Abstract
1. Uptake of 3H-nicotine by isolated rat superior cervical sympathetic (SCG) and nodose (NG) ganglia was measured in vitro. Depolarization of the ganglia by nicotine was measured electrically.
2. Nicotine depolarized the SCG but not the NG. The mean ED50 for depolarization was 5·3 × 10-6M.
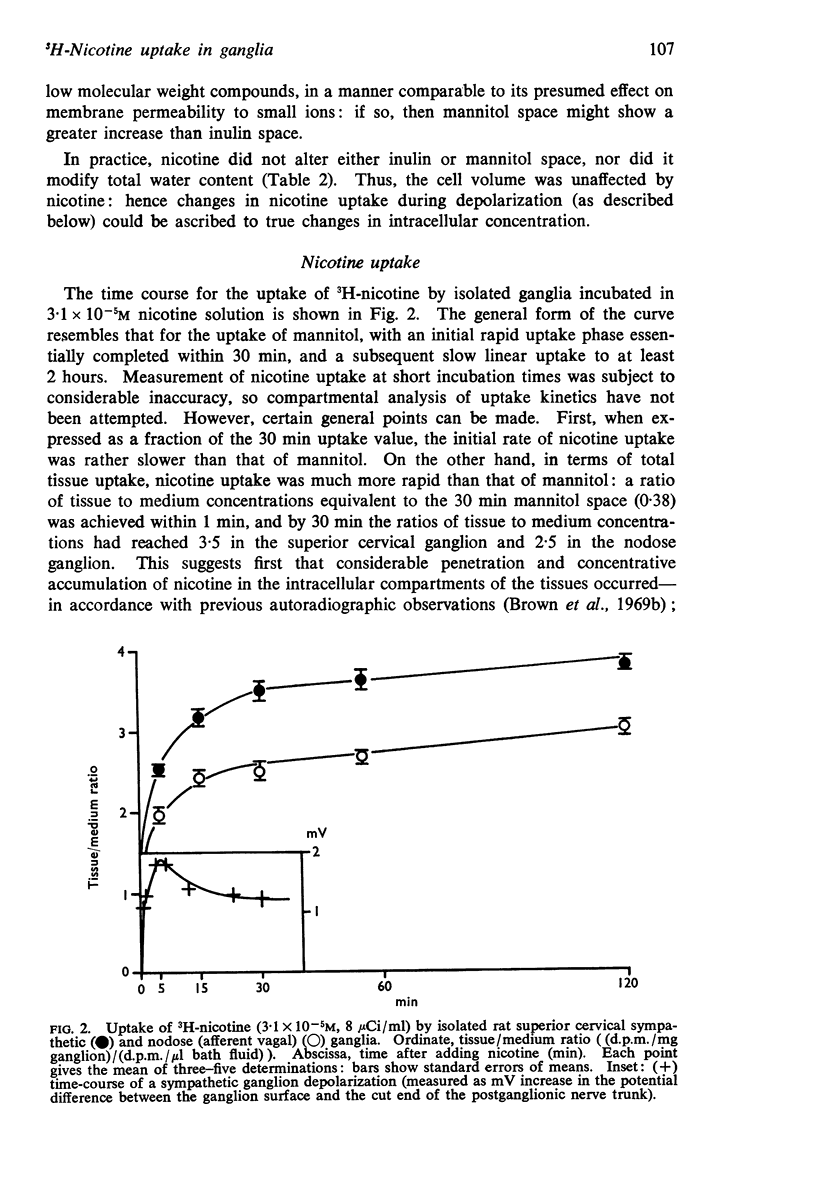
3. Both ganglia accumulated nicotine when incubated in 3·1 × 10-5M 3H-nicotine: after 30 min incubation the ratios of tissue to medium concentrations were (mean ± S.E. of mean): SCG, 3·49 ± 0·13; NG, 2·50 ± 0·09.
