Abstract
We have investigated what limits demand-driven de novo glutathione (GSH) biosynthesis in green Arabidopsis suspension culture cells. GSH is the most abundant low-molecular weight thiol in most plants and can be quantified using monochlorobimane to fluorescently label GSH in live cells. Progress curves for labeling reached a plateau as all the cytoplasmic GSH was conjugated. In the presence of excess monochlorobimane, a second, almost linear phase of labeling was observed, after a lag of 2 to 3 h, that was then maintained for an extended period. The increase in fluorescence was shown to be because of de novo GSH biosynthesis by high-performance liquid chromatography analysis and was eliminated by dl-buthionine-[S,R]-sulfoximine, a specific inhibitor of GSH biosynthesis, or reduced by inhibitors of transcription and translation. The rate of GSH biosynthesis during the linear phase was 8.9 ± 1.4 nmol g fresh weight−1 min−1 and was not affected by addition of glutamate, glycine, or cysteine, the immediate precursors needed for GSH biosynthesis. Likewise, the synthesis rate was not affected by pretreatment with aminotriazole, menadione, jasmonic acid, or cadmium, all of which cause oxidative stress and up-regulate expression of GSH biosynthetic genes. The lag phase was markedly reduced by aminotriazole and menadione and marginally by jasmonic acid, suggesting the system was primed to react faster after mild stress. In contrast to the other feeding experiments, exclusion of SO42− from the medium abolished the second phase completely. This suggests demand-driven GSH biosynthesis is directly coupled to uptake of SO42− and that the linear increase in fluorescence reflects flux through the entire SO42− assimilation pathway.
Plant cells are able to respond to adverse environmental conditions by up-regulating key metabolic pathways that ameliorate or counteract the physiological consequences of the imposed stress. Reduced glutathione (GSH) is the most abundant nonprotein thiol in cells and its nucleophilic activity is exploited in several stress response pathways to detoxify active oxygen species (AOS), xenobiotics, and certain heavy metals (May et al., 1998a; Noctor and Foyer, 1998; Noctor et al., 1998). For example, GSH and its oxidized form, GSSG, form one of a series of redox couples that transfer reducing equivalents from NADPH to AOS. Typically, AOS, such as hydrogen peroxide, initially oxidize ascorbic acid to monodehydroascorbate and/or dehydroascorbate using ascorbate peroxidases (Noctor and Foyer, 1998; Conklin, 2001). Rereduction of dehydroascorbate is driven by cycling of GSH to GSSG, catalyzed by dehydroascorbate reductase. GSSG is then rereduced by GSH reductase at the expense of NADPH (Foyer and Halliwell, 1976; Noctor and Foyer, 1998). In this pathway, the redox poise of the GSH pool alters in response to stress, but there is no net consumption of GSH. In contrast, detoxification of electrophilic xenobiotics via glutathione S-transferase (GST)-dependent conjugation or detoxification of heavy metals via formation of phytochelatins with the general structure (γ-Glu-Cys)nGly (n = 2–11) both lead to an immediate decrease of the cellular GSH pool (Coleman et al., 1997b; Cobbett, 2000). In these cases, cells respond to the imposed stress with de novo biosynthesis of GSH from its constituent amino acids to maintain the resting level of GSH. It has been proposed that both the resting level of GSH and the efficiency at which cells can refill the cytoplasmic GSH pool after depletion may influence their degree of stress tolerance (May et al., 1998a). It is known that reduction in GSH levels in mutants or transgenic plants reduces stress tolerance (e.g. Howden et al., 1995; Xiang et al., 2001); however, the protective role of elevated GSH levels and/or increased biosynthetic capacity is more controversial. In different systems, elevated GSH is reported to reduce the effects of stress (Zhu et al., 1999; Gullner et al., 2001), confer no additional tolerance (Arisi et al., 1999; Xiang et al., 2001), or even lead to greater oxidative damage (Creissen et al., 1999).
The underlying control mechanisms leading to up-regulation of GSH biosynthesis in planta are not well defined and probably operate at multiple levels depending on the severity of the stress and the time period considered. The importance of each step in the pathway can be investigated by sequentially modifying the activity of each enzyme involved using transgenic techniques. This approach has already revealed information on the role of ATP sulfurylase (Hatzfeld et al., 1998; Pilon-Smits et al., 1999), γ-Glu-Cys synthetase (γ-ECS; Noctor et al., 1996; Xiang et al., 2001), and glutathione synthetase (Strohm et al., 1995; Creissen et al., 1999). An alternative and complementary approach is to measure changes in flux through the pathway in the intact system as demand or supply alters (Roscher et al., 2000). Previously, addition of cadmium has been used to achieve an elevated demand for GSH through consumption during phytochelatin synthesis (Schneider and Bergmann, 1995). GSH can also be depleted by conjugation to model xenobiotics such as monochlorobimane (MCB) or 1-chloro-2,4-dinitrobenzene (CDNB; e.g. Coleman et al., 1997a, 1997b). We have shown previously that short-term (1–3 h) labeling with MCB in vivo follows a progress curve for a GST-catalyzed conjugation reaction in a variety of different cell types and tends toward a plateau value as all the GSH is reacted (Fricker et al., 2000; Gutiérrez-Alcalá et al., 2000; Meyer and Fricker, 2000; Fricker and Meyer, 2001; Meyer et al., 2001). In this paper, we have used an extended period of in vivo labeling with MCB, to create and maintain a “sink” for GSH in Arabidopsis suspension culture cells. The assay provides a continuous readout of the level of GSH and fine temporal resolution of the kinetics of the cellular response leading to de novo GSH biosynthesis.
RESULTS
Long-Term Incubation of Cells with MCB Triggers Demand-Driven GSH Biosynthesis
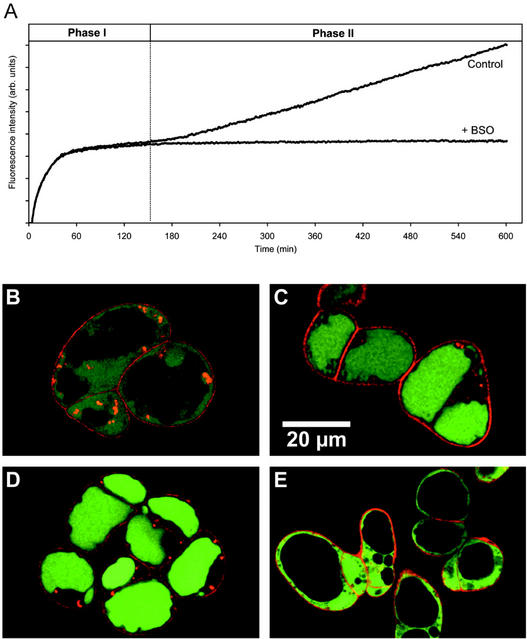
Fluorescence from conjugation of MCB to GSH increased rapidly after incubation of Arabidopsis suspension culture cells with 100 μm MCB until a plateau was reached after 60 to 120 min (Fig. 1A). Size exclusion chromatography showed that virtually all the fluorescence was present in the low-Mr fraction, with negligible protein labeling (data not shown). At the plateau phase, GSH was the major low-Mr compound labeled (92.7%), with much smaller amounts of Cys and γ-Glu-Cys (γ-EC; 6% and 1.3%, respectively; Table I). The absolute amount of GSH labeled in situ corresponded well with the amount measured by conventional extraction and in vitro bimane labeling (Table I).
