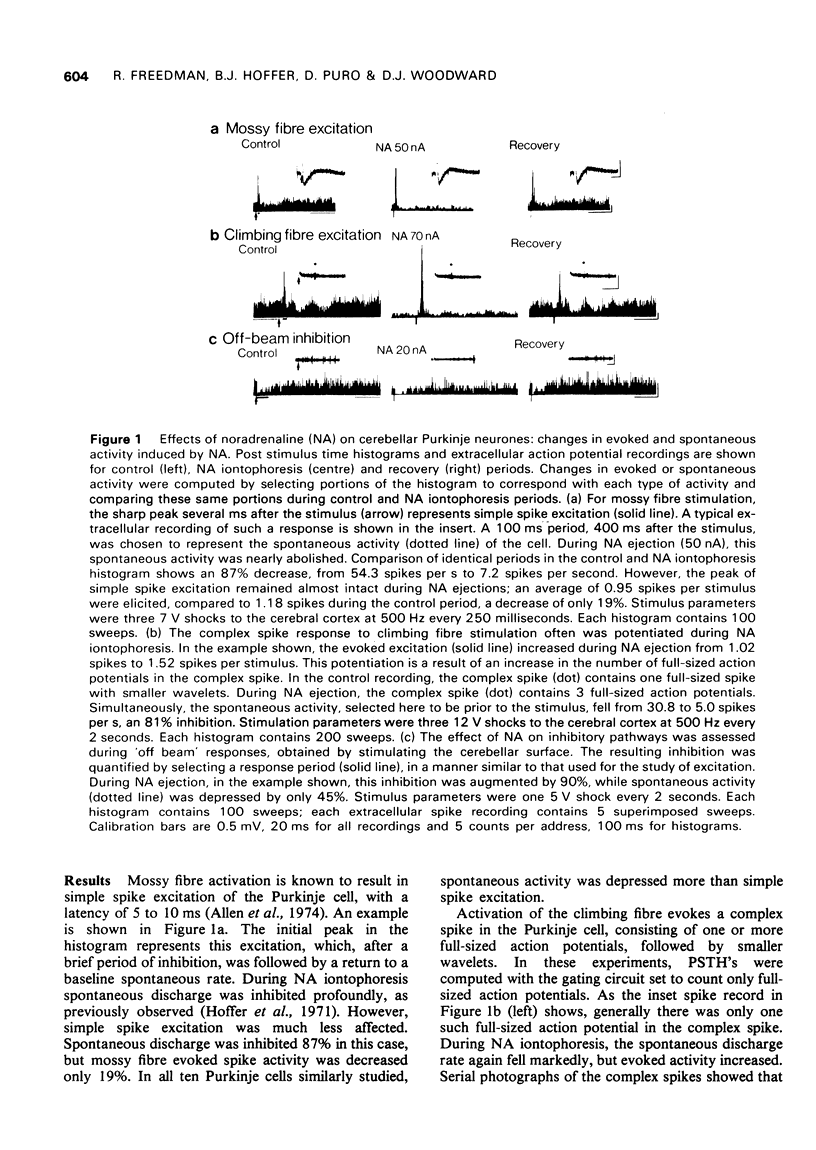
Abstract
Noradrenaline, applied by microiontophoresis to rat cerebellar Purkinje neurones, selectively depressed spontaneous neuronal discharge. Simple spike and complex spike excitations, evoked by stimulation of the mossy and climbing fibres, were relatively preserved during the inhibition of spontaneous activity, and the number of full-sized action potentials in the complex spike increased. Inhibition mediated by the basket and stellate cells was augmented. Thus, relative to the change in spontaneous activity, noradrenaline increased the responsiveness of the Purkinje cell to afferent input.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. I., Azzena G. B., Ohno T. Cerebellar Purkyne cell responses to inputs from sensorimotor cortex. Exp Brain Res. 1974;20(3):239–254. doi: 10.1007/BF00238315. [DOI] [PubMed] [Google Scholar]
- Bloom F. E., Hoffer B. J., Siggins G. R. Studies on norepinephrine-containing afferents to Purkinje cells of art cerebellum. I. Localization of the fibers and their synapses. Brain Res. 1971 Feb 5;25(3):501–521. doi: 10.1016/0006-8993(71)90457-4. [DOI] [PubMed] [Google Scholar]
- Foote S. L., Freedman R., Oliver A. P. Effects of putative neurotransmitters on neuronal activity in monkey auditory cortex. Brain Res. 1975 Mar 21;86(2):229–242. doi: 10.1016/0006-8993(75)90699-x. [DOI] [PubMed] [Google Scholar]
- Freedman R., Hoffer B. J., Woodward D. J. A quantitative microiontophoretic analysis of the responses of central neurones to noradrenaline: interactions with cobalt, manganese, verapamil and dichloroisoprenaline. Br J Pharmacol. 1975 Aug;54(4):529–539. doi: 10.1111/j.1476-5381.1975.tb07601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller H. M., Woodward D. J. Responses of cultured cerebellar neurons to iontophoretically applied amino acids. Brain Res. 1974 Jul 5;74(1):67–80. doi: 10.1016/0006-8993(74)90112-7. [DOI] [PubMed] [Google Scholar]
- Hoffer B. J., Siggins G. R., Oliver A. P., Bloom F. E. Activation of the pathway from locus coeruleus to rat cerebellar Purkinje neurons: pharmacological evidence of noradrenergic central inhibition. J Pharmacol Exp Ther. 1973 Mar;184(3):553–569. [PubMed] [Google Scholar]
- Hoffer B., Seiger A., Ljungberg T., Olson L. Electrophysiological and cytological studies of brain homografts in the anterior chamber of the eye: maturation of cerebellar cortex in oculo. Brain Res. 1974 Oct 18;79(2):165–184. doi: 10.1016/0006-8993(74)90409-0. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Fuxe K. Cerebellar monoamine nerve terminals, a new type of afferent fibers to the cortex cerebelli. Exp Brain Res. 1969 Aug 19;9(1):63–72. doi: 10.1007/BF00235452. [DOI] [PubMed] [Google Scholar]
- Olson L., Fuxe K. On the projections from the locus coeruleus noradrealine neurons: the cerebellar innervation. Brain Res. 1971 Apr 16;28(1):165–171. doi: 10.1016/0006-8993(71)90533-6. [DOI] [PubMed] [Google Scholar]