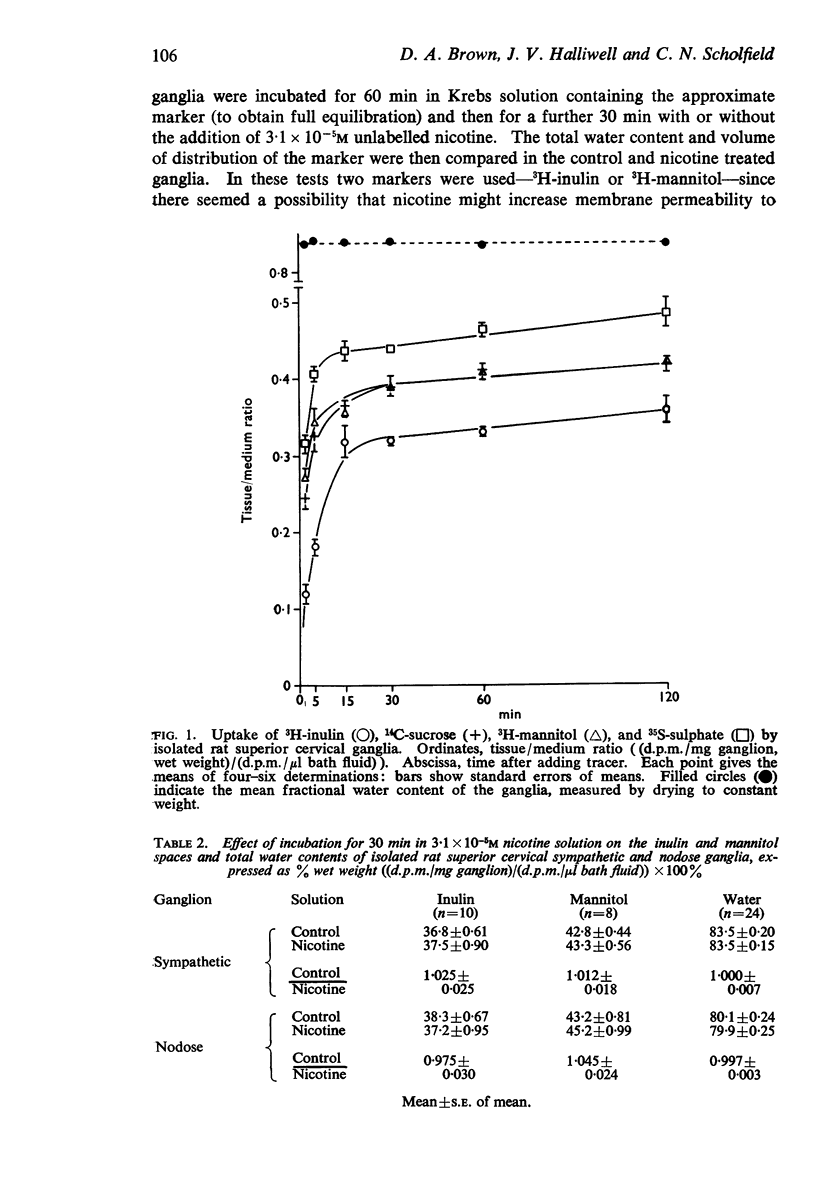
4. Total water contents, estimated by drying to constant weight, were: SCG, 83·8 ± 0·12%; NG, 80·1 ± 0·21%. Extracellular spaces, measured as 3H-mannitol space, were: SCG, 38·8 ± 1·3; NG, 40·3 ± 0·8% wet weight. These values were not significantly altered by nicotine.