Figure 1.
Long-term in vivo labeling of Arabidopsis suspension culture cells with MCB. Cells were incubated with 100 μm MCB and fluorescence recorded continuously for 8 h or stopped at different time points for further analysis of the labeled compounds. A, Continuous recording of the increasing fluorescence on a fluorescence microplate reader in the absence or presence of 1 mm dl-buthionine-[S,R]-sulfoximine (BSO). Excitation wavelength (λex) = 390 nm; emission wavelength (λem) = 460 nm. B through D, Two-photon laser scanning microscopy (TPLSM) images of cells labeled with MCB for 30 min (B), 90 min (C), and 6 h (D). MCB-dependent fluorescence (green) was initially generated in the cytoplasm and then subsequently sequestered into the vacuole. Fifty micromolar propidium iodide (PI) was added to cells immediately before imaging and labeled only the cell walls (red). Red fluorescent spots in the cytoplasm are because of red autofluorescence from the chloroplasts. E, TPLSM images of cells labeled with MCB for 60 min in the presence of 5 mm azide with excitation at λex = 770 nm. Azide completely blocked vacuolar sequestration of fluorescent GSH-bimane conjugates.
Table I.
Amounts of bimane-labeled low-Mr thiols at different time points during in situ labeling of Arabidopsis cells with 100 μm MCB
| In Situ Labeling Time | Cys | CysGly | λ-EC | GSH |
|---|---|---|---|---|
| nmol g fresh wt−1 | ||||
| 15 ± 3 | nd | 7 ± 1 | 589 ± 32 | |
| 120 min | 37 ± 6 | nd | 8 ± 2 | 560 ± 37 |
| 240 min | 69 ± 14 | nd | 11 ± 2 | 685 ± 51 |
| 360 min | 171 ± 29 | 8 ± 1 | 23 ± 4 | 857 ± 73 |
| Amount measured after in vitro labeling | 25 ± 4 | nd | 19 ± 4 | 590 ± 43 |
nd, Not detected. Values are given as the mean ± sd, n = 5.
Signal was initially observed in the cytoplasm and was subsequently transferred to the vacuole (Fig. 1, B and C). Quantitative analysis of the fluorescence signal from the TPLSM images corresponded to an initial cytoplasmic GSH concentration of 2.1 ± 0.3 mmol GSH-bimane conjugates (GSB) (lcytoplasm)−1.
A second, nearly linear increase in fluorescence was observed after 120 to 150 min that continued for at least 6 to 10 h. More than 99% (n = 340 cells in seven experiments) of the cells remained viable during this extended labeling period as judged from the absence of PI labeling of the nuclei (Fig. 1D). The additional red spots within the cytoplasm were not caused by PI labeling, but rather show autofluorescence from chloroplasts that were also excited with TPLSM at 770 nm. HPLC analysis of cells labeled for 6 h showed an increase in the total thiol fluorescence from the plateau value to 173%. The fraction of label in Cys and γ-EC increased to 16% and 2.2%, respectively, and a small amount (0.7%) of Cys-Gly was also detected. These thiols might become labeled through nonspecific reactions of MCB in the cytoplasm or result from partial degradation of GSB by vacuolar peptidases after transport into the vacuole. To distinguish between these possibilities, vacuolar transport of GSB was completely inhibited by 1 to 5 mm sodium azide (Fig. 1E). Overall labeling of Cys, γ-EC, and Cys-Gly was concomitantly reduced to <1% of the total (data not shown). We infer that in situ labeling with MCB is highly specific for GSH and the other thiol derivatives arise from the action of vacuolar peptidases that clip off the Glu and Gly residues.
The second phase of labeling was completely blocked by BSO, an inhibitor of γ-ECS (Fig. 1A), suggesting that the second phase of labeling represents de novo biosynthesis of GSH that is subsequently conjugated to MCB, rather than labeling of another thiol or a different subcellular GSH pool that labels with different kinetics. In the presence of BSO, the fluorescence signal remained stable for an extended period, suggesting that there was no further degradation of the fluorescent tag beyond Cys-S-bimane and consequent disruption of the fluorophore.
Characterization of Lag Phase before Demand-Driven GSH Biosynthesis Is Initiated
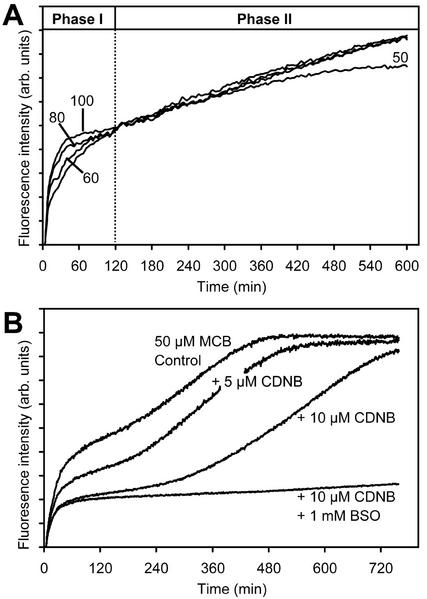
To determine whether the extent of the lag phase was linked to the rate of GSH depletion, the effect of manipulating the rate of GSH removal was examined. Concentrations of MCB lower than 100 μm resulted in a slower rate of GSB formation but did not affect the plateau level reached, the duration of the lag phase, or the slope of the second phase (Fig. 2A), provided sufficient MCB was present in the assay. For example, the tail off in the rate of labeling after 5 to 6 h of incubation with 50 μm MCB was because of depletion of MCB in the medium around the cells (Fig. 2A). The extent that this occurred depended on the ratio of cytoplasmic volume to total assay volume and varied depending on the configuration of the experimental system used (multiwell plate reader or fluorimeter cuvette) and the volume ratio of cells to medium.
Figure 2.
The rate of depletion of GSH during phase I does not affect the lag before start of the demand-driven GSH biosynthesis in phase II. A, Increasing concentrations of MCB increase the rate of GSB formation, and GSH depletion, but do not affect the lag before the start of phase II or subsequent rate of de novo GSH biosynthesis. The tail off in phase II for 50 μm MCB reflects depletion of MCB in the assay. B, Simultaneous incubation of cells with 50 μm MCB and 5 and 10 μm CDNB and effect of BSO in the presence of 10 μm CDNB. CDNB is conjugated more rapidly than MCB and, therefore, reduces the GSH pool available to form GSB until all the CDNB is consumed. Despite the very rapid removal of half the GSH pool with 10 μm CDNB, initiation of phase II is not triggered earlier.
To increase the rate of GSH depletion, cells were incubated in CDNB as a second substrate for GSTs that is very rapidly conjugated to GSH, thereby increasing demand at a constant level of MCB. The presence of CDNB resulted in a lower level of fluorescence at the plateau, as might be expected if conjugation of CDNB competes for the available GSH (Fig. 2B). However, the lag phase before start of the second phase appeared to be extended rather than reduced at higher CDNB concentrations (Fig. 2B). There was a very small difference between the CDNB treatment alone and CDNB plus BSO that might hint at a very low rate of GSH biosynthesis earlier than 120 to 180 min. The addition of CDNB did not cause an increase in the slope of the second phase, even though all the CDNB was conjugated by this stage, but rather slightly reduced the slope, particularly at higher concentrations (Fig. 2B).
