Abstract
Acclimation to irradiance was measured in terms of light-saturated photosynthetic carbon assimilation rates (Pmax), Rubisco, and pigment content in mature field-grown rice (Oryza sativa) plants in tropical conditions. Measurements were made at different positions within the canopy alongside irradiance and daylight spectra. These data were compared with a second experiment in which acclimation to irradiance was assessed in uppermost leaves within whole-plant shading regimes (10% low light [LL], 40% medium light [ML], and 100% high light [HL] of full natural sunlight). Two varieties, japonica (tropical; new plant type [NPT]) and indica (IR72) were compared. Values for Rubisco amount, chlorophyll a/b, and Pmax all declined from the top to the base of the canopy. In the artificial shading experiment, acclimation of Pmax (measured at 350 μL L−1 CO2) occurred between LL and ML for IR72 with no difference observed between ML and HL. The Rubisco amount increased between ML and HL in IR72. A different pattern was seen for NPT with higher Pmax (measured at 350 μL L−1 CO2) at LL than IR72 and some acclimation of this parameter between ML and HL. Rubisco levels were higher in NPT than IR72 contrasting with Pmax. Comparison of data from both experiments suggests a leaf aging effect between the uppermost two leaf positions, which was not a result of irradiance acclimation. Results are discussed in terms of: (a) acclimation of photosynthesis and radiation use efficiency at high irradiance in rice, and (b) factors controlling photosynthetic rates of leaves within the canopy.
A plant's light environment will commonly exhibit large changes in both intensity and spectral quality. In response, there are alterations in the composition of chloroplasts that adjust photosynthesis to the prevailing conditions. This is termed photosynthetic acclimation (Anderson et al., 1995) and results in optimization of light utilization and protects against the potential stress from excess light (Anderson and Osmond, 1987). Differences in anatomy can also be observed between high light- and low light-grown leaves (Weston et al., 2000). A correlation has been shown between the capacity of a plant species for acclimation and the range of habitats in which it is found (Murchie and Horton, 1997), and species inhabiting extreme environments may express particular aspects of acclimation to an exaggerated extent (Maxwell et al., 1999).
On a chloroplast level, most plant species acclimate to irradiance over the long term by altering relative amounts of photosynthetic enzymes and pigment-protein complexes. Growth or long-term presence in high irradiance results in a progressive loss of light-harvesting pigment proteins and a synthesis of electron transport and carbon assimilation components, compared with leaves exposed to low light (Anderson and Osmond, 1987; Anderson et al., 1995). As a result, the photosynthetic capacity (Pmax) of high light-acclimated leaves is often consistently higher than low light leaves. Acclimation maintains ambient photosynthesis at a point below low light saturation, so enhancing photosynthetic quantum efficiency. However, as growth irradiance increases, the increase in Pmax approaches an acclimation ceiling, and exposure to light beyond this increasingly results in saturation of photosynthesis. When the acclimation of Pmax is at or close to the ceiling, the response of the plant is to increase its protection against excess light. Such responses can include an increase in the xanthophyll cycle pool size (Thayer and Björkman, 1990; Bilger et al., 1995), and a regulated loss of chlorophyll (Chl) and pigment proteins per chloroplast (Anderson, 1986). The latter processes may be induced at lower irradiances in studies in which the acclimation ceiling is lowered by withholding another resource, such as N (Verhoeven et al., 1997) or water (Maxwell et al., 1999). The range of irradiance over which acclimation occurs, therefore, is dependent not only on species but on other growth conditions. Furthermore, the irradiance response is exceedingly complex, with the contents of different proteins changing over particular ranges of irradiance, making it possible to suggest that it includes both a low-light and a high-light response (Bailey et al., 2001).
There is little understanding of acclimation of photosynthesis to irradiance under field conditions. For a cereal crop species such as rice (Oryza sativa), acclimation will depend upon the intensity of incident sunlight and the attenuation of sunlight by the canopy. For the former, the extent of acclimation will depend on whether the acclimation ceiling has been reached. For the latter, the extent of acclimation will also be determined by the position of the leaf in the canopy, the canopy structure, and leaf age. Acclimation of photosynthetic components to low irradiance within canopies is known to occur in rice (Okada and Katoh, 1998; Yamazaki et al., 1999) and other species such as alfalfa (Medicago sativa; Evans, 1993). However, Pmax in rice grown under high irradiance is also dependent on the age of the leaf (Makino et al., 1985; Hidema et al., 1991, 1992). In laboratory experiments, after full leaf expansion of preflag leaves, light-saturated rates of photosynthesis (Pmax) can begin to decline after a short period: Under high irradiance, this is accelerated further (Makino et al., 1985; Hidema et al., 1991). Such a response has been observed in other species, e.g. Lolium temulentum (Mae et al., 1993). This is associated with a decline in amounts of photosynthetic components and total leaf N and is probably related to remobilization of nutrients to rapidly developing parts of the plant (Mae and Ohira, 1981). The influence of decreased irradiance on the Pmax of lower leaves in rice has not been proven. Gradients of N content and photosynthetic rate within plant canopies have been well studied, however, and in plants such as vine (Ipomea tricolor Cav.; Ackerly, 1992; Hikosaka et al., 1994; Hikosaka, 1996), irradiance was shown to be a primary factor rather than any specific effects of leaf age. However, the irradiance-independent decline of Pmax such as that which occurs in rice leaves shortly after full leaf expansion (above) was not shown for vine, suggesting these species respond in largely different ways.
Recently, we determined levels of photosynthetic efficiency in irrigated field-grown rice (Murchie et al., 1999a). Photosynthesis at the top of the canopy was saturated during full sunlight, and there was extensive reduction of the electron transport chain and the engagement of photoprotective energy dissipation. This suggested that the acclimation ceiling had been reached. Little is known about the process of acclimation of photosynthesis to irradiances as high as those used in this field study. Therefore, the current paper describes an extension to this in which a detailed examination of acclimation was undertaken, examining factors that determine Pmax both within and at the top of the canopy. Two sets of experiments were carried out: (a) We measured irradiance transmission and alterations in daylight spectra within a rice canopy in the field, utilizing cultivars with contrasting canopy structure for comparison followed by measurement of photosynthetic parameters. (b) We manipulated the irradiance level in the field at two points during leaf development. From these data, we were able to establish that acclimation of photosynthesis to irradiance in the field does occur in rice leaves. Furthermore, by identification of the upper limit to the irradiance acclimation process with regard to Pmax, we could provide a “physiologically relevant” measurement of the proportion of photons that are in excess of that required in photosynthesis. By comparing data from the two sets of experiments, we could establish whether alterations in Pmax throughout the canopy are consistent with those arising from acclimation of photosynthesis.
RESULTS
Acclimation to Canopy Depth
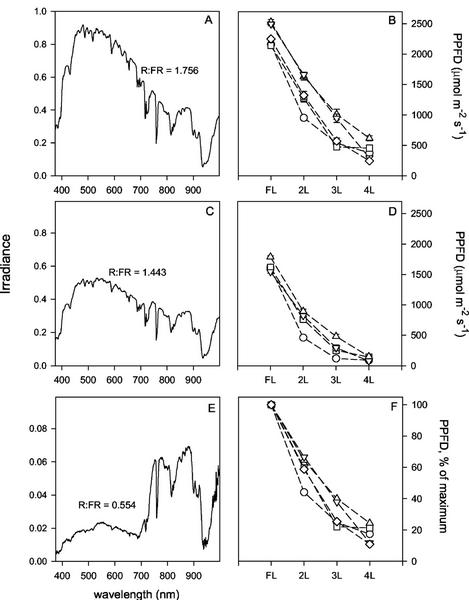
New plant type (NPT) rice plants possess fewer, larger, upright leaves (per plant) which permit a high degree of penetration of irradiance to lower leaves in the canopy (Peng et al., 1994). Although this results in a lower leaf area index, grain yields are equivalent or higher than traditional cultivars (International Rice Research Institute [IRRI], 2000). Figure 1, A, C, and E, show the effect of leaf position on radiation within the rice canopy, showing both an attenuation of total radiation and a distortion of the spectral composition caused by canopy-dependent reflection and shading. Measurements were made at the FL, and the next three leaves 2L, 3L, and 4L. At the spectral maximum (around 500 nm), there was a 50-fold reduction at leaf 3L (Fig. 1E) compared with leaf 1L (Fig. 1A). There was also a decrease in the ratio of R (taken as 660 nm) relative to FR (taken as 730 nm) in the lower points in the canopy. These wavelengths are close to the action spectrum maxima for activation and inactivation of phytochrome, which is involved in a number of shade-type responses (Fankhauser and Chory, 1997). Exposed leaves had an R:FR approaching 1.76 and this declined to near 1.44 for leaf 2L (Fig. 1C) and 0.55 for leaf 3L (Fig. 1E). The change in R to FR ratio at the lowest position was greater for the canopy arrangement of NPT compared with that of IR72 (0.55 and 0.40, respectively; data not shown for latter).
