Abstract
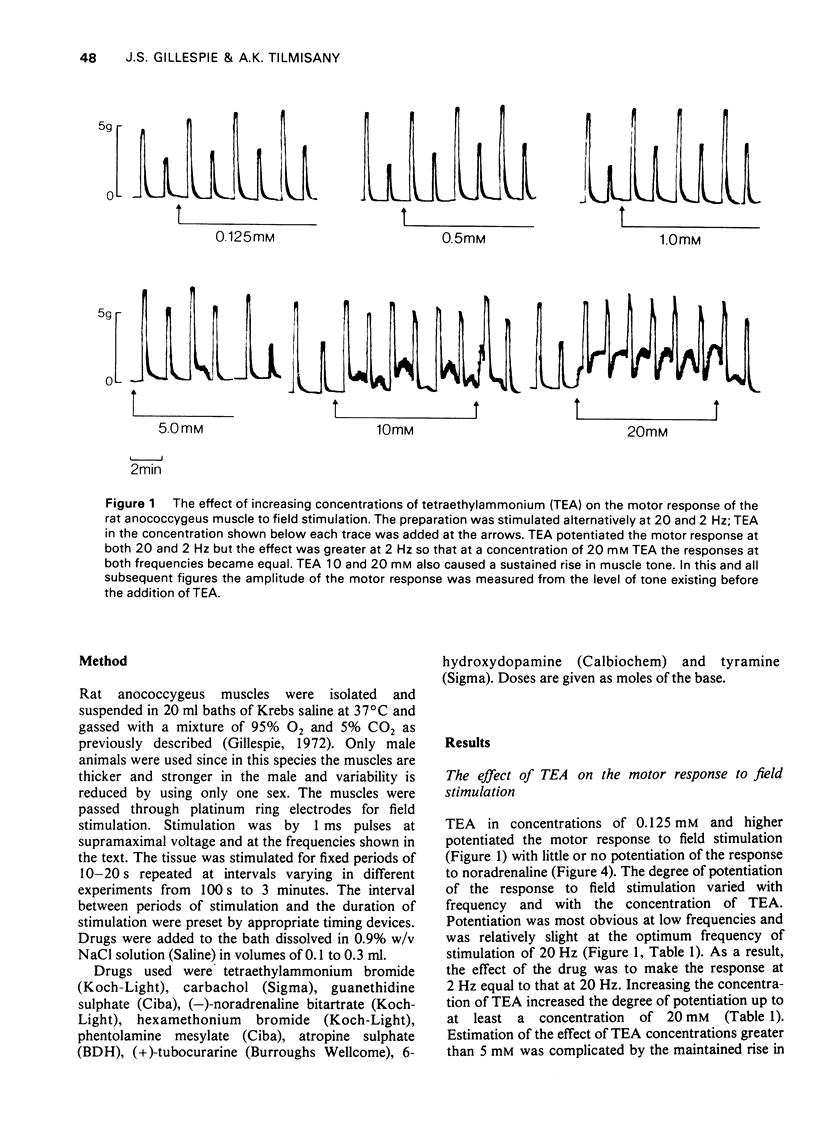
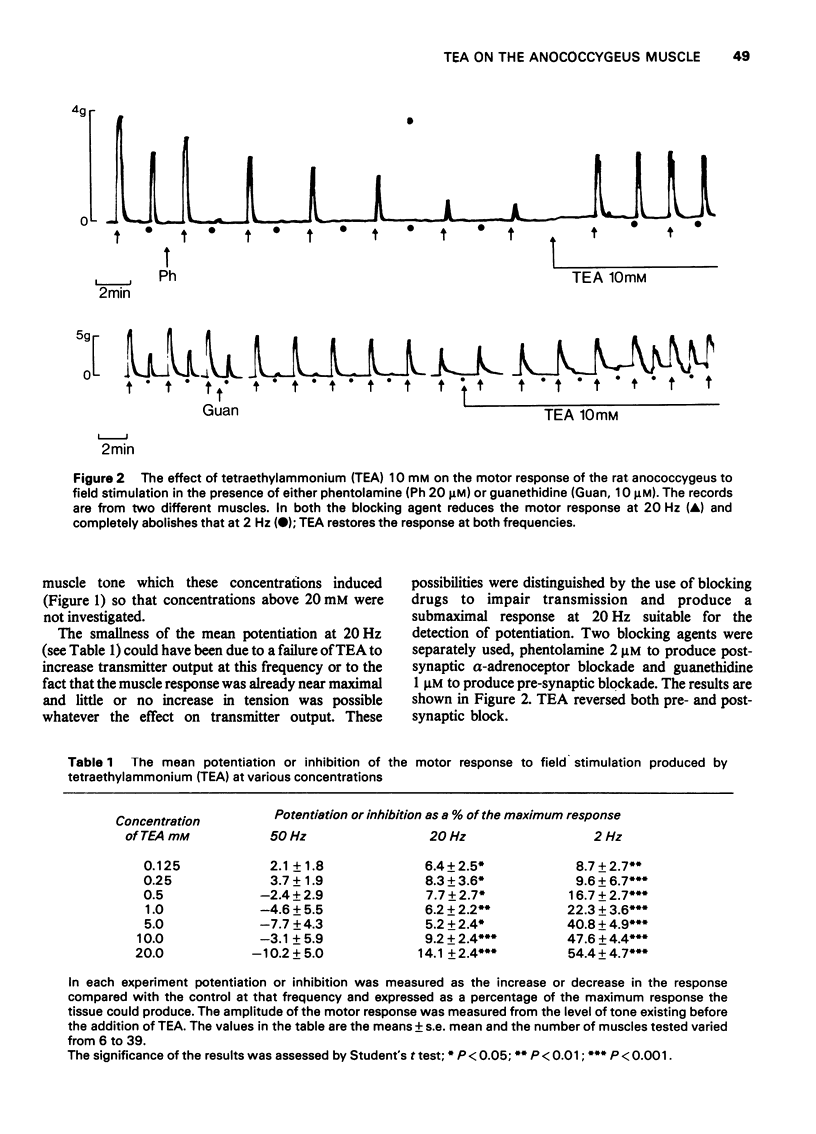
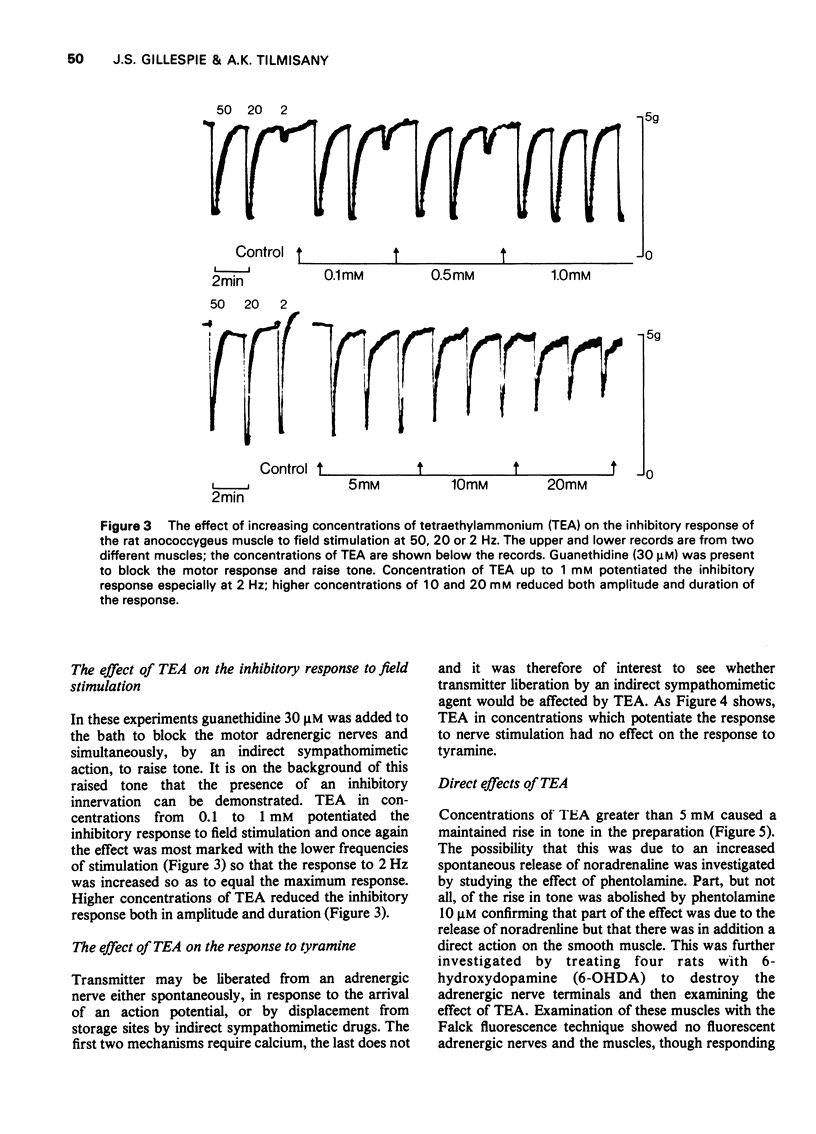
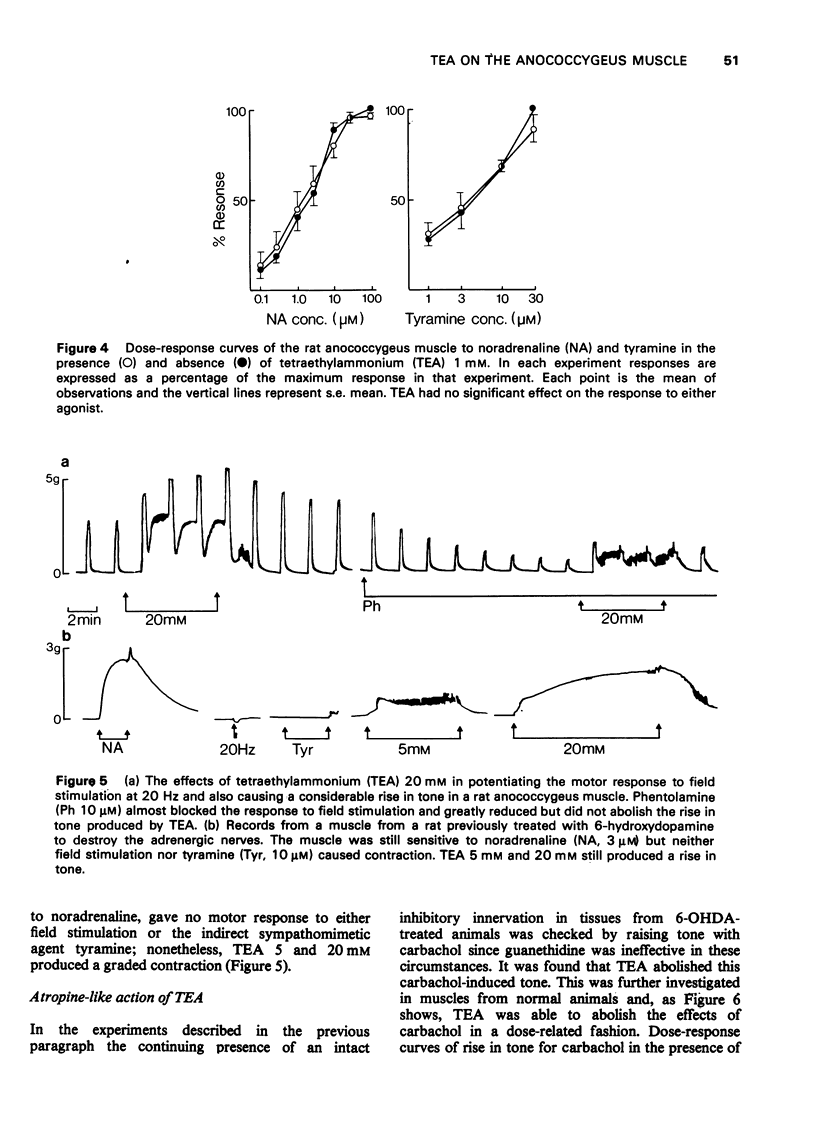