The Lag Phase and Rate of de Novo Synthesis Are Relatively Insensitive to the Addition of Precursors for GSH Biosynthesis
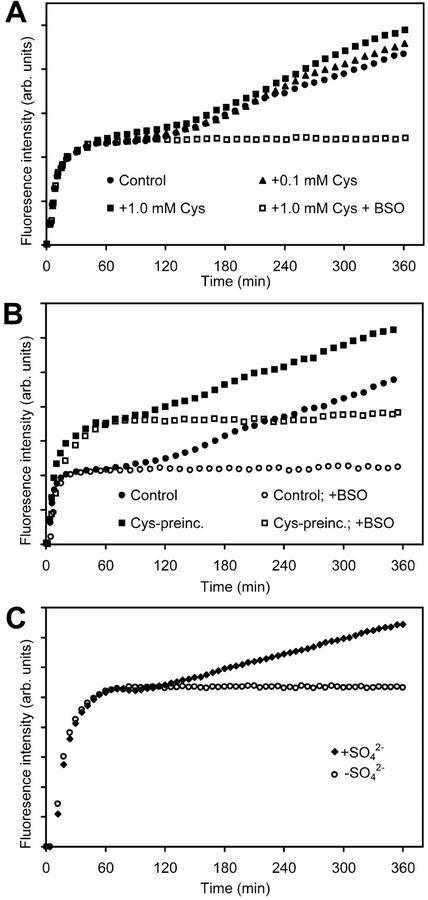
The rate of GSH biosynthesis observed during the second phase of labeling would place a considerable demand for precursors of intermediates earlier in the pathway. To test which, if any, of these components might be limiting flux through the pathway, the medium was supplemented with high levels of precursors and the rate of GSH biosynthesis determined. The slope of the second phase without supplements corresponded to a synthesis rate for GSH of 8.9 ± 1.4 nmol g fresh weight−1 min−1 (Table II). Exogenous Glu, Gly, and Cys were rapidly taken up by suspension culture cells at rates corresponding to 20 ± 3.8, 25.7 ± 5.3, and 23 ± 8.6 nmol g fresh weight−1 min−1 (n = 4), respectively, over a 2-h period. Glu and Gly at 1 mm had no effect on the rate of GSH biosynthesis, even after pre-incubation for several hours (Table II). Addition of Cys at 0.1 mm had no effect on the rate of GSH biosynthesis, whereas 1 mm Cys gave a very slight (20%) stimulation (Fig. 3A; Table II). Pre-incubation with 1 mm Cys for 12 h, followed by assay in normal medium, gave an approximate 2-fold increase in the resting level of GSH, but did not reduce the delay before the onset of the second phase or increase the rate of GSH biosynthesis (Fig. 3B). Interestingly, the response to exogenous supply of both N-acetyl-l-Cys, a potential source of Cys, and O-acetyl-l-Ser, one of the immediate precursors of Cys, could not be followed as both caused bleaching of the cells within 8 h.
Table II.
Effect of GSH precursors on demand-driven de novo GSH biosynthesis during incubation of Arabidopsis cells with MCB
| Treatment | Resting Level of GSH | Estimated Lag Time | Rate of GSH Biosynthesis |
|---|---|---|---|
| % | min | nmol g fresh wt−1 min−1 | |
| Murashige and Skoog medium supplemented with 3% (w/v) Suc (control) | 100 | 120–180 | 8.9 ± 1.4 (6) |
| +1 mm Glu | 102 ± 6 | 120–180 | 8.9 ± 1.5 (5) |
| +1 mm Gly | 97 ± 8 | 120–180 | 8.8 ± 1.4 (5) |
| +1 mm Cys | 105 ± 15 | 120–180 | 10.6 ± 1.8 (5) |
| +0.1 mm Cys | 95 ± 12 | 120–180 | 9.3 ± 1.7 (5) |
| +1 mm Glua | 101 ± 5 | 120–180 | 8.8 ± 1.9 (5) |
| +1 mm Glya | 102 ± 9 | 120–180 | 8.7 ± 1.6 (5) |
| +1 mm Cysa | 169 ± 17 | 120–180 | 10.3 ± 2.0 (5) |
| −SO42− | 102 ± 5 | nd | 0.0 ± 0.0 (6) |
| +1 mm SO42− | 101 ± 7 | 120–180 | 8.9 ± 1.4 (7) |
| +10 mm SO42− | 99 ± 11 | 120–180 | 9.0 ± 1.2 (7) |
Cells were always labeled with 50 to 100 μm MCB over 6 h. Precursors were removed from or added to the labeling solution at the start of labeling. All values are given as mean ± sd with the no. of experiments in parentheses. The control medium contained 1.73 mm SO42−. nd, Not detected.
Cells were pre-incubated for 10 to 12 h.
Figure 3.
Treatment of cells with precursors for the biosynthesis of GSH. A, Typical progress curves in the presence of Cys in the exogenous medium. B, Effect of pre-incubation of cells with 1 mm Cys for 12 h on the resting level of GSH and the rate of GSH biosynthesis during the second phase of labeling. C, Impact of exogenous SO42− on the biphasic labeling of cells with 100 μm MCB.
Demand-Driven GSH Biosynthesis Requires Sulfate Uptake from the External Medium
Intermediates earlier in the sulfur assimilation pathway, such as sulfide and SO32−, are known to be toxic to cells when added exogenously. Thus, we then focused on the effect of changing SO42− availability on GSH biosynthesis. The normal growth and assay media contained 1.73 mm SO42−. Further addition of SO42− up to 10 mm had no effect on GSH biosynthesis (Table II); however, reconstitution of the assay in SO42−-free media completely abolished the second phase of labeling (Fig. 3C). We infer from this result that these culture cells do not maintain or utilize intracellular pools of SO42− for Cys biosynthesis, but rely entirely on uptake of SO42− from the medium during rapid, demand-driven GSH biosynthesis.
Demand-Driven GSH Synthesis Requires Transcription and Translation
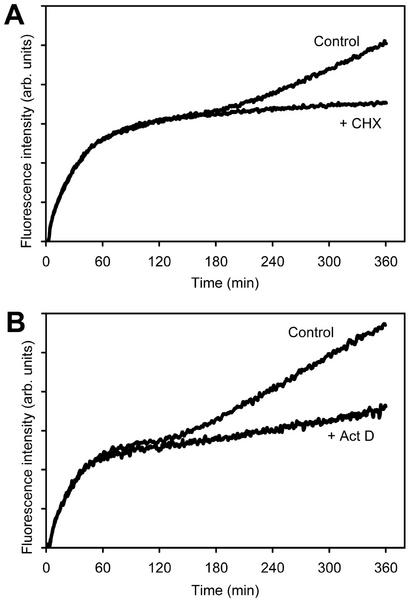
To elucidate whether the lag phase before start of the second phase of labeling required de novo protein biosynthesis, the translation inhibitor cycloheximide (CHX) or the transcription inhibitor actinomycin D (Act D) were included in the labeling medium. Both inhibitors did not affect the first phase of labeling but substantially inhibited the second phase of labeling (Fig. 4, A and B).