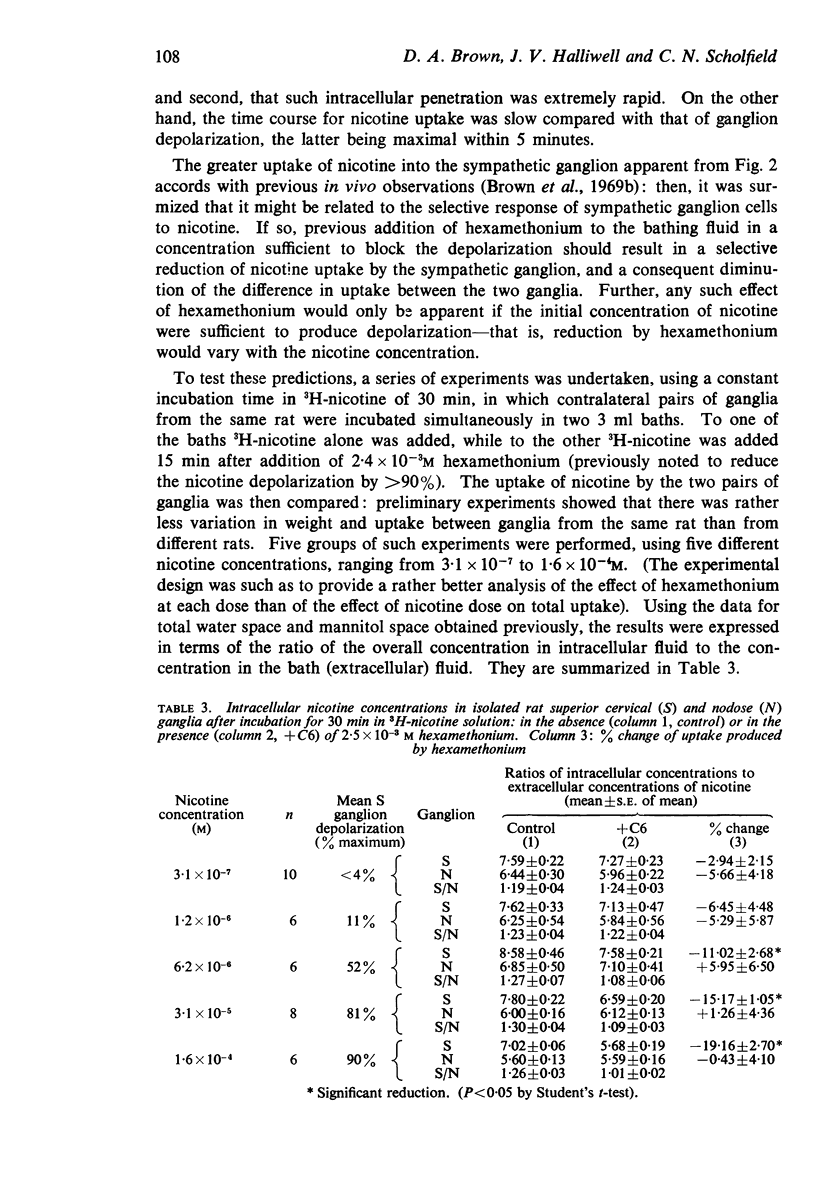
5. Correction for tissue fluid spaces indicated that the ratio of the mean intracellular fluid concentration to the extracellular fluid concentration for 3H-nicotine at 3·1 × 10-5M were: SCG, 7·4; NG, 5·6. The ratios were not altered in any consistent manner on varying the nicotine concentration between 3·1 × 10-7 and 1·6 × 10-4M.