Figure 1.
Spectra and photosynthetic photon flux density (PPFD) according to canopy position. Measurements were taken at midday at the flag leaf (FL), and the next three leaves (2L, 3L, and 4L). A, C, and E, Spectra for NPT variety IR65598-112-2 measured using a portable spectroradiometer and data here are expressed as arbitrary units. A, Top of the canopy (FL); C, 2L position; and E, 3L position. R:FR, Ratio of irradiance at 660 nm to that at 730 nm. B, D, and F, Decline in PPFD according to canopy position for the indica (IR72; ○) and four NPT varieties: IR655998-112-2 (□), IR65600-42-5-2 (▿), IR65600-129-1-1-2 (▵), and IR68544-29-2-1-3-1-2 (⋄). B and D, PPFD measured at 12 pm (i.e. sun directly overhead) and 3 pm, respectively. F, Values calculated as the percentage of that for the top canopy position measured at 12 pm. Means ± se, n = 15 to 20.
Figure 1(B, D, and F) shows the attenuation of irradiance (here measured as PPFD) through mature canopies of NPT and IR72 rice. Figure 1, B and D, show actual values of irradiance, whereas Figure 1F gives values as a proportion of that at the top of the canopy. At midday, the reduction in irradiance was significantly greater in the IR72 compared with all the NPT varieties at 2L and two of the NPT varieties at 3L (Fig. 1, B and F). In both types, the irradiance was reduced by 80% to 90% at the base of the canopy. Measurements made at 3 pm (Fig. 1D) demonstrated a marked reduction in irradiance both at the top of the canopy and lower leaves. The attenuation of light was greater than at midday, dropping to approximately 30% at position 2L for IR72, and being reduced by over 90% at the base of the canopy, with irradiance values of less than 100 μmol m−2 s−1. Attenuation at this time was greater for IR72 than NPT at 2L and 3L.
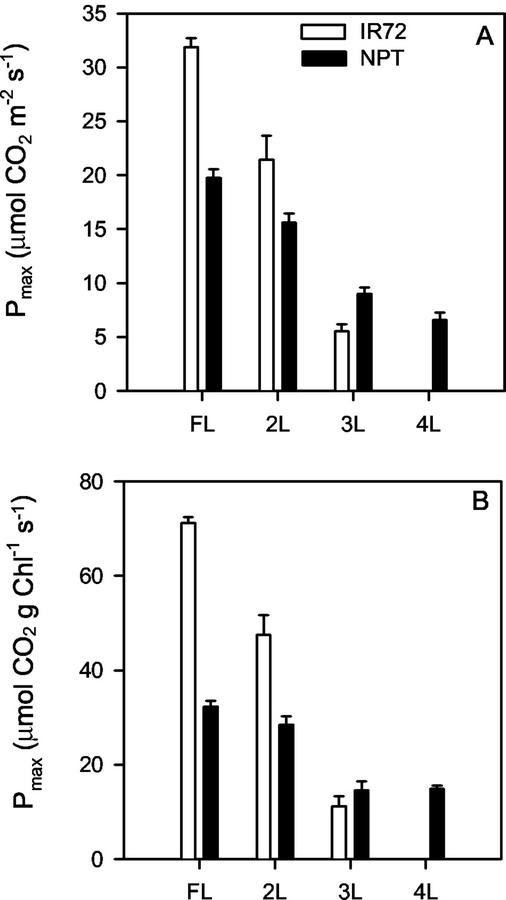
Figure 2 shows the light-saturated rates of photosynthesis (Pmax) for NPT and IR72, measured using an integrated light-emitting diode (LED) as a light source. Murchie et al. (1999a) showed that 1,800 μmol m−2 s−1 and 350 μL L−1 CO2 was sufficient to completely saturate photosynthesis in NPT and 93% of photosynthesis in IR72 and rates taken at this PPFD will be referred to here as being light saturated (Pmax). It is clear that Pmax declined steadily from the exposed FL to the leaves lower in the canopy, a trend consistent with acclimation to the different irradiances shown in Figure 1. This was observed for both varieties. A lower Pmax for the NPT has been noted previously (E.H. Murchie, unpublished data), and this was observed in the top two leaf positions, although the proportional difference between the two varieties was less in 2L than FL. In fact, at the 3L leaf position, Pmax per unit leaf area in the NPT was actually higher than that of IR72. It was apparent that the Pmax in NPT was generally less responsive to canopy position than IR72. The decline in Pmax of IR72 was dramatic, with no photosynthetic activity being detectable in the senescent 4L of IR72. In contrast, the 4L leaf position of NPT still showed approximately 30% of the photosynthetic capacity of the FL.
Figure 2.
Light-saturated rates of photosynthesis (Pmax) expressed per unit leaf area (A) and per unit Chl (B) according to canopy position measured at ambient CO2 levels (350 μL L−1) for IR72 (white bars) and NPT variety IR65598-112-2 (black bars). Measurements were made at the FL, and the next three leaves (2L, 3L, and 4L). Means ± se, n = 5 to 10 (individual plants).
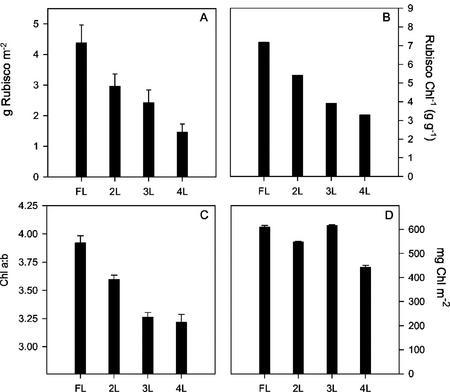
Two key indicators of acclimation to the light environment, the Rubisco content and pigment composition, were measured for NPT (Fig. 3). The values for Rubisco in upper leaves (and HL-grown plants in experiment 2) are within the range seen in the literature for rice: Makino et al. (1994) showed values up to 6 g m−2 in fully expanded leaves using a similar method for Rubisco determination. A value of approximately 4.4 g m−2 was found in the FL, this declining steadily through the canopy to approximately 1.5 g m−2 at 4L. When expressed on a unit Chl basis, the difference between 3L and 4L is reduced because of the decline in Chl in the latter leaf. The total Chl content was maintained at a high level in all the leaves, with a decline only in 4L. The Chl a/b declined from near 4.00 in FL to 3.2 in 4L, indicating significant alteration in thylakoid composition as found for a juvenile rice canopy (Yamazaki et al., 1999). Specifically, this data is consistent with a change in the content of peripheral LHCII, a key attribute of acclimation. This strongly resembles the pattern of changes in Rubisco content.
Figure 3.
Rubisco content expressed per unit leaf area (A) and per unit Chl (B). C, Ratio of Chl a to b. D, Chl content per unit leaf area of leaves according to canopy position for NPT variety IR65598-112-2. Measurements were made at the FL, and the next three leaves (2L, 3L, and 4L). Means ± se, n = 5 to 8 (individual plants).