Figure 4.
Transcriptional and translational inhibitors block demand-driven GSH biosynthesis. Labeling reached a plateau after 120 to 180 min in the presence and absence of 20 mg L−1 CHX (A) or 2 mg L−1 Act D (B); however, the second phase of labeling was substantially reduced by both inhibitors.
Effect of Chemical Stress Agents on GSH Status and Demand-Driven GSH Biosynthesis
To determine whether treatment of cells with chemical stress agents caused changes in the resting level of GSH and the biphasic progress curve for labeling with MCB, cells were exposed to several different compounds and heavy metals that are known to induce parts of the stress response pathway. After pre-incubation of cells for 10 h with the catalase inhibitor aminotriazole (AT) or the superoxide-generating menadione (MQ), which both cause mild oxidative stress, subsequent labeling with MCB did not result in the normal biphasic progress curve. In these cases, no clear lag phase was observed even though cells still entered the linear phase of demand-driven GSH biosynthesis (Table III). Although the estimated resting GSH levels were slightly enhanced after pretreatment with MQ and AT, the rate of GSH biosynthesis was not markedly changed. Jasmonic acid (JA) is known to induce accumulation of GSH metabolic gene transcripts (Xiang and Oliver, 1998). However, the only detectable effect of pre-incubation of cells with 100 μm JA for 10 h was a slight reduction in the lag phase (Table III).
Table III.
Effect of chemical stress agents on the progress curves for the labeling of Arabidopsis cells with MCB
| Treatment | Resting Level of GSH | Estimated Lag Time | Rate of Labeling in Phase II |
|---|---|---|---|
| % | min | nmol g−1 min−1 | |
| MQ, 20 μma | 153 ± 16 | No lag | 8.7 ± 1.6 |
| AT, 2 mma | 122 ± 18 | No lag | 7.9 ± 2.8 |
| JA, 100 μma | 95 ± 9 | 90–150 | 8.3 ± 1.2 |
| Cd2+, 100 μmb | 91 ± 15 | 120–180 | 6.8 ± 1.0 |
Cells were labeled with 100 μm MCB for 6 h. Resting levels of GSH are given as percentage of control values. Values for the rate of GSH biosynthesis are means ± sd from at least three experiments.
Pretreatment for 10 h.
Pretreatment for 15 h.
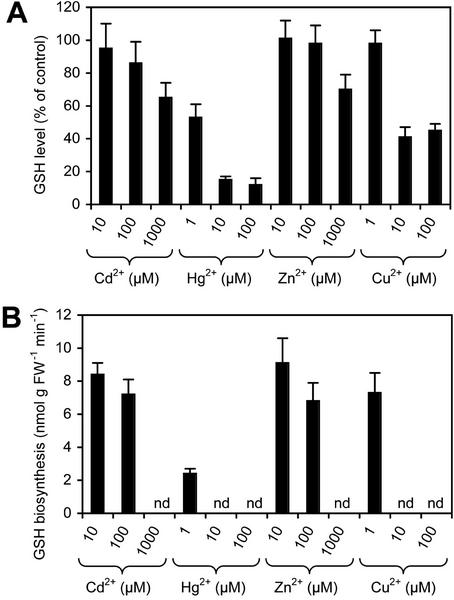
Arabidopsis plants respond to heavy metal challenge by synthesizing phytochelatins (Cobbett, 2000). Besides triggering the synthesis of phytochelatins, heavy metals also might form chelates with GSH and cause oxidative stress. Pretreatment with 100 μm CdCl2 for 10 to 12 h caused a very slight reduction in GSH levels and a slight reduction of the rate of labeling during phase II, but did not significantly affect the overall biphasic behavior of the progress curve (Table III). Co-incubation of cells with MCB and various other heavy metals resulted in a reduction of the plateau level of labeling of between 10% and 60% at various concentrations and reduced labeling during the second phase (Fig. 5, A and B). The reduction in the rate of GSH labeling during the second phase was concentration dependent and most pronounced with HgCl2 and CuCl2 (Fig. 5). Although 1 μm HgCl2 reduced the rate to less than 30%, addition of 10 to 100 μm HgCl2 or CuCl2 caused cell death within a few minutes after start of the treatment as judged by microscopic observation of the cell morphology (data not shown). Cell death prevented further labeling of the GSH pool. Similar effects could be observed with millimolar concentrations of Cd2+ and Zn2+ (Fig. 5). In all other cases, the second phase started after around 2 to 3 h. It should be noted, however, that the reduced rates of labeling in the second phase observed in the presence of heavy metals do not necessarily reflect the entire amount of GSH synthesized during the course of the experiment. As seen in the presence of high concentrations of CDNB (Fig. 2B), competition between heavy metals and/or heavy metal-induced phytochelatin synthesis and MCB for the GSH pool results in a reduction in the amount of GSB formed.
Figure 5.
Effect of heavy metals on the labeling of Arabidopsis cells with MCB. A, Levels of GSH determined after simultaneous addition of 100 μm MCB and various heavy metals at various concentrations. B, Rates of labeling during the second phase of labeling after long term (>6 h) incubation of cells with 100 μm MCB and various heavy metals at various concentrations. nd, Not detected.
DISCUSSION
Long-Term Incubation of Cells with MCB Triggers de Novo GSH Biosynthesis
Conjugation of GSH to MCB to give fluorescent GSB was originally used to track xenobiotic detoxification pathways in vivo (Coleman et al., 1997a, 1997b). We recently have exploited this reaction to quantify cytoplasmic GSH concentrations in a variety of living cells and tissues (Fricker et al., 2000; Gutiérrez-Alcalá et al., 2000; Meyer and Fricker, 2000; Meyer et al., 2001) and to analyze the kinetics for GST and glutathione S-xenobiotic conjugate pump activity in vivo (Fricker and Meyer, 2001). In other systems, GSH-S conjugates are further degraded to the Cys-S conjugate after sequestration in the vacuole (Wolf et al., 1996, Coleman et al., 1997a). Consistent with this view, we report here that removal of Gly and Glu from GSB requires vacuolar transport in Arabidopsis. Labeling with MCB rapidly depleted the GSH pool, leading to a massive demand for de novo biosynthesis of GSH, similar to the approach adopted by Schneider and Bergmann (1995) using cadmium. However, the time resolution of the continuous fluorescence assay used here was sufficient to reveal more detailed kinetics on activation of GSH biosynthesis, most notably the presence of an extended lag period before de novo GSH biosynthesis was triggered.
