHILLIS J. W. Actions of certain amines on cerebral cortical neurones. Br J Pharmacol Chemother. 1963 Jun;20:471–490. doi: 10.1111/j.1476-5381.1963.tb01484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostopoulos G. K., Yarbrough G. G., Straughan D. W. Proceedings: Iontophoretic studies with 'false transmitters' on cerebellar Purkinje cells. Br J Pharmacol. 1974 Sep;52(1):136P–136P. [PMC free article] [PubMed] [Google Scholar]
- Lassen J. B. The effect of amantadine and (+)-amphetamine on motility in rats after inhibition of monoamine synthesis and storage. Psychopharmacologia. 1973 Feb 27;29(1):55–64. doi: 10.1007/BF00421211. [DOI] [PubMed] [Google Scholar]
- Lindvall O., Björklund A., Moore R. Y., Stenevi U. Mesencephalic dopamine neurons projecting to neocortex. Brain Res. 1974 Dec 6;81(2):325–331. doi: 10.1016/0006-8993(74)90947-0. [DOI] [PubMed] [Google Scholar]
- MOORE K. E. TOXICITY AND CATECHOLAMINE RELEASING ACTIONS OF D- AND L-AMPHETAMINE IN ISOLATED AND AGGREGATED MICE. J Pharmacol Exp Ther. 1963 Oct;142:6–12. [PubMed] [Google Scholar]
- McLennan H., York D. H. The action of dopamine on neurones of the caudate nucleus. J Physiol. 1967 Apr;189(3):393–402. doi: 10.1113/jphysiol.1967.sp008175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papeschi R. Amantadine may stimulate dopamine and noradrenaline receptors. Neuropharmacology. 1974 Jan;13(1):77–83. doi: 10.1016/0028-3908(74)90009-4. [DOI] [PubMed] [Google Scholar]
- SMITH C. B. ENHANCEMENT BY RESERPINE AND ALPHA-METHYL DOPA OF THE EFFECTS OF D-AMPHETAMINE UPON THE LOCOMOTOR ACTIVITY OF MICE. J Pharmacol Exp Ther. 1963 Dec;142:343–350. [PubMed] [Google Scholar]
- Scatton B., Cheramy A., Besson M. J., Glowinski J. Increased synthesis and release of dopamine in the striatum of the rat after amantadine treatment. Eur J Pharmacol. 1970;13(1):131–133. doi: 10.1016/0014-2999(70)90194-9. [DOI] [PubMed] [Google Scholar]
- Schwab R. S., England A. C., Jr, Poskanzer D. C., Young R. R. Amantadine in the treatment of Parkinson's disease. JAMA. 1969 May 19;208(7):1168–1170. [PubMed] [Google Scholar]
- Sinclair J. G. Interaction of amantadine with tyramine and noradrenaline on the cat nictitating membrane. Can J Physiol Pharmacol. 1973 Apr;51(4):305–308. doi: 10.1139/y73-045. [DOI] [PubMed] [Google Scholar]
- Stone T. W. Cholinergic mechanisms in the rat somatosensory cerebral cortex. J Physiol. 1972 Sep;225(2):485–499. doi: 10.1113/jphysiol.1972.sp009951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone T. W. Cortical pyramidal tract interneurones and their sensitivity to L-glutamic acid. J Physiol. 1973 Aug;233(1):211–225. doi: 10.1113/jphysiol.1973.sp010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone T. W. Further evidence for a dopamine receptor stimulating action of an ergot alkaloid. Brain Res. 1974 May 31;72(1):177–180. doi: 10.1016/0006-8993(74)90664-7. [DOI] [PubMed] [Google Scholar]
- Stone T. W. Proceedings: Responses of cortical pyramidal tract cells to amantadine and amphetamine after depletion of central catecholamines. Br J Pharmacol. 1975 Jun;54(2):244P–245P. [PMC free article] [PubMed] [Google Scholar]
- Stromberg U., Svensson T. H., Waldeck B. On the mode of action of amantadine. J Pharm Pharmacol. 1970 Dec;22(12):959–962. doi: 10.1111/j.2042-7158.1970.tb08487.x. [DOI] [PubMed] [Google Scholar]
- Strömberg U., Svensson T. H. Further studies on the mode of action of amantadine. Acta Pharmacol Toxicol (Copenh) 1971;30(3):161–171. doi: 10.1111/j.1600-0773.1971.tb00646.x. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971;367:69–93. doi: 10.1111/j.1365-201x.1971.tb11000.x. [DOI] [PubMed] [Google Scholar]
- VAN ROSSUMJ, HURKMANS A. T. MECHANISM OF ACTION OF PSYCHOMOTOR STIMULANT DRUGS. SIGNIFICANCE OF DOPAMINE IN LOCOMOTOR STIMULANT ACTION. Int J Neuropharmacol. 1964 May;3:227–239. doi: 10.1016/0028-3908(64)90012-7. [DOI] [PubMed] [Google Scholar]
- VOGT M. The concentration of sympathin in different parts of the central nervous system under normal conditions and after the administration of drugs. J Physiol. 1954 Mar 29;123(3):451–481. doi: 10.1113/jphysiol.1954.sp005064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernier V. G., Harmon J. B., Stump J. M., Lynes T. E., Marvel J. P., Smith D. H. The toxicologic and pharmacologic properties of amantadine hydrochloride. Toxicol Appl Pharmacol. 1969 Nov;15(3):642–665. doi: 10.1016/0041-008x(69)90066-0. [DOI] [PubMed] [Google Scholar]
- Von Voigtlander P. F., Moore K. E. Involvement of nigro-striatal neurons in the in vivo release of dopamine by amphetamine, amantadine and tyramine. J Pharmacol Exp Ther. 1973 Mar;184(3):542–552. [PubMed] [Google Scholar]
- York D. H. Dopamine receptor blockade--a central action of chlorpromazine on striatal neurones. Brain Res. 1972 Feb 11;37(1):91–99. doi: 10.1016/0006-8993(72)90348-4. [DOI] [PubMed] [Google Scholar]
- van Rossum J. M. The significance of dopamine-receptor blockade for the mechanism of action of neuroleptic drugs. Arch Int Pharmacodyn Ther. 1966 Apr;160(2):492–494. [PubMed] [Google Scholar]