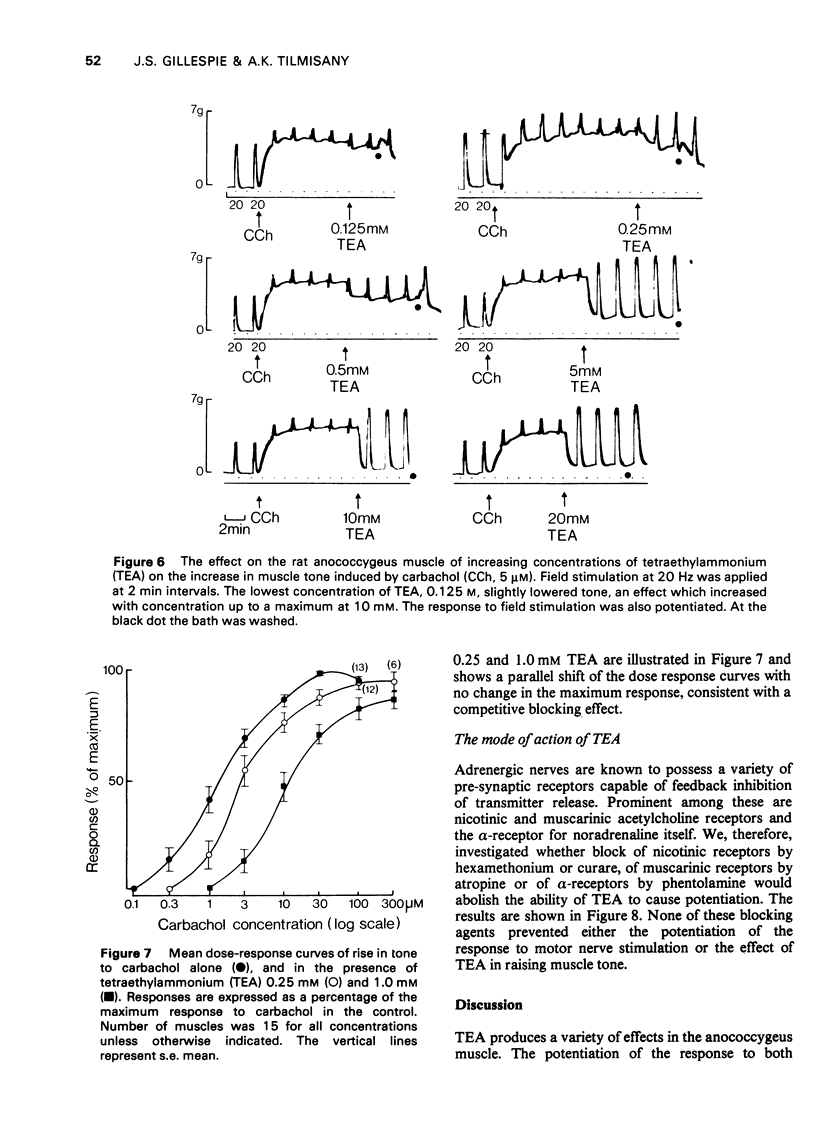
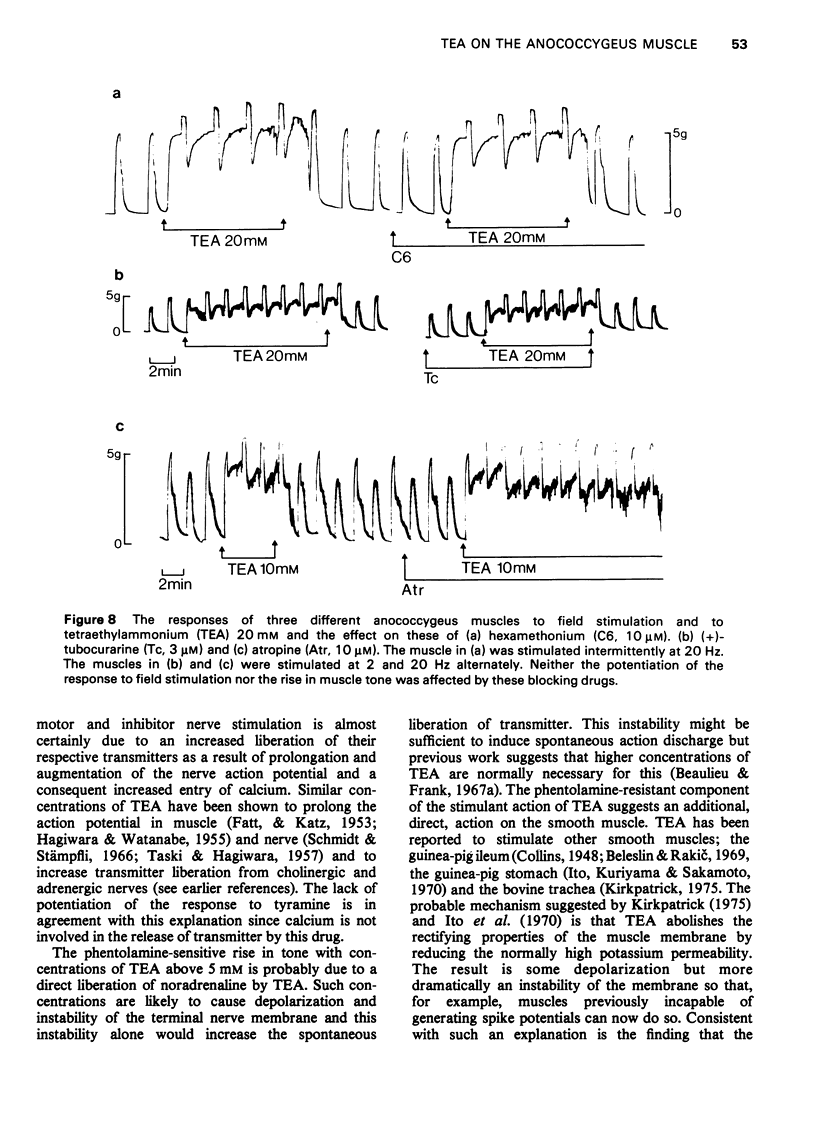
1 Tetraethylammonium chloride (TEA) 0.125 mM to 20 mM potentiates the response of the anococcygeus muscle to field stimulation of the motor adrenergic nerves without affecting the response to noradrenaline suggesting a pre-synaptic origin of potentiation. The potentiation is greatest at low, submaximal, frequencies (2 Hz) of stimulation and only slight at the higher frequency