GSH Biosynthesis Is Tightly Regulated at the Level of γ-ECS
In animal cells, activity of γ-ECS, the first dedicated enzyme of GSH biosynthesis, is normally maintained at a low level through feedback inhibition by high concentrations of cytoplasmic GSH (Ki[GSH] = 1.8 mm; Huang et al., 1993). In plant cells, γ-ECS is also inhibited by high concentrations of GSH in vitro, with a Ki[GSH] of 0.27 to 0.42 mm (Hell and Bergmann, 1990; Schneider and Bergmann, 1995), suggesting that γ-ECS activity would increase as the cytoplasmic GSH level was reduced. In this study, the presence of a lag period before de novo GSH biosynthesis was initiated suggests that feedback inhibition was not the major control operating on γ-ECS in this system. Furthermore, the length of the lag period was relatively unaffected by a range of treatments that altered the rate of GSH depletion, including varying the MCB concentration, addition of heavy metals, or CDNB. If the major control of γ-ECS was through feedback inhibition by GSH, we would have predicted that de novo biosynthesis would have been initiated at different times and a different rates as the rate of GSH depletion was varied.
It is possible that γ-ECS activity did increase as feedback inhibition was alleviated, but the flux to GSH was still limited by substrate availability further upstream in the pathway. Cells were used at the end of the light period when high (millimolar) levels of Glu and Gly might be anticipated (Winter et al., 1993, 1994; Noctor et al., 1999). Furthermore, no increase in labeling was observed after addition of exogenous Glu and Gly, even though these amino acids were taken up rapidly. In contrast, resting levels of Cys were very low, as reported for other species and cell types (Noctor et al., 1998; Leustek et al., 2000) and the availability of Cys has been shown to limit the rate that the GSH pool can be replenished both in wild-type maize (Zea mays; Rüegsegger and Brunold, 1992) and transgenic poplars (Populus tremula × Populus alba) with enhanced GSH levels arising from overexpression of γ-ECS (Noctor et al., 1996). In this study, pre-incubation with Cys increased GSH levels 2-fold and co-incubation with MCB slightly increased the rate of GSH synthesis in the second phase. However, the effect was small and Cys feeding did not reduce the length of the lag period. We infer that the resting level of γ-ECS activity was low, even in the presence of excess substrate, until the start of the second phase. Cys feeding in parsley (Petroselinum crispum) and tobacco (Nicotiana tabacum) suspension culture cells also resulted in rapid increases in intracellular Cys, but gave limited or no stimulation of GSH biosynthesis (Schneider and Bergmann, 1995). Both sets of data would be consistent with tight control of γ-ECS activity as a major factor limiting the overall flux through the GSH biosynthesis pathway, but our data do not provide clear support that this control operates via feedback inhibition by GSH.
De Novo GSH Biosynthesis Requires Transcription and Translation
The second phase was inhibited almost completely by inhibitors of transcription and translation, suggesting that at least one enzyme or key regulatory component in the pathway requires de novo gene expression. The delay inherent in gene induction may also explain the lag before new GSH biosynthesis was initiated and the apparently constant lag period. The most obvious target for such regulation would be γ-ECS itself, and increases in γ-ECS transcript level (Xiang and Oliver, 1998) and extractable γ-ECS activity (Rüegsegger and Brunold, 1992; Chen and Goldsbrough, 1994; Kocsy et al., 1996) are well documented in response to a wide range of stresses. However, in a similar Arabidopsis suspension culture, May et al. (1998b) found that steady-state mRNA levels for γ-ECS did not alter during treatments with cadmium, MQ, AT, or the safener fenchlorazole, over a short term (6 h), although the enzyme activity increased markedly. Therefore, they suggested that posttranslational modification of γ-ECS might be responsible for the increased activity.
In this study, pretreatment with both MQ and AT reduced the lag phase before de novo biosynthesis was triggered, although the subsequent synthesis rate was not affected. We infer that pretreatment with MQ and AT may have primed cells to activate control of the GSH biosynthesis pathway, including the components that might require de novo gene expression, if this is the source of the lag phase.
A similar effect might be anticipated after treatment with JA, cadmium, and copper, which are known to induce both γ-ECS and glutathione synthetase transcript levels (Xiang and Oliver, 1998). However, in this study, pretreatment with JA only resulted in a slight reduction in the lag phase. Cadmium at 100 μm showed a limited (nonsignificant) reduction in the resting level of GSH and the rate of GSH biosynthesis with no effect on the lag phase. Co-incubation with Cd2+ at 1 mm or Cu2+ at 10 μm showed a greater reduction of labeling of the GSH pool and eliminated labeling of the second phase altogether. Unfortunately, the effect of heavy metals is quite difficult to interpret in this study because they have several potential effects in the assay. Formation of heavy metal-GSH chelates (Martell and Smith, 1982) or the synthesis of heavy metal-binding phytochelatins from GSH (Cobbett, 2000) will both compete directly for the available GSH, and the level of GSB fluorescence can no longer be regarded as a reliable indicator of the total amount of GSH synthesized. Quantitatively, this explanation cannot account for the profound effect of Hg2+ at 1 μm because the cells contain approximately 20-fold excess total amount of GSH. Treatment of plants with heavy metals may also result in the generation of free radicals and AOS (Clemens, 2001). These do not necessarily affect the total level of GSH but are likely to shift the balance from GSH toward GSSG (Noctor and Foyer, 1998). However, if this detoxification is not sufficient for rapid AOS removal, additional lipid peroxidation and concomitantly the loss of membrane integrity can occur (Noctor and Foyer, 1998).
Supply of Reduced Sulfur Is Directly Coupled to Uptake of SO42−
Although the concentration of Cys does not appear to limit GSH biosynthesis under the conditions used here, an adequate supply of reduced sulfur must be maintained to support the synthesis rates observed. Cys is synthesized by the transfer of sulfide, derived from reduction of SO42− in plastids, to O-acetyl-l-Ser, derived from Ser. Multiple control points are thought to exist in these pathways to couple SO42− assimilation and Cys synthesis with the subsequent utilization of Cys for synthesis of proteins, other amino acids, or GSH (Leustek et al., 2000; Saito, 2000). Because removal of SO42− from the medium abolished the second phase of labeling, we infer that demand-driven biosynthesis of GSH is directly coupled to SO42− uptake from the medium. Sulfate uptake involves high-affinity SO42− transporters with Km values in the low micromolar range (Takahashi et al., 2000). The level of SO42− in the medium (1.73 mm) would be sufficient to saturate such a system under normal conditions; therefore, it is not surprising that supply of cells with additional SO42− above this level did not increase the rate of GSH biosynthesis.
Both GSH and Cys are reported to repress SO42− uptake from the medium in other systems (Lappartient and Touraine, 1996; Bolchi et al., 1999; Lappartient et al., 1999; Hawkesford, 2000). This repression does not affect SO42− transporters directly, but rather takes place at the transcriptional level. In principle, induction of SO42− transporters might also contribute the delay before de novo biosynthesis of GSH. However, this does not appear to be a major limitation because it would have been overcome by the Cys feeding experiments.