It is important to assess the operational photosynthetic characteristics of the leaves. Chl fluorescence was used to measure the efficiency of PSII electron transport and the redox state of PSII during periods of full sunlight (Table I). Actinic levels of irradiance for these measurements are those given in Figure 1B. ΦPSII was low for the upper two leaf levels (0.2–0.3), indicating a large degree of saturation of photosynthesis. This was also reflected in qP, a parameter that measures the proportion of PSII reaction centers that are in a closed or reduced state. A decrease in qP monitors the increase in “excitation pressure” and a value of below 0.6 has been suggested to indicate a potential for “chronic photo-inhibition” (Öquist et al., 1992). A qP of below 0.5 was found for the FL and 2L leaves, indicating significant saturation of photosynthetic electron transport and potential for photo-inhibition. In contrast, leaf 3L had values around 0.9. Dark-adapted values of Fv/Fm were measured. Ten minutes of dark-adaptation is sufficient to remove the rapidly relaxing type of non-photochemical quenching of Chl fluorescence, qE. Most of the remaining quenching is designated as being photo-inhibitory, and may be caused by either damage to PSII reaction centers or an as-yet-unidentified quenching process. Previous work using rice leaves grown at the same site in the dry season showed complete recovery of Fv/Fm overnight (Murchie et al., 1999a) and the same was observed in these experiments (data not shown). Therefore, photo-inhibition in these experiments was dynamic rather than chronic (Osmond, 1994). The FL was clearly the most susceptible to a sustained decline in Fv/Fm, with recorded values approaching 0.7. This is generally in agreement with previous studies of photo-inhibition in rice (Huang et al., 1989; Murchie et al., 1999a). In contrast, leaves 2L and 3L had values close to the observed maximum of 0.83.
Table I.
In situ fluorescence characteristics of leaves of IR72 plants
| IR72 | Fv/Fm | qP | ΦPSII |
|---|---|---|---|
| FL | 0.717 ± 0.010 | 0.443 ± 0.039 | 0.217 ± 0.025 |
| 2L | 0.785 ± 0.011 | 0.514 ± 0.032 | 0.292 ± 0.026 |
| 3L | 0.817 ± 0.002 | 0.901 ± 0.031 | 0.630 ± 0.022 |
Measurements were made at the FL, 2L, and 3L and taken during mid-morning and mid-afternoon when incident irradiance was greatest (as dictated by the upright leaf posture). Maximum photochemical efficiency of PSII in the dark-adapted state (Fv/Fm) was measured following a 10-min dark adaptation period. Photochemical quenching (qP) and quantum yield of PSII reaction centers (ΦPSII) were calculated as described in the text. Values represent means of measurements taken over a 2- to 3-d period ± se, n > 20.
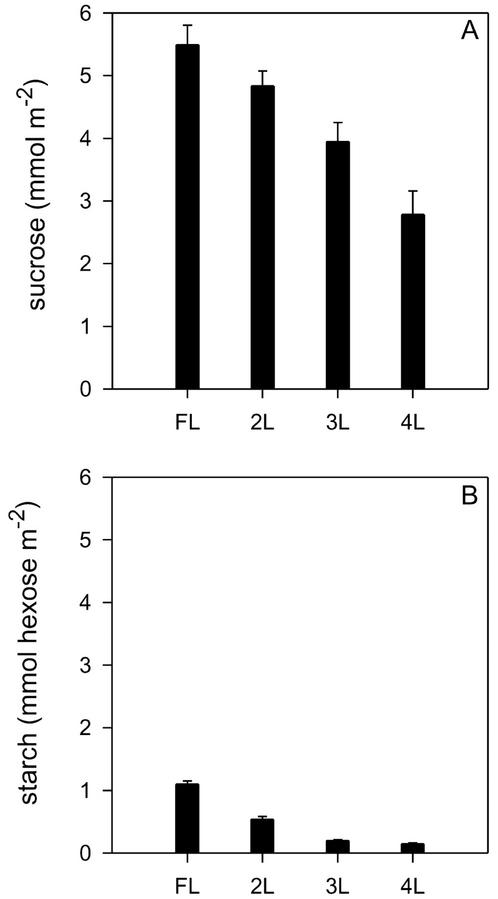
Figure 4 shows end of day Suc and starch content for leaves at different positions in the canopy. These are the major nonstructural leaf carbohydrates in rice leaves and Suc levels were greater than starch levels as has been reported previously for rice leaves (Vu et al., 1998). Amounts of both Suc and starch progressively declined from the top to the base of the canopy, suggesting that the rate of daily photosynthesis was lower at the base of the canopy (Conocono et al., 1998). Other work showed that in rice leaves under these conditions, Suc and starch were mostly exported or respired overnight.
Figure 4.
Nonstructural carbohydrate content of rice leaves according to canopy position for NPT variety IR65598-112-2. A, Suc. B, Starch. Measurements were made at the FL, and the next three leaves (2L, 3L, and 4L). Samples were frozen for measurement at the same time (5 pm) on the same day (sunset took place at 6 pm). Means ± se, n = 5 (individual plants).
Acclimation to Irradiance
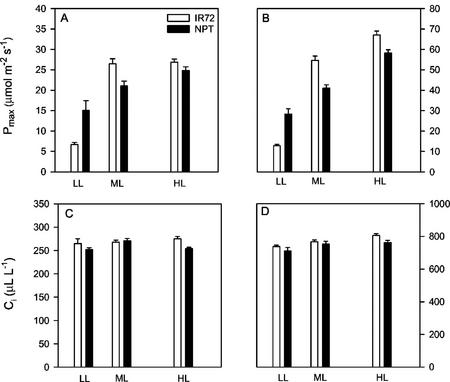
In the second experiment, we directly determined the capacity for acclimation by imposing changes in irradiance on the rice canopy. Significant acclimation of photosynthetic capacity in uppermost leaves was found between LL and medium light (ML) for both varieties (Fig. 5). In leaves of IR72, there was no significant difference in Pmax between leaves grown at ML and those at 100% sunlight when measured at ambient CO2 concentrations (350 μL L−1). For leaves of NPT grown at HL, Pmax was 14% higher than those at ML. There are some other striking differences between the characteristics of IR72 and NPT: in particular, the Pmax at intermediate and LL intensities. At LL, Pmax in NPT was twice that of IR72 (Fig. 5, A and B). At ML, IR72 had a higher Pmax than NPT. At 900 μL L−1 CO2, a similar pattern was seen for NPT, although at higher rates of CO2 assimilation. For IR72 measured at 900 μL L−1, Pmax was higher in HL compared with ML. For IR72, intercellular [CO2] (Ci) was similar at all growth irradiances when measured at ambient chamber [CO2] (Fig. 5, C and D), although values at HL were slightly higher (by approximately 7 μL L−1) than ML and LL. In NPT, the highest Ci was seen at ML. However, in general, changes in Ci were small (<15 μL L−1). When measured at a chamber [CO2] of 900 μL L−1, small increases in Ci were seen between LL and ML for NPT and progressively from LL to ML to HL in IR72.
Figure 5.
Pmax (A and B) and intercellular [CO2] (Ci; C and D) for IR72 (white bars) and NPT (IR65600-42-5-2; black bars) grown under LL, ML, and HL (10%, 40%, and 100% of full sunlight, respectively). Pmax and Ci were measured at: ambient (350 μL L−1; A and C) and high (900 μL L−1; B and D) CO2 levels. Means ± se, n =20 to 30 (individual plants).
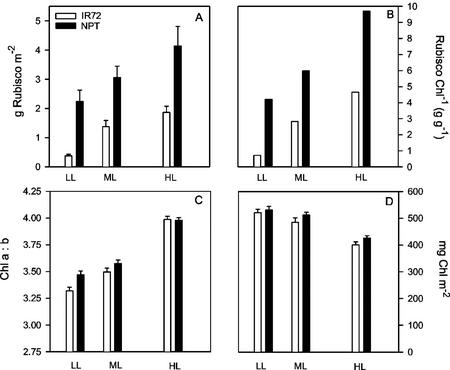
Both varieties showed an increase in Rubisco content per unit leaf area with increasing irradiance (Fig. 6). Significant differences were obtained between HL and ML, as well as between LL and ML. Rubisco per unit Chl showed a similar trend to that of Rubisco per unit leaf area. The Rubisco contents of NPT were markedly higher than that of IR72, on a leaf area or leaf Chl basis at all irradiances, despite being measured at the same number of days after full leaf expansion. The Chl content of HL plants was approximately 20% lower than that of LL plants. A large increase in Chl a to b ratio was found at full sunlight compared with either LL or ML, clearly demonstrating the loss of peripheral LHCII characteristic of growth under HL intensities. The loss in LHCII may have contributed to the reduction in Chl content per unit leaf area.
Figure 6.
Rubisco content expressed per unit leaf area (A) and per unit Chl (B) for NPT (IR65600-42-5-2; black bars) and IR72 (white bars). Plants were grown under LL, ML, and HL (10%, 40%, and 100% of full sunlight, respectively). C, Chl a to b ratio. D, Chl content per unit leaf area. Means ± se, n = 5 (individual plants).