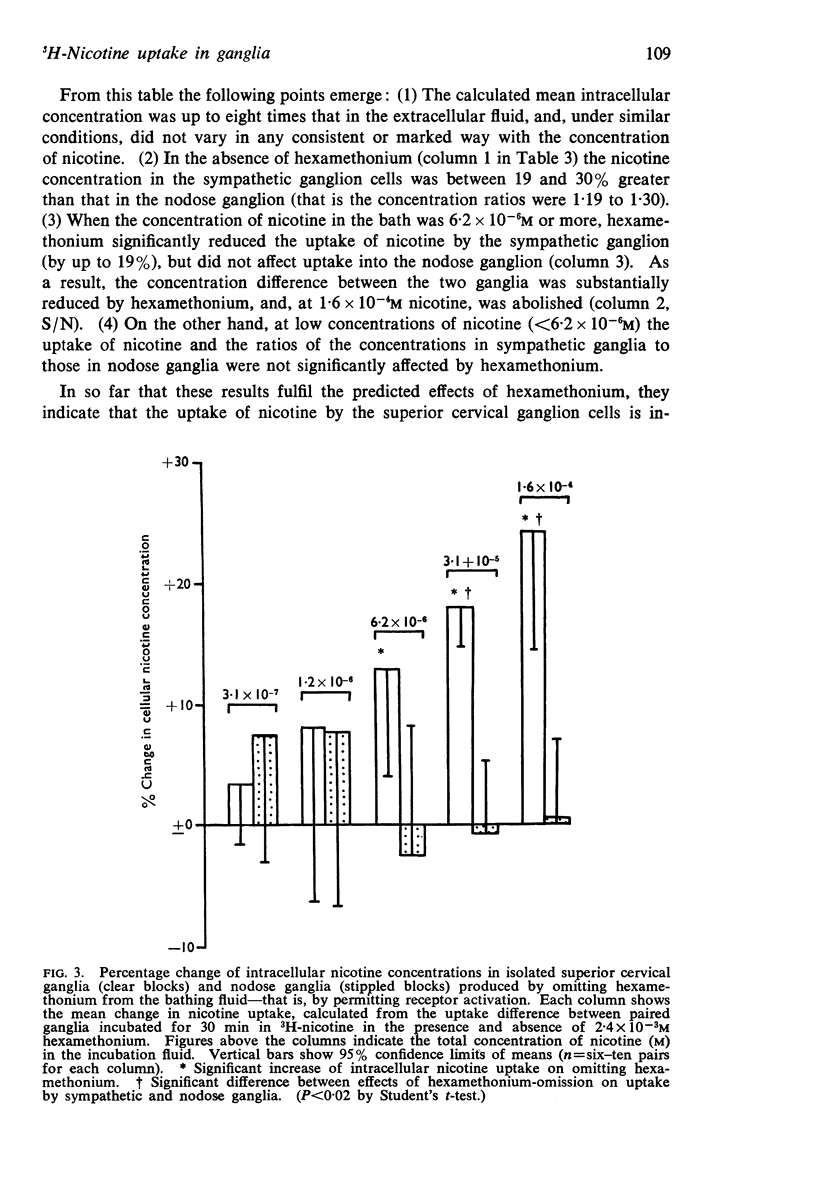
6. When the nicotine concentration was sufficiently great (6·2 × 10-6M or more) to evoke large SCG depolarizations, hexamethonium (2·5 × 10-3M) reduced 3H-nicotine uptake by the SCG by up to 19% without affecting uptake by the NG, and thereby reduced the uptake difference between the two ganglia. With nicotine concentrations <6·2 × 10-6M, hexamethonium did not modify uptake by either ganglion.
7. It was concluded that nicotine may be concentrated within neurones, and that such intracellular accumulation may be augmented during depolarization induced by nicotine.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPELGREN L. E., HANSSON E., SCHMITERLOEW C. G. LOCALIZATION OF RADIOACTIVITY IN THE SUPERIOR CERVICAL GANGLION OF CATS FOLLOWING INJECTION OF C14-LABELLED NICOTINE. Acta Physiol Scand. 1963 Dec;59:330–336. doi: 10.1111/j.1748-1716.1963.tb02748.x. [DOI] [PubMed] [Google Scholar]
- ARMETT C. J., RITCHIE J. M. The action of acetylcholine and some related substances on conduction in mammalian non-myelinated nerve fibres. J Physiol. 1961 Feb;155:372–384. doi: 10.1113/jphysiol.1961.sp006634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman J. G., Purves R. D. Intracellular recordings from ganglia of the thoracic sympathetic chain of the guinea-pig. J Physiol. 1969 Jul;203(1):173–198. doi: 10.1113/jphysiol.1969.sp008858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A. Depolarization of normal and preganglionically denervated superior cervical ganglia by stimulant drugs. Br J Pharmacol Chemother. 1966 Mar;26(3):511–520. doi: 10.1111/j.1476-5381.1966.tb01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A. Effects of hexamethonium and hyoscine on the drug-induced depolarization of isolated superior cervical ganglia. Br J Pharmacol Chemother. 1966 Mar;26(3):521–537. doi: 10.1111/j.1476-5381.1966.tb01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Hoffmann P. C., Roth L. J. 3H-Nicotine in cat superior cervical and nodose ganglia after close-arterial injection in vivo. Br J Pharmacol. 1969 Mar;35(3):406–417. doi: 10.1111/j.1476-5381.1969.tb08282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Stumpf W. E., Roth L. J. Location of radioactively labelled extracellular fluid indicators in nervous tissue by autoradiography. J Cell Sci. 1969 Jan;4(1):265–288. doi: 10.1242/jcs.4.1.265. [DOI] [PubMed] [Google Scholar]
- Creese R., England J. M. Decamethonium in depolarized muscle and the effects of tubocurarine. J Physiol. 1970 Sep;210(2):345–361. doi: 10.1113/jphysiol.1970.sp009214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese R., Taylor D. B. Entry of labeled carbachol in brain slices of the rat and the action of d-tubocurarine and strychnine. J Pharmacol Exp Ther. 1967 Aug;157(2):406–419. [PubMed] [Google Scholar]