Once inside the cell, SO42− reduction takes place exclusively in the plastids, and requires supply of reductant. Notably, GSH is needed to reduce adenosine 5′-phosphosulfate (APS) to SO32− by APS reductase (Bick et al., 1998) and ferredoxin (Fd) is required by SO32− reductase to reduce SO32− to sulfide (Yonekura-Sakakibara et al., 2000). This provides us with a paradox in that the HPLC analysis indicates that essentially all the GSH pool is conjugated to bimane by the time the second phase is initiated, yet sufficient GSH must remain in the plastids to allow reduction of APS. Using stereological techniques, we estimate the volume of the plastids is at most 5% to 8% of these photoheterotrophic Arabidopsis suspension culture cells. If the plastidic GSH concentration was around the Km[GSH] of APS reductase of 0.6 to 1.2 mm (Bick et al., 1998), this would equate to less than 10% of the cytoplasmic GSH content. From these considerations, we infer that MCB does not label the plastidic GSH pool and artificial depletion of GSH in the cytosol does not cause a substantial efflux of GSH from the chloroplasts. This implies that reduced sulfur is exported from the chloroplasts in the form of HS−, Cys, or γ-EC, rather than GSH per se. Appropriate transporters for these compounds have not been reported yet; however, isoforms of all enzymes necessary to synthesize GSH from HS− are present in the cytosol of Arabidopsis and could contribute to GSH biosynthesis.
Because the assay is effectively conducted in darkness with intermittent illumination only during the fluorescent measurement, we infer that most of the NADPH required to rereduce plastidic GSH is derived from the oxidative pentose phosphate pathway, which is located in the plastids (Neuhaus and Emes, 2000). Likewise, the NADPH from the oxidative pentose phosphate pathway has to drive rereduction of Fdox via non-photosynthetic Fd-NADP oxidoreductase (Yonekura-Sakakibara et al., 2000).
The overall results from our study reveal that demand-driven activation of the GSH biosynthesis pathway is tightly regulated in vivo at the level of γ-ECS, but the dominant form of control is not through feedback inhibition by high levels of GSH. Activation of the pathway shows a pronounced lag phase and requires de novo protein expression, possibly of a regulatory component. Pre-exposure to certain stress agents can reduce or eliminate this lag period. Once GSH synthesis is initiated, the demand for reduced sulfur is directly coupled to SO42− uptake from the medium. In normal growth medium for suspension culture cells, the relatively high levels of exogenous sulfate and the capacity for sulfur reduction do not appear to be limiting. However, if similar mechanisms operate in planta, when levels of available SO42− may be considerably lower, the effect of SO42− uptake might have a much more significant impact, suggesting that SO42− transporters might be a potential target for genetic modification to remove tight metabolic control over SO42− uptake, while leaving other regulatory mechanisms in the sulfur assimilation pathway intact (Hawkesford, 2000).
MATERIALS AND METHODS
Arabidopsis Cell Cultures
Green Arabidopsis suspension cultures (Columbia) established by May and Leaver (1993) were grown in basal Murashige and Skoog medium supplemented with 3% (w/v) Suc, 0.5 mg L−1 naphthalene acetic acid, and 0.05 mg L−1 kinetin at pH 5.8 and subcultured every week. Cell cultures were kept under a 16-h-light/8-h-dark illumination regime at 21°C ± 1°C. For all experiments, cells from exponentially growing cultures were used 4 d after subculture. At this stage, 1 mL of cell suspension contained 70 mg of cells. Cells were used at the end of the light phase.
Fluorescent Dyes and Other Chemicals
Stock solutions of 100 mm MCB or monobromobimane (Molecular Probes, Eugene, OR) in dimethyl sulfoxide (DMSO) were stored at −20°C. Aliquots were thawed immediately before use and diluted with Murashige and Skoog medium supplemented with 3% (w/v) Suc or deionized water. PI (Molecular Probes) was solved as a 5 mm stock in distilled water and used at a final concentration of 50 μm. Stock solutions of CHX and Act D were made up in ethanol and stored at −20°C. BSO was dissolved in deionized water and stored as 100 mm stock at −20°C. A 100 mm CDNB stock solution was made up in DMSO and stored at room temperature in the dark. All chemicals apart from the fluorescent dyes were purchased from Sigma (Poole, UK).
TPLSM
Cells were labeled in Eppendorf tubes (Eppendorf UK Ltd., Cambridge, UK) with 50 μm MCB in Murashige and Skoog medium supplemented with 3% (w/v) Suc at room temperature (21°C). The tubes were continuously agitated by placing them on a shaker at 120 rpm. At different time points, 50-μL samples were transferred to a slide and PI was added to a final concentration of 50 μm. Single optical sections through the midplane of groups of cells were captured by TPLSM using an MRC1024MP (Bio-Rad Microscience Ltd., Hemel Hempstead, UK) attached to an upright microscope (BX50WI, Olympus, Southall, UK). GSB and PI were simultaneously excited at 770 nm with a femtosecond pulsed mode-locked Ti:Sapphire laser (Mira900, Coherent, Cambridge, UK) equipped with a 5-W solid-state pump laser (Verdi, Coherent). Images were collected with a 60× 1.2 numerical aperture water immersion lens and Kalman averaged over four frames. The (x, y) pixel size was 0.192 × 0.192 μm2. For quantification of the fluorescence, images were calibrated against GSB standards imaged under the same conditions as cells. Vacuolar GSB concentrations were converted into cytoplasmic concentrations after measurement of the volume of both compartments using a stereological approach (Meyer et al., 2001).
Continuous Recording of GSH Labeling
Continuous recordings of progress curves for the labeling of Arabidopsis cells with MCB were collected either on a fluorimeter equipped with a temperature-controlled four-position cuvette holder (LS50B, Perkin-Elmer, Beauconsfield, UK) or on a fluorescence microplate reader (Polarstar, BMG LabTechnologies, Aylesbury, UK). For experiments conducted on the fluorimeter, 3-mL cuvettes were used in which cells were continuously agitated with a stirrer bar. One hundred microliters of cell suspension was added to 2.9 mL of Murashige and Skoog medium supplemented with 3% (w/v) Suc containing different concentrations of MCB. Fluorescence was recorded with λex = 442 ± 2.5 nm and λem = 477 ± 2.5 nm. For experiments on the plate reader, either 48- or 96-well plates were used (Falcon, Becton-Dickinson, Franklin Lakes, NJ). Fifty microliters of cell suspension was added to 150 μL of Murashige and Skoog medium supplemented with 3% (w/v) Suc for small wells or 100 μL of cell suspension to 300 μL of Murashige and Skoog medium supplemented with 3% (w/v) Suc for large wells, respectively, and labeling reaction was started by automated injection of different concentrations of MCB. Fluorescence was recorded with λex = 390 ± 6 nm and λem = 460 ± 10 nm with both excitation and detection of fluorescence from the flat bottom of the wells by fiber optics. All experiments were conducted at 25°C.
Measurement of Protein Labeling
Arabidopsis cells were incubated for 4 h in Murashige and Skoog medium supplemented with 3% (w/v) Suc containing 100 μm MCB. After, cells were washed twice with fresh Murashige and Skoog medium supplemented with 3% (w/v) Suc to remove free MCB. The medium was removed and cells resuspended in 1 mL of extraction buffer (100 mm TRIS/KOH [pH 8.0], 2 mm MgCl2, and 1 mm EDTA). This suspension was ground in a chilled mortar with addition of 0.1 g of polyvinylpyrrolidone with an Mr of 40,000. After centrifugation at 25,000g for 10 min, the supernatant was taken off and fractionated on Sephadex G-25 (PD10 columns, Supelco, Bellefonte, PA) with extraction buffer as eluent. Two hundred-fifty-microliter fractions were collected on multiwell plates and the fluorescence measured on a plate reader with λex = 395 nm. Protein containing fractions were identified according to Bradford (1976).