Table II shows the carotenoid composition of leaves acclimated to different irradiances. Significant shifts in the ratio of lutein to β-carotene were observed, changing from 1.1 in HL (IR72 and NPT) to 1.5 in LL (IR72) and 1.3 (NPT), indicating differences in the composition of the pigment protein complexes of the thylakoid membrane. An increase in the amounts of xanthophyll cycle carotenoids (zeaxanthin, violaxanthin, and antheraxanthin) is observed in HL-acclimated leaves (Thayer and Björkman, 1990; Logan et al., 1998) and indicative of photoprotective acclimation to excess irradiance. In IR72, there were increases in the Car to Chl ratio in HL plants compared with LL, which were because of the decreases in Chl content. In NPT, the changes in Car to Chl were smaller, indicating that the carotenoid content also fell in HL plants. The composition of carotenoids differed markedly in both varieties. For IR72, the content of xanthophyll cycle carotenoids increased from below 18% in LL to nearly 30% in HL plants, with most of the difference being between ML and HL. The changes were smaller in the case of NPT leaves; hence, the xanthophyll cycle pool size was higher in LL and lower in HL compared with IR72.
Table II.
Carotenoid content of leaves of varieties IR72 and IR65600-42-5-2 (NPT) in LL, ML, and HL conditions (10%, 40%, and 100% of full sunlight, respectively)
| Variety and Growth Condition | Lutein | Neoxanthin | β -Carotene | XC | Ratio of Total Carotenoid to Total Chlorophyll |
|---|---|---|---|---|---|
| IR72 | |||||
| HL | 32.4 ± 0.37 | 12.58 ± 0.15 | 29.15 ± 0.42 | 28.81 ± 1.94 | 0.512 ± 0.012 |
| ML | 37.91 ± 0.32 | 14.17 ± 0.15 | 28.56 ± 0.27 | 18.41 ± 0.78 | 0.443 ± 0.008 |
| LL | 40.41 ± 0.90 | 15.07 ± 0.15 | 26.91 ± 0.42 | 17.61 ± 0.01 | 0.395 ± 0.005 |
| NPT | |||||
| HL | 32.03 ± 0.41 | 12.58 ± 0.15 | 28.75 ± 0.45 | 26.23 ± 1.25 | 0.474 ± 0.015 |
| ML | 33.49 ± 1.95 | 13.91 ± 0.56 | 30.12 ± 1.13 | 22.98 ± 0.95 | 0.481 ± 0.020 |
| LL | 36.08 ± 1.10 | 14.67 ± 0.52 | 28.03 ± 0.79 | 19.93 ± 0.77 | 0.437 ± 0.014 |
Lutein, neoxanthin, and β -carotene levels are expressed as percentage of total carotenoid. Xanthophyll cycle (XC) content is expressed as percentage of total carotenoid content. Means ± se, n = 5.
Chl fluorescence was again used to assess the efficiency of PSII electron transport and the redox state of PSII in situ (Table III). Dark-adapted Fv/Fm measurements were taken at midday. Again, complete recovery of Fv/Fm overnight was observed (data not shown). There was little difference in levels of photo-inhibition between the two varieties. No photo-inhibition was observed in the LL plants. Levels of photo-inhibition were not substantially greater in HL plants compared with those at ML. However larger differences were seen in other fluorescence parameters: qP was lowest in exposed plants with values equivalent to those observed previously (approximately 0.4; Murchie et al., 1999a). Values for the ML plants were above 0.6. ΦPSII showed a similar response with extremely low values in the exposed plants and increasing as the growth irradiance was reduced.
Table III.
In situ fluorescence characteristics of varieties IR72 and IR65600-42-5-2 (NPT) plants in LL, ML, and HL conditions (10%, 40%, and 100% of full sunlight, respectively)
| Variety and Growth Condition | Fv/Fm | qP | Φ PSII |
|---|---|---|---|
| IR72 | |||
| LL | 0.805 ± 0.002 | 0.884 ± 0.016 | 0.707 ± 0.013 |
| ML | 0.765 ± 0.003 | 0.607 ± 0.047 | 0.424 ± 0.024 |
| HL | 0.752 ± 0.005 | 0.461 ± 0.025 | 0.255 ± 0.013 |
| NPT | |||
| LL | 0.803 ± 0.002 | 0.923 ± 0.022 | 0.711 ± 0.025 |
| ML | 0.773 ± 0.002 | 0.714 ± 0.025 | 0.468 ± 0.013 |
| HL | 0.736 ± 0.003 | 0.519 ± 0.015 | 0.268 ± 0.015 |
Measurements were taken during mid-morning and midafternoon when incident irradiance was greatest (as dictated by the upright leaf posture). Fv/Fm was measured following a 10-min dark adaptation period. qP and quantum yield of PSII reaction centers (ΦPSII) were calculated as described in the text. Values represent means of measurements taken over a 2- to 3-d period ± se, n > 20.
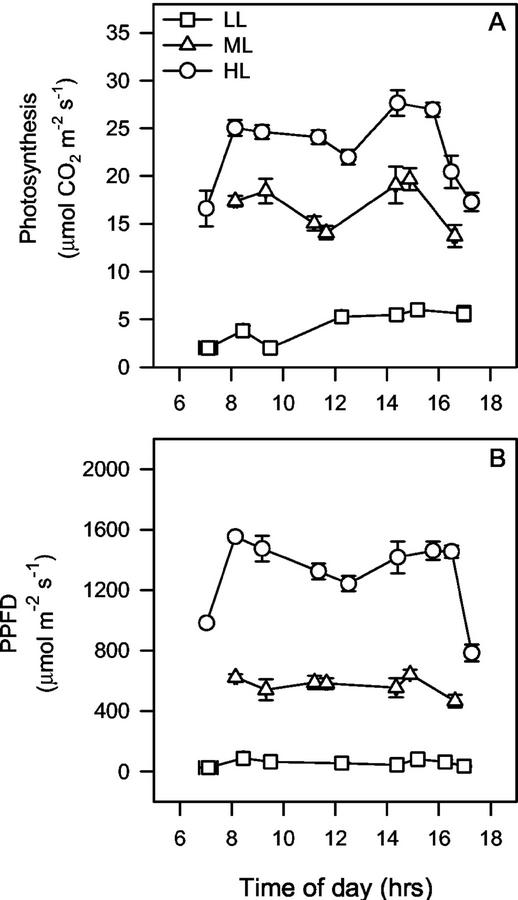
Figure 7A shows the diurnal course of in situ photosynthesis in the HL, ML, and LL conditions for IR72 plants. Measurements took into account the natural upright angle of the rice leaves so irradiance was highest during the hours of the morning, approximately 8 to 11 am (Murchie et al., 1999a) and between 4 and 6 pm as shown in Figure 7B. In the case of these measurements peak PPFD was just over 1,600 μmol m−2 s−1 (mid-morning). Although this is lower than that shown for midday (Fig. 1), it must be pointed out that this irradiance is: (a) still saturating or nearly saturating for photosynthesis (Murchie et al., 1999a), and (b) takes into account the reduction in incident irradiance at midday that occurs as a result of the upright leaf posture. Although no change was observed in Pmax (350 μL L−1) between ML and HL (Fig. 5A), there was a distinct increase in the daily level of photosynthesis: Integration of the area under the data in Figure 7 reveals that daily photosynthesis was 33% higher in HL. The reason for this probably lies in the convexity of light saturation curves for leaves: Murchie et al. (1999a) showed data for rice leaves in which photosynthesis measured at 800 μmol m−2 s−2 was less than that measured at 1,800 μmol m−2 s−2, sufficient to explain the difference in daily photosynthesis seen in Figure 7. Therefore, although the upper limit for Pmax had been reached in both HL and ML, photosynthesis was operating at a lower level in situ in ML conditions.
Figure 7.
A, Diurnal changes in photosynthesis for leaves of IR72 grown under LL, ML, and HL (10%, 40%, and 100% of full sunlight, respectively). Photosynthesis was measured as in “Materials and Methods” except that natural sunlight was used as the light source. This was achieved by replacing the LED source in the leaf chamber with a clear-topped unit (“sun and sky,” LI-COR, Lincoln, NE). Attention was paid to the natural upright leaf posture of rice leaves such that at midday the incident light was lowered (Murchie et al., 1999a). B, Incident sunlight for this diurnal course, measured using the external light sensor on the LI-COR 6400. Values are means ± se, n = 5. Each value is the mean of measurements taken over a consecutive 3-d period.