- DOUGLAS W. W., RITCHIE J. M. Cardiovascular reflexes produced by electrical excitation of non-medullated afferents in the vagus, carotid sinus and aortic nerves. J Physiol. 1956 Oct 29;134(1):167–178. doi: 10.1113/jphysiol.1956.sp005632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., SCHAUMANN W. A study of the depressor and pressor components of the cat's carotid sinus and aortic nerves using electrical stimuli of different intensities and frequencies. J Physiol. 1956 Apr 27;132(1):173–186. doi: 10.1113/jphysiol.1956.sp005512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M. Intracellular potentials recorded from a mammalian sympathetic ganglion. J Physiol. 1955 Dec 29;130(3):572–584. doi: 10.1113/jphysiol.1955.sp005428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodford P. J., Leach E. H. The extracellular space of the smooth muscle of the guinea-pig taenia coli. J Physiol. 1966 Sep;186(1):1–10. doi: 10.1113/jphysiol.1966.sp008016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSSON E., HOFFMANN P. C., SCHMITERLOEW C. G. METABOLISM OF NICOTINE IN MOUSE TISSUE SLICES. Acta Physiol Scand. 1964 Aug;61:380–392. [PubMed] [Google Scholar]
- Hancock J. C., Volle R. L. Blockade of conduction in vagal fibers by nicotinic drugs. Arch Int Pharmacodyn Ther. 1969 Mar;178(1):85–98. [PubMed] [Google Scholar]
- Law R. O., Phelps C. F. The size of the sucrose, raffinose, and inulin spaces in the gastrocnemius muscle of the rat. J Physiol. 1966 Oct;186(3):547–557. doi: 10.1113/jphysiol.1966.sp008055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls J. G., Wolfe D. E. Distribution of 14C-labeled sucrose, inulin, and dextran in extracellular spaces and in cells of the leech central nervous system. J Neurophysiol. 1967 Nov;30(6):1574–1592. doi: 10.1152/jn.1967.30.6.1574. [DOI] [PubMed] [Google Scholar]
- Waddell W. J., Bates R. G. Intracellular pH. Physiol Rev. 1969 Apr;49(2):285–329. doi: 10.1152/physrev.1969.49.2.285. [DOI] [PubMed] [Google Scholar]
- Weiss G. B. Dependence of nicotine-C14 distribution and movements upon pH in frog sartorius muscle. J Pharmacol Exp Ther. 1968 Mar;160(1):135–147. [PubMed] [Google Scholar]
- Weiss G. B. The effect of pH on nicotine-induced contracture and Ca45 movements in frog sartorius muscle. J Pharmacol Exp Ther. 1966 Dec;154(3):605–612. [PubMed] [Google Scholar]
- Woodward J. K., Bianchi C. P., Erulkar S. D. Electrolyte distribution in rabbit superior cervical ganglion. J Neurochem. 1969 Mar;16(3):289–299. doi: 10.1111/j.1471-4159.1969.tb10367.x. [DOI] [PubMed] [Google Scholar]