HPLC of Bimane-Labeled Low-Mr Thiols
For separation and identification of low-Mr thiols labeled with MCB, cells were ground in ice-cold methanesulfonic acid (200 mm) after in vivo labeling for different times. Cell extracts were centrifuged at 12,000g for 10 min and immediately analyzed by reverse-phase HPLC (Hichrom 5C18, 300 × 4.6 mm, Hichrom, Reading, UK) with 0.25% (v/v) acetic acid (pH 3.9) as solvent A and methanol as solvent B. A linear gradient from 92% (v/v) A to 85% (v/v) A over 10 min and a subsequent hold of 20 min with a constant flow rate of 1 mL min−1 was employed to give good separation of GSB from the possible degradation products γ-EC-S-bimane, Cys-Gly-S-bimane and Cys-S-bimane. Bimane derivatives were detected on a fluorescence detector (RF 2000, Dionex, Germering, Germany) with λex = 395 nm and λem = 477 nm. Standards for bimane derivatives of GSH, γ-EC, Cys-Gly, and Cys were prepared in vitro by mixing excess thiol with a final concentration of 10 mm monobromobimane at pH 8.5. These isolated thiol-bimane standards were used for peak identification and quantification. Resting levels of reduced low-Mr thiols were analyzed after labeling as described by Fahey and Newton (1987) and HPLC analysis with parameters given above.
Uptake of Exogenous Amino Acids
Uptake of Cys, Glu, and Gly was measured by depletion from the external medium. Ten-milliliter aliquots of 3- to 4-d-old cell suspensions were washed twice and resuspended in 3% (w/v) Suc with or without 1 mm of each amino acid, and incubated at room temperature with gentle shaking. One-milliliter samples were taken at 30-min intervals over 120 min, centrifuged (300g for 5 min), and the level of amino acid remaining in the supernatant determined using Ninhydrin reagent in DMSO according to Moore (1968) with absorbance measurement at 570 nm for Gly and Glu and 450 nm for Cys in a 96-well plate reader (Dynatech MRX, Dynex Technologies, Ashford, UK).
Footnotes
This work was partially supported by Aventis Crop Science Ltd.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.008243.
LITERATURE CITED
- Arisi A-CM, Mocquot B, Lagriffoul A, Mench M, Foyer CH, Jouanin L. Responses to cadmium in leaves of transformed poplars overexpressing γ-glutamylcysteine synthetase. Physiol Plant. 1999;109:143–149. [Google Scholar]
- Bick J-A, Aslund F, Chen Y, Leustek T. Glutaredoxin function for the carboxyl-terminal domain of the plant-type 5′-adenylsulphate reductase. Proc Natl Acad Sci USA. 1998;95:8404–8409. doi: 10.1073/pnas.95.14.8404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolchi A, Petrucco S, Tenca PL, Foroni C, Ottonello S. Coordinate modulation of maize sulphate permease and ATP sulphurylase mRNAs in response to variations in sulphur nutritional status: stereospecific downregulation by l-cysteine. Plant Mol Biol. 1999;39:527–537. doi: 10.1023/a:1006148815106. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle protein-dye-binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen J, Goldsbrough PB. Increased activity of γ-glutamylcysteine synthetase in tomato cells selected for cadmium tolerance. Plant Physiol. 1994;106:233–239. doi: 10.1104/pp.106.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S. Molecular mechanisms of plant metal tolerance and homeostasis. Planta. 2001;212:475–486. doi: 10.1007/s004250000458. [DOI] [PubMed] [Google Scholar]
- Cobbett CS. Phytochelatins and their role in heavy metal detoxification. Plant Physiol. 2000;123:825–832. doi: 10.1104/pp.123.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JOD, Blake-Kalff MMA, Davies TGE. Detoxification of xenobiotic by plants: chemical modification and vacuolar compartmentation. Trends Plant Sci. 1997a;2:144–151. [Google Scholar]
- Coleman JOD, Randall R, Blake-Kalff MMA. Detoxification of xenobiotics in plant cells by glutathione conjugation and vacuolar compartmentalization: a fluorescent assay using monochlorobimane. Plant Cell Environ. 1997b;20:449–460. [Google Scholar]
- Conklin P. Recent advances in the role and biosynthesis of ascorbic acid in plants. Plant Cell Environ. 2001;24:383–394. [Google Scholar]
- Creissen G, Firmin J, Fryer M, Kular B, Leyland N, Reynolds H, Pastori G, Wellburn F, Baker N, Wellburn A et al. Elevated glutathione biosynthetic capacity in the chloroplasts of transgenic tobacco plants paradoxically causes increased oxidative stress. Plant Cell. 1999;11:1277–1292. doi: 10.1105/tpc.11.7.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey RC, Newton GL. Determination of low-molecular weight thiols using monobromobimane fluorescent labelling and high-performance liquid chromatography. Methods Enzymol. 1987;143:85–96. doi: 10.1016/0076-6879(87)43016-4. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Fricker MD, May M, Meyer AJ, Sheard N, White NS. Measurement of glutathione levels in intact roots of Arabidopsis. J Microsc. 2000;198:162–173. doi: 10.1046/j.1365-2818.2000.00696.x. [DOI] [PubMed] [Google Scholar]
- Fricker MD, Meyer AJ. Confocal imaging of metabolism in vivo: pitfalls and possibilities. J Exp Bot. 2001;52:631–640. [PubMed] [Google Scholar]
- Gullner G, Kömives T, Rennenberg H. Enhanced tolerance of transgenic poplar plants overexpressing gamma-glutamylcysteine synthetase towards chloroacetanilide herbicides. J Exp Bot. 2001;52:971–979. doi: 10.1093/jexbot/52.358.971. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Alcalá G, Gotor C, Meyer AJ, Fricker M, Vega JM, Romero LC. Glutathione biosynthesis in Arabidopsis trichome cells. Proc Natl Acad Sci USA. 2000;97:11108–11113. doi: 10.1073/pnas.190334497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzfeld Y, Cathala N, Grignon C, Davidian J-C. Effect of ATP sulphurylase overexpression in Bright Yellow 2 tobacco cells. Regulation of ATP sulphurylase and SO42− transport activities. Plant Physiol. 1998;116:1307–1313. doi: 10.1104/pp.116.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkesford MJ. Plant responses to sulphur deficiency and the genetic manipulation of sulphate transporters to improve S-utilization efficiency. J Exp Bot. 2000;51:131–138. [PubMed] [Google Scholar]
- Hell R, Bergmann L. γ-Glutamylcysteine synthetase in higher plants: catalytic properties and subcellular localization. Planta. 1990;180:603–612. doi: 10.1007/BF02411460. [DOI] [PubMed] [Google Scholar]
- Howden R, Andersen CR, Goldsbrough PB, Cobbett CS. A cadmium-sensitive, glutathione-deficient mutant of Arabidopsis thaliana. Plant Physiol. 1995;107:1067–1073. doi: 10.1104/pp.107.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CS, Chang LS, Anderson ME, Meister A. Catalytic and regulatory properties of the heavy subunit of rat kidney gamma-glutamylcysteine synthetase. J Biol Chem. 1993;268:19675–19680. [PubMed] [Google Scholar]