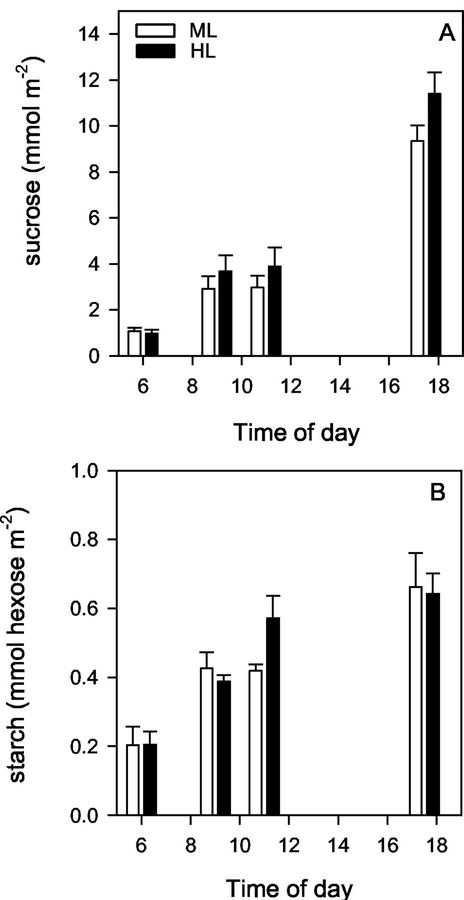
The level of major nonstructural carbohydrates in rice leaves, Suc and starch, did not show a significant difference between ML and HL (Fig. 8). Given the differences in daily rate of photosynthesis, this implies that export rates of carbon from leaves were higher in HL compared with ML.
Figure 8.
Diurnal changes in nonstructural carbohydrate levels for leaves of IR72 grown at ML (40% of full sunlight, white bars) or HL (100% of natural sunlight, black bars). A, Suc. B, Starch. Samples were taken at 6 am, 9 am, 11 am, and 5:30 pm. Values are means ± se of means, n = 4 (individual plants).
DISCUSSION
Irradiance, Acclimation, and Position within the Canopy
As expected, irradiance decreased dramatically from the top to the bottom of the rice canopy. The decline was steeper in the canopy of IR72 than in NPT. At midday, irradiance values at the lowest leaves of the canopy may be less than 100 μmol m−2 s−1, whereas the upper leaves are exposed to over 2,000 μmol m−2 s−1. These changes had major effects on the degree of saturation of photosynthesis and the extent of photo-inhibition. The large changes in penetration of irradiance were accompanied by changes in leaf composition: Declines in Pmax, Rubisco, and Chl a/b were observed with increasing depth through the canopy. Such differences are indicative of strong photosynthetic acclimation to irradiance. Similar changes were found when the level of sunlight reaching the exposed leaves was manipulated by imposed shading. The magnitude of the changes was smaller in NPT than IR72 in both cases. However, it is important to consider whether all of these observed changes are attributable to acclimation to the light environment. The data suggest that acclimation of Pmax in IR72 at ambient [CO2] above 800 μmol m−2 s−1 was limited. It was unlikely that this was caused by stomatal limitations (Ci, Fig. 5). Despite this, there was a large drop in Pmax between FL and 2L, which, therefore, would not be predicted from the difference in irradiance between these positions (2,200 and 950 μmol m−2 s−1, respectively). For NPT, when irradiance in the artificial shading experiment increased from 800 to over 2,000 μmol m−2 s−1 (a 2.5-fold increase), Pmax increased by only 14%. However, in NPT, when comparing leaves at the position of 2L with those at the top of the canopy (1,250–2,200 μmol m−2 s−1, a 1.8-fold increase), an increase in Pmax of 25% and an increase in Rubisco content of 45% was observed (Figs. 2A and 3A).
Previous work with other species suggested that light is a dominant factor in determining both the N profile and photosynthetic characteristics of leaves in mature canopies (Hikosaka, 1996). However, leaf age (i.e. changes in photosynthetic characteristics of a seemingly endogenous origin after full leaf expansion) becomes more important with increasing N deficiency (Hikosaka et al., 1994). In this study using N-sufficient plants, we have seen that toward the top of the rice canopy, the reduction in Pmax at 2L in IR72 was too great to be accounted for by acclimation to irradiance levels. We suggest that a leaf aging effect was involved. At lower points in the canopy, the photosynthetic characteristics were consistent with having resulted from acclimation alone, although leaf age will have determined Pmax at earlier stages in leaf development. The data also suggest that leaf age effects were less prevalent in the determination of levels of photosynthetic enzymes and Pmax in NPT compared with IR72.
A decline in rice Pmax with leaf age has been observed that also coincided with changes in the Rubisco content (Makino et al., 1985; Hidema et al., 1991). The age at which this occurs depends upon the irradiance during development: At a high irradiance, the decline in Pmax can begin 3 to 4 d after the maximum is attained (Hidema et al., 1991; E.H. Murchie, S. Hubbart, and P. Horton, unpublished data). Therefore, the period of time in which photosynthesis can operate at highest Pmax can be a small proportion of the entire life span of the leaf. This decline has been attributed specifically to senescence (Hidema et al., 1991, 1992) and possesses appropriate characteristics, with a decline in Rubisco content and a decline in Pmax. An important question is whether this represents reallocation of minerals and/or reduced carbon to rapidly growing tissue, as has been shown for flag leaves during the grain-filling period (Mae and Ohira, 1981).
Acclimation of Photosynthesis Saturates at a Relatively Low Irradiance
Acclimation of photosynthesis to irradiance is a dramatic example of the functional plasticity of plants. We have found large changes in the photosynthetic capacity of rice that result from the response to the intensity of solar radiation. In a separate field experiment (data not shown), similar differences in Pmax were seen when rice leaves had emerged within a light regime. Developmental constraint (i.e. the dominance of leaf aging after full leaf expansion) over the process of photosynthetic acclimation in this field experiment was not as great as previous work may suggest (Hidema et al., 1991; Mae, 1997). Levels of light-harvesting pigment proteins, cytochrome f, and the CF1 unit of chloroplast ATPase are responsive to a reduction in irradiance imposed after full leaf expansion in rice (Hidema et al., 1991, 1992). In these studies, the effect of shading seemed to be a slowing of the rate of aging of the rice leaf; hence, a retardation of the decline in Rubisco. Makino et al. (1997a) found only minimal differences in Pmax between rice leaves grown under 350 compared with 1,000 μmol m−2 s−1 and concluded that acclimation to irradiance in rice was based on a morphological alteration of leaf area ratio rather than of photosynthetic components per se. Mae (1997) suggested that the level of N might be more important than irradiance in determining leaf Rubisco amounts in rice. The different results observed may reflect variation between genotypes and different conditions of cultivation.
We have shown clear differences between genotypes that indicate an ability to acclimate over different ranges of irradiances in the field. IR72 showed strong acclimation of photosynthesis over low to intermediate ranges, whereas the NPT studied appeared to show less acclimation than IR72 over low irradiances but increased Pmax by about 14% between the two higher irradiance levels. Irrespective of these differences, we conclude that in IR72 at an irradiance of 2,000 μmol m−2 s−1 (full sunlight) a large proportion (often approaching 50%) of incident photons can be considered excess and represents a significant decrease in the light use efficiency of rice plants during the tropical dry season. In addition, even though acclimation of Rubisco and Pmax occurred for NPT between ML and HL, this was not proportional to the difference in irradiance (800, ML; and 2,000, HL), which implies that NPT had also reached, or was reaching, its upper point of acclimation at a level well below that of full sunlight. This has relevance to a recent survey that concluded that radiation use efficiency (aboveground dry mass/MJ intercepted photosynthetically active radiation) is low in rice compared with other C3 crops (Mitchell et al., 1998). In the case of NPT, the increment in Pmax between ML and HL (at ambient CO2 levels) was much smaller than the increase in irradiance levels. This effect (seen in both genotypes) is a result of the inability of the photosynthetic system to acclimate to the highest irradiances in terms of carbon assimilation rates. Acclimation of Pmax to irradiance would normally result in an optimization of the proportion of absorbed versus utilized photons so that light saturation is avoided. The failure of Pmax to acclimate is reflected in the fact that the increase in daily photosynthesis between ML and HL was only 33% despite a greater than 2-fold difference in daily radiation. Similarly, the HL leaves showed increased reduction states of PSII, decreased PSII efficiency, and more photo-inhibition than the ML or LL leaves.
The differences in acclimation characteristics seen in the two varieties may be important: Light levels in the tropical dry season are typically much higher than those in the wet season. This is reflected in the generally lower yields in the latter (IRRI, 1998). However, NPT yields are consistently and significantly higher than IR72 in the wet season. We suggest that this is at least partially because of the higher Pmax of the NPT when acclimated to LL: These leaves would be able to exploit temporary periods of high irradiance to better effect than the IR72.
Data for the artificial shading experiment in IR72 showed that acclimation of the thylakoid membrane and Rubisco occurred at irradiance levels where no changes in Pmax (measured at ambient CO2) were seen. For the thylakoid, this may indicate that these responses were specifically to the excess irradiance: The large change in Chl a/b indicates a selective degradation of light-harvesting complexes, which would increase the quantum efficiency of photosynthesis at HL. Total Chl also decreased in HL. Similarly, in IR72, the shift in carotenoid composition in favor of the operation of the xanthophyll cycle, responsible for high-energy state quenching of Chl fluorescence, is indicative of photoprotection. Interestingly, the shift in xanthophyll cycle content in NPT was more consistent with Pmax, unlike IR72. Species differences between these two parameters are known (Bilger et al., 1995). Previous work would predict that at 900 μL L−1 CO2 and saturating irradiance, Rubisco activity exerts minimal control over the rate of photosynthesis. The increase in Pmax measured at high CO2 between ML and HL conditions indicates that acclimation of some component(s) other than Rubisco was occurring such as electron transport capacity or end product biosynthesis capacity. Under similar measurement conditions using N-replete rice plants, Makino et al. (1994) provided evidence that Suc synthesis rather than electron transport limited carbon assimilation rates.
Irradiance-Dependent Changes in Rubisco Level
Measurements of Rubisco amount presented here pose a number of questions concerning the regulation of its accumulation within rice leaves. First, as growth irradiance increases from ML to HL conditions, why did leaves of IR72 continue to accumulate Rubisco? The extra protein was not needed to maintain a higher Pmax at ambient CO2 levels. In this experiment, Rubisco amount and Pmax were not strongly linked, at least at all stages of leaf development. One possibility is that the accumulation of Rubisco protein serves another purpose, such as storage. We speculate that the mechanism by which this occurs may be related to the higher daily rates of photosynthesis (Fig. 7) or the higher degree of saturation of the electron transport chain in HL compared with ML (table III). Whatever the mechanism, it is unclear whether this secondary role would result in accumulation to “excess” as has been shown for tobacco (Nicotiana tabacum; Lauerer et al., 1993; see also Makino et al., 1994, 1997b). An accumulation of this type would have some physiological basis in rice. High leaf N content is needed in this species to attain maximum grain yields not only by optimization of photosynthesis, but also by the reallocation of N: It is known that a large proportion of rice leaf protein is broken down and transported to the grain (Mae and Ohira, 1981), where it makes up a significant proportion of total grain N content. It would need to be determined whether any “excess” Rubisco is present in leaves of NPT in the active or inactive form.
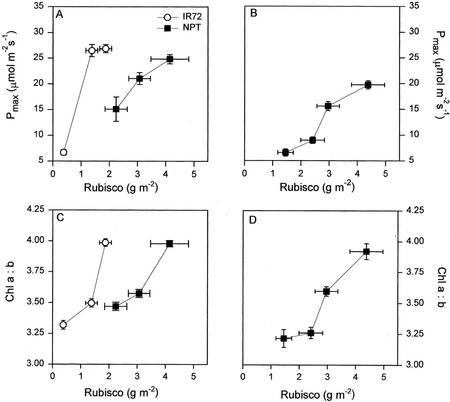
The levels of Rubisco in leaves of NPT in comparison with IR72 are notable because this was not reflected in Pmax. When comparing the two genotypes, the relationship between Pmax (ambient CO2) and Rubisco content was very different (Fig. 9, A and B). Ci values for NPT were slightly lower in HL compared with ML, suggesting a possible stomatal limitation at high irradiances, as noted for similar tropical japonica cultivars by Peng et al. (1998). Also shown in Figure 9 are the relationships between Chl a/b and Rubisco content, which demonstrate that the relationship between light harvesting and carboxylation capacity was not conserved between the varieties. The high content of Rubisco in NPT when compared with IR72 can be explained by the high leaf thickness of the former, a characteristic considered desirable in the NPT breeding program (Peng et al., 1994). In this case, it is concluded that the resulting leaf Rubisco content did not coincide with higher photosynthesis.
Figure 9.
The relationship between Pmax at ambient (350 μL L−1) CO2 levels and Rubisco content (A and B) and between Rubisco content and Chl a to b ratio (C and D) for NPT (▪) and IR72 (○); for LL, ML, and HL (A and C) as in Figures 5 and 6; and for leaves at different canopy positions (B and D) as in Figures 2 and 3.
Recently, a theory was proposed that the function of the high leaf area index in rice is primarily to provide a large enough leaf area so that N can be stored before retranslocation to the grain (Sinclair and Sheehy, 1999). This being the case, the upright posture of the NPT varieties would permit greater light penetration to lower parts of the canopy, increasing the efficiency of this area to act as an N store. Clearly, there is a complex and poorly understood interplay between the irradiance, leaf age, plant morphology, and canopy structure in determining the Rubisco content of a rice leaf.
SUMMARY
Previous work has shown that acclimation of photosynthesis and Rubisco content to irradiance in rice leaves is limited. By shading plants in field conditions, we have demonstrated that this type of acclimation does exist in rice. We have also shown that the acclimation process was less responsive at higher irradiances, showing that acclimation of photosynthesis to full tropical irradiance in rice plants was restricted. This has implications for improvement of radiation use efficiency. In an IR72 variety, this was associated with the induction of photoprotective processes. We also examined photosynthetic acclimation as a factor controlling photosynthetic activity of lower leaves in a field canopy and conclude that leaf age was dominant in determining Pmax in upper leaves, whereas acclimation to irradiance levels was of greater importance in the lower leaves.
MATERIALS AND METHODS
Growth of Plant Material
Experiments were carried out in the dry season of 1998 and 1999 at the IRRI farm (Los Baños, Philippines). Tropical rice (Oryza sativa) Japonica (NPT) varieties were used that possessed an “open” canopy structure and compared with IR72, which possessed a more “closed” canopy structure. NPT varieties used were IR65598-112-2, IR65600-42-5-2, IR65600-129-1-1-2, and IR68544-29-2-1-3-1-2. Experiment 1 measurements were made during the first week after flowering to ensure that the canopy was fully closed. Nutrients were supplied throughout the growing period. This plot was transplanted on December 22, 1998 at 10- × 15-cm spacing. Total N application was 120 kg ha−1. The following nomenclature for numbering leaves is used: FL, and next three leaves in order of increasing age: 2L, 3L, and 4L. In the case of IR72, 4L was senesced and not used for any measurement. Experiment 2 was conducted on a field that had been divided into plots of 4 × 6 m. These were transplanted on February 4, 1998 at 20 × 15 cm with four seedlings per hill. We applied 60 kg ha−1 N, 30 kg ha−1 P, 40 kg ha−1 K, and 5 kg ha−1 Zn 1 d before transplanting. We applied 45 kg ha−1 N at mid-tillering, 60 kg ha−1 N at panicle initiation, and 45 kg ha−1 N at flowering. To reduce positional effects, data were combined from three plots per variety, each plot at a different position within the field. To manipulate the level of irradiance during growth, cloches were constructed (1.5 × 1.5 × 1.5 m) consisting of a wooden frame covered with a neutral density netting (pore size 2 × 2 mm). This provided shading to the level of the base of the plant and a space of 0.5 to 0.75 m above the plant. One or two layers of netting provided daily PPFDs of 24.84 ± 0.42 and 3.86 ± 0.21 mol m−2 (ML and LL), respectively. Exposed plants (HL) received 58.66 ± 2.2 mol m−2 d−1 and were measured in plots next to, but not shaded by, the cloches. Midday (peak) PPFDs were typically 1,600 to 1,900 μmol m−2 s−1 (HL), 600 to 800 μmol m−2 s−1 (ML), and 50 to 200 μmol m−2 s−1 (LL). Midday leaf temperatures in these conditions were 32.97°C ± 0.38°C (LL), 32.52°C ± 0.17°C (ML), and 32.86°C ± 0.31°C (HL) for IR72 and 32.34°C ± 0.16°C (LL), 32.73°C ± 0.21°C (ML), and 33.67°C ± 0.36°C (HL) for NPT (n = 7, means ± se) Measurements of leaf temperature were made with the thermocouple attached to the PAM-2000 fluorometer leaf clip 2030B (Walz, Effeltrich, Germany), which was positioned on the underside of the leaf. Cloches were placed in the field 15 d before measurements began. At this time, the penultimate leaf to the FL was fully developed and the emerging FL was one-half the length of the penultimate leaf. The penultimate leaf to the FL is referred to as the first leaf, on which all measurements were made.
In both experiments, “irradiance” was measured as PPFD. Experiment 1 utilized a Sunfleck Ceptometer (Decagon Devices, Inc., Pullman, WA). This device consisted of 80 individual light sensors arranged on a rod of dimensions 1 × 0.02 m so that measurements could be integrated across a large surface area to accommodate spatial variation. The sensors were maintained in a horizontal position at all times: A spirit level was incorporated into the sensor to level the device for accurate readings. Approximately 20 measurements were made at each position in the canopy and results averaged. Measurements were taken at midday when solar penetration of the canopy would be greatest and also at 3 pm when the solar angle is reduced. The midpoint of each leaf measured (i.e. halfway along the leaf length) was the position at which both measurements of PPFD and photosynthesis were taken. The distances of these points from the top of the canopy taken as a proportion of the total height of the canopy were found to be similar for all varieties. Daylight spectra within and at the top of the canopy were measured in two of the varieties (IR65598-112-2, IR72) using a handheld portable spectroradiometer (Analytical Spectral Devices Inc., Boulder, CO). In Experiment 2, “irradiance” was measured using the external light sensor mounted on the leaf chamber of the LI-COR 6400 infrared gas analyzer.
Gas Exchange
Leaf gas exchange measurements were made using a LI-COR 6400 infrared gas analyzer (LI-COR) as described by Murchie et al. (1999a). The light source used was a red LED (Li64002, LI-COR). Temperature of the leaf chamber was maintained at 30°C.
Chl Fluorescence
Chl fluorescence was carried out using a portable PAM 2000 fluorometer (Walz) as described by Murchie et al. (1999a).
Assays for Pigment, Rubisco, and Carbohydrates
Assays for Chl and carotenoids were as described by Murchie et al. (1999a). Rubisco was assayed by SDS-PAGE essentially as described by Makino et al. (1994), except that densitometry was used for analysis of the gels rather than formamide extraction. Leaf discs were ground to powder in liquid N2 and 1 mL of a buffer was added that contained 100 mm HEPES/HCl (pH 7.6), 1 mm EDTA, 5 mm MgCl2, 5 mm dithiothreitol, 1 mm phenyl methyl sulfanilamide, 0.1% (v/v) Triton X-100, and 20 mg mL−1 polyvinylpolypyrrolidone. These were centrifuged at 13,000g for 15 min at 4°C and the supernatant removed. Leaf protein content was assayed by the binding of Coomassie Blue and measurement of A595 (Bradford, 1976). For this, Bio-Rad protein assay dye reagent was used (Bio-Rad Laboratories, Munich): 70 μL of undiluted dye reagent was added to 30 μL of sample and total volume made up to 1 mL with purified water. This was left for 15 min and A595 was measured. For calibration of this assay, purified Rubisco was used (wheat Rubisco, kindly provided by Martin Parry, Institute of Arable Crops Research, Rothamstead, UK). Portions of the extract to be assayed were denatured completely by incubation for 3 min at 100°C after diluting 1:1 (v/v) with a solution containing 0.0625 m Tris/HCl (pH 6.8), 10% (w/v) glycerol, 5% (w/v) SDS, 5% (v/v) β-mercaptoethanol, and 0.1% (w/v) bromophenol blue. Rubisco amount was assayed by SDS-PAGE on a Bio-Rad Mini-Protean II apparatus. Gel dimension was 100 × 80 × 1 mm. The order of samples loaded on to the gels was randomized. A sample volume corresponding to 7 μg of total protein was resolved on a 10% (w/v) polyacrylamide gel alongside 5.6 μg of purified Rubisco. Gels were stained with Coomassie Brilliant Blue R-250, dried, and scanned on a flatbed scanner (Scanjet 4c, Hewlett-Packard, Palo Alto, CA), and images analyzed using the software package Optimas (version 5.2) with the profile 1 gel densitometer application (version 1.1, Optimas Corporation, Silver Spring, MD). Gaussian transformations were performed for the peaks in each gel lane, and the area under each peak compared with that of the purified standards. When tests were carried out using varying amounts of either purified Rubisco or crude extract, a linear relationship was seen between amount per lane and area under each Rubisco peak for the range 0.5 to 10 μg of total protein applied to gel. A sample of pellets after the initial centrifugation was tested for the presence of membrane-bound Rubisco: Pellets were washed in extraction buffer and then incubated in the presence of 0.1% (v/v) Triton X-100 for 5 min, recentrifuged, and the supernatant analyzed for the presence of Rubisco. Contamination of the insoluble fraction was negligible (data not shown). Nonstructural carbohydrates were assayed as in Murchie et al. (1999b).
ACKNOWLEDGMENTS
We are grateful to the staff at IRRI for their help during this work, particularly all the members of Dr. Shaobing Peng's laboratory and to Dr. Gurdev Khush for use of his experimental plots. We also thank Dr. Mark Wentworth and Dr. Sasha Ruban for useful discussions.
Footnotes
This work was supported by the UK Department for International Development (contract no. ARP505H) and by the UK Biotechnology and Biological Sciences Research Council (grant no. 50/P13990).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.011098.
LITERATURE CITED
- Ackerly DD. Light, leaf age and leaf nitrogen concentration in a tropical vine. Oecologia. 1992;89:596–600. doi: 10.1007/BF00317169. [DOI] [PubMed] [Google Scholar]
- Anderson JM. Photoregulation of the composition, function and structure of thylakoid membranes. Annu Rev Plant Physiol Plant Mol Biol. 1986;37:93–136. [Google Scholar]
- Anderson JM, Chow WS, Park YI. The grand design of photosynthesis: acclimation of the photosynthetic apparatus to environmental cues. Photosynth Res. 1995;46:129–139. doi: 10.1007/BF00020423. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Osmond CB. Shade-sun responses: compromises between acclimation and photoinhibition. In: Kyle DJ, Osmond CB, Arntzen CJ, editors. Photoinhibition. Amsterdam: Elsevier Science Publishers; 1987. pp. 1–36. [Google Scholar]
- Bailey S, Walters RG, Jansson S, Horton P. Acclimation of Arabidopsis thaliana to the light environment: the existence of separate low light and high light responses. Planta. 2001;293:794–801. doi: 10.1007/s004250100556. [DOI] [PubMed] [Google Scholar]
- Bilger W, Fisahn J, Brummet W, Kossmann J, Willmitzer L. Violaxanthin cycle pigment contents in potato and tobacco plants with genetically reduced photosynthetic capacity. Plant Physiol. 1995;108:1479–1486. doi: 10.1104/pp.108.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Conocono EA, Egdane JA, Setter TL. Estimation of canopy photosynthesis in rice by means of daily increases in leaf carbohydrate concentrations. Crop Sci. 1998;38:987–995. [Google Scholar]
- Evans JR. Photosynthetic acclimation and nitrogen partitioning within a lucerne canopy: I. Canopy characteristics. Aust J Plan Physiol. 1993;20:55–67. [Google Scholar]
- Fankhauser C, Chory J. Light control of plant development. Annu Rev Cell Dev Biol. 1997;13:203–229. doi: 10.1146/annurev.cellbio.13.1.203. [DOI] [PubMed] [Google Scholar]
- Hidema J, Makino A, Kurita Y, Mae T, Ojima K. Changes in the levels of chlorophyll and light-harvesting chlorophyll a/b protein of PSII in rice leaves aged under different irradiances from full expansion through senescence. Plant Cell Physiol. 1992;33:1209–1214. [Google Scholar]
- Hidema J, Makino A, Mae T, Ojima K. Photosynthetic characteristics of rice leaves aged under different irradiances from full expansion through senescence. Plan Physiol. 1991;97:1287–1293. doi: 10.1104/pp.97.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka K. Effects of leaf age, nitrogen nutrition and photon flux density on the organisation of the photosynthetic apparatus in leaves of vine (Ipomea tricolor Cav.) grown horizontally to avoid mutual shading of leaves. Planta. 1996;198:144–150. doi: 10.1007/BF00325881. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Terashima I, Katoh S. Effects of leaf age, nitrogen nutrition and photon flux density on the distribution of nitrogen among leaves of a vine (Ipomea tricolor Cav.) grown horizontally to avoid mutual shading of leaves. Oecologia. 1994;97:451–457. doi: 10.1007/BF00325881. [DOI] [PubMed] [Google Scholar]
- Huang L-K, Osmond CB, Terashima I. Chilling injury in mature leaves of rice: II. Varietal differences in the response to interactions between low temperature and light measured by chlorophyll fluorescence at 77K and the quantum yield of photosynthesis. Aust J Plant Physiol. 1989;16:339–352. [Google Scholar]
- IRRI (1998) IRRI Research Report for 1998: Irrigated Rice Ecosystems. IRRI, Manila, Philippines
- IRRI (2000) IRRI Research Report for 2000: Irrigated Rice Ecosystems. IRRI, Manila, Philippines
- Lauerer M, Saftic D, Quick WP, Labate C, Fichtner K, Schulze E-D, Rodermel SR, Bogorad L, Stitt M. Decreased ribulose-1,5-bisphosphate carboxylase-oxygenase in transgenic tobacco transformed with “antisense” rbcS: VI. Effect on photosynthesis in plants grown at different irradiance. Planta. 1993;190:332–345. [Google Scholar]
- Logan BA, Demmig-Adams B, Adams WW, Grace SC. Antioxidants and xanthophyll cycle-dependent energy dissipation in Cucurbita pepo L. and Vinca major L. acclimated to four growth PPFDs in the field. J Exp Bot. 1998;49:1869–1879. [Google Scholar]
- Mae T. Physiological nitrogen efficiency in rice: nitrogen utilisation, photosynthesis and yield potential. Plant Soil. 1997;196:201–210. [Google Scholar]
- Mae T, Ohira K. The remobilisation of nitrogen related to leaf growth and senescence in rice plants (Oryza sativa L.) Plant Cell Physiol. 1981;22:1067–1074. [Google Scholar]
- Mae T, Thomas H, Gay AP, Makino A, Hidema J. Leaf development in Lolium temulentum: photosynthesis and photosynthetic proteins in leaves senescing under different irradiances. Plant Cell Physiol. 1993;34:391–399. [Google Scholar]
- Makino A, Mae T, Ohira K. Photosynthesis and ribulose-1,5-bisphosphate carboxylase/oxygenase in rice leaves from emergence through senescence. Quantitative analysis by carboxylation/oxygenation and regeneration of ribulose-1,5-bisphosphate. Planta. 1985;166:414–420. doi: 10.1007/BF00401181. [DOI] [PubMed] [Google Scholar]
- Makino A, Nakano H, Mae T. Responses of ribulose-1,5-bisphosphate carboxylase, cytochrome f, and sucrose synthesis enzymes in rice leaves to leaf nitrogen and their relationships to photosynthesis. Plant Physiol. 1994;105:173–179. doi: 10.1104/pp.105.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Sato T, Nakano H, Mae T. Leaf photosynthesis, plant growth and nitrogen allocation in rice under different irradiances. Planta. 1997a;203:390–398. [Google Scholar]
- Makino A, Shimada T, Takumi S, Kaneko K, Matsuoka M, Shimamoto K, Nakano H, Miyao-Tokutomi M, Mae T, Yamamoto N. Does decrease in ribulose-1,5-bisphosphate carboxylase by antisense RbcS lead to a higher N-use efficiency of photosynthesis under conditions of saturating CO2 and light in rice plants? Plant Physiol. 1997b;114:483–491. doi: 10.1104/pp.114.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell K, Marrison JL, Leech RM, Griffiths H, Horton P. Chloroplast acclimation in leaves of Guzmania monostachia in response to high light. Plant Physiol. 1999;121:89–95. doi: 10.1104/pp.121.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PL, Sheehy JE. Performance of a potential C4 rice: overview from quantum yield to grain yield. In: Mitchell PL, Sheehy JE, Hardy B, editors. Redesigning Rice Photosynthesis to Increase Yield. Amsterdam: International Rice Research, Makati City, Philippines, and Elsevier Science Publishers; 2000. pp. 145–163. [Google Scholar]
- Murchie EH, Chen Y-Z, Hubbart S, Peng S, Horton P. interactions between senescence and leaf orientation determine in situ patterns of photosynthesis and photoinhibition in field-grown rice. Plant Physiol. 1999a;119:553–563. doi: 10.1104/pp.119.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchie EH, Horton P. Acclimation of photosynthesis to irradiance and spectral quality in British plant species: chlorophyll content, photosynthetic capacity and habitat preference. Plant Cell Environ. 1997;20:438–448. [Google Scholar]
- Murchie EH, Sarrobert C, Contard P, Betsche T, Foyer CH, Galtier N. Overexpression of sucrose-phosphate synthase in tomato plants grown with CO2 enrichment leads to decreased foliar carbohydrate accumulation relative to untransformed controls. Plant Physiol Biochem. 1999b;37:251–260. [Google Scholar]
- Okada K, Katoh S. Two long term effects of light that control the stability of proteins related to photosynthesis during senescence of rice leaves. Plant Cell Physiol. 1998;39:394–404. [Google Scholar]
- Öquist G, Chow WS, Anderson JM. Photoinhibition of photosynthesis represents a mechanism for the long term regulation of photosystem II. Planta. 1992;186:450–460. doi: 10.1007/BF00195327. [DOI] [PubMed] [Google Scholar]
- Osmond CB. What is photoinhibition? Some insights from comparison of sun and shade plants. In: Baker NR, Boyer JR, editors. Photoinhibition: Molecular Mechanisms to the Field. Oxford: Bios Scientific Publications; 1994. pp. 1–24. [Google Scholar]
- Peng S, Khush GS, Cassman KG. Evolution of the new plant ideotype for increased yield potential. In: Cassman KG, editor. Breaking the Yield Barrier: Proceedings of a Workshop on Rice Yield Potential in Favorable Environments. IRRI, Manila, Philippines. 1994. [Google Scholar]
- Peng S, Laza RC, Khush GS, Sanico AL, Visperas RM, Garcia FV. Transpiration efficiencies of indica and improved tropical japonica rice grown under irrigated conditions. Euphytica. 1998;103:103–108. [Google Scholar]
- Sinclair TR, Sheehy JE. Erect leaves and photosynthesis in rice. Science. 1999;283:1455–1456. [Google Scholar]
- Thayer SS, Björkman O. Leaf xanthophyll content and composition in sun and shade determined by HPLC. Photosynth Res. 1990;23:331–343. doi: 10.1007/BF00034864. [DOI] [PubMed] [Google Scholar]
- Verhoeven AS, Demmig-Adams B, Adams WW. Enhanced employment of the xanthophyll cycle and thermal energy dissipation in spinach exposed to high light and N stress. Plant Physiol. 1997;113:817–824. doi: 10.1104/pp.113.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu JCV, Baker JT, Pennanen AH, Allen LH, Jr, Bowes G, Boote KJ. Elevated CO2 and water deficit effects on photosynthesis, ribulose bisphosphate carboxylase-oxygenase and carbohydrate metabolism in rice. Physiol Plant. 1998;103:327–339. [Google Scholar]
- Weston E, Thorogood K, Vinti G, López-Juez E. Light quantity controls leaf-cell and chloroplast development in Arabidopsis thaliana wild type and blue-light perception mutants. Planta. 2000;211:807–815. doi: 10.1007/s004250000392. [DOI] [PubMed] [Google Scholar]
- Yamazaki, J-y, Kamimura Y, Okada M, Sugimara Y. Changes in photosynthetic characteristics and photosystem stoichiometries in the lower leaves in rice seedlings. Plant Sci. 1999;148:155–163. [Google Scholar]