- Kocsy G, Brunner M, Rüegsegger A, Stamp P, Brunold C. Glutathione synthesis in maize genotypes with different sensitivity to chilling. Planta. 1996;198:365–370. [Google Scholar]
- Lappartient AG, Touraine B. Demand-driven control of root ATP sulphurylase activity and SO42− uptake in intact canola. Plant Physiol. 1996;111:147–157. doi: 10.1104/pp.111.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappartient AG, Vidmar JJ, Leustek T, Glass ADM, Touraine B. Interorgan signalling in plants: regulation of ATP sulphurylase and sulphate transporter genes expression in roots mediated by phloem-translocated compound. Plant J. 1999;18:89–95. doi: 10.1046/j.1365-313x.1999.00416.x. [DOI] [PubMed] [Google Scholar]
- Leustek T, Martin MN, Bick J-A, Davies JP. Pathways and regulation of sulphur metabolism revealed through molecular and genetic studies. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:141–165. doi: 10.1146/annurev.arplant.51.1.141. [DOI] [PubMed] [Google Scholar]
- Martell AE, Smith RM. Critical Stability Constants. 5, Suppl 1. New York: Plenum Press; 1982. [Google Scholar]
- May MJ, Leaver CJ. Oxidative stimulation of glutathione synthesis in Arabidopsis thaliana suspension cultures. Plant Physiol. 1993;103:621–627. doi: 10.1104/pp.103.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May MJ, Vernoux T, Leaver C, Van Montagu M, Inzé D. Glutathione homeostasis in plants: implications for environmental sensing and plant development. J Exp Bot. 1998a;49:649–667. [Google Scholar]
- May MJ, Vernoux T, Sánchez-Fernández R, Van Montagu M, Inzé D. Evidence for posttranscriptional activation of γ-glutamylcysteine synthetase during plant stress response. Proc Nat Acad Sci USA. 1998b;95:12049–12054. doi: 10.1073/pnas.95.20.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AJ, Fricker MD. Direct measurement of glutathione in epidermal cells of intact Arabidopsis roots by two-photon laser scanning microscopy. J Microsc. 2000;198:174–181. doi: 10.1046/j.1365-2818.2000.00697.x. [DOI] [PubMed] [Google Scholar]
- Meyer AJ, May M, Fricker M. Quantitative in vivo measurement of glutathione in Arabidopsis cells. Plant J. 2001;27:67–78. doi: 10.1046/j.1365-313x.2001.01071.x. [DOI] [PubMed] [Google Scholar]
- Moore S. Amino acid analysis: aqueous dimethyl sulfoxide as solvent for the ninhydrin reaction. J Biol Chem. 1968;243:6281–6283. [PubMed] [Google Scholar]
- Neuhaus HE, Emes MJ. Nonphotosynthetic metabolism in plastids. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:111–140. doi: 10.1146/annurev.arplant.51.1.111. [DOI] [PubMed] [Google Scholar]
- Noctor G, Arisi ACM, Jouanin L, Foyer CH. Photorespiratory glycine enhances glutathione accumulation in both the chloroplastic and cytosolic compartments. J Exp Bot. 1999;50:1157–1167. [Google Scholar]
- Noctor G, Arisi A-CM, Jouanin L, Kunert K-J, Rennenberg H, Foyer CH. Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot. 1998;49:623–647. [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Noctor G, Strohm M, Jouanin L, Kunert K-J, Foyer CH, Rennenberg H. Synthesis of glutathione in leaves of transgenic poplar overexpressing γ-glutamylcysteine synthetase. Plant Physiol. 1996;112:1071–1078. doi: 10.1104/pp.112.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon-Smits EA, Hwang S, Mel Lytle C, Zhu Y, Tai JC, Bravo RC, Chen Y, Leustek T, Terry N. Overexpression of ATP sulphurylase in Indian mustard leads to increased selenate uptake, reduction, and tolerance. Plant Physiol. 1999;119:123–132. doi: 10.1104/pp.119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscher A, Kruger NJ, Ratcliffe RG. Strategies for metabolic flux analysis in plants using isotope labelling. J Biotechnol. 2000;77:81–102. doi: 10.1016/s0168-1656(99)00209-6. [DOI] [PubMed] [Google Scholar]
- Rüegsegger A, Brunold C. Effect of cadmium on γ-glutamylcysteine synthesis in maize seedlings. Plant Physiol. 1992;99:428–433. doi: 10.1104/pp.99.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K. Regulation of sulphate transport and synthesis of sulphur-containing amino acids. Curr Opin Plant Biol. 2000;3:188–195. [PubMed] [Google Scholar]
- Schneider S, Bergmann L. Regulation of glutathione synthesis in suspension cultures of parsley and tobacco. Bot Acta. 1995;108:34–40. [Google Scholar]
- Strohm M, Jouanin L, Kunert KJ, Pruvost C, Polle A, Foyer CH, Rennenberg H. Regulation of glutathione synthesis in leaves of transgenic poplar (Populus tremula × P. alba) overexpressing glutathione synthetase. Plant J. 1995;7:141–145. [Google Scholar]
- Takahashi H, Watanabe-Takahashi A, Smith FW, Blake-Kalff M, Hawkesford MJ, Saito K. The roles of three functional sulphate transporters involved in uptake and translocation of sulphate in Arabidopsis thaliana. Plant J. 2000;23:171–182. doi: 10.1046/j.1365-313x.2000.00768.x. [DOI] [PubMed] [Google Scholar]
- Winter H, Robinson DG, Heldt HW. Subcellular volumes and metabolite concentrations in barley leaves. Planta. 1993;191:180–190. [Google Scholar]
- Winter H, Robinson DG, Heldt HW. Subcellular volumes and metabolite concentrations in spinach leaves. Planta. 1994;193:530–535. [Google Scholar]
- Wolf AH, Dietz KJ, Schröder P. Degradation of glutathione S-conjugates by a carboxypeptidase in the plant vacuole. FEBS Lett. 1996;384:31–34. doi: 10.1016/0014-5793(96)00272-4. [DOI] [PubMed] [Google Scholar]
- Xiang C, Oliver DJ. Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell. 1998;10:1539–1550. doi: 10.1105/tpc.10.9.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang C, Werner BL, Christensen EM, Oliver DJ. The biological functions of glutathione revisited in Arabidopsis transgenic plants with altered glutathione levels. Plant Physiol. 2001;126:564–574. doi: 10.1104/pp.126.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekura-Sakakibara K, Onda Y, Ashikari T, Tanaka Y, Kusumi T, Hase T. Analysis of reductant supply systems for ferredoxin-dependent sulfite reductase in photosynthetic and nonphotosynthetic organs of maize. Plant Physiol. 2000;122:887–894. doi: 10.1104/pp.122.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YL, Pilon-Smits EA, Tarun AS, Weber SU, Jouanin L, Terry N. Cadmium tolerance and accumulation in Indian mustard is enhanced by overexpressing gamma-glutamylcysteine synthetase. Plant Physiol. 1999;121:1169–1178. doi: 10.1104/pp.121.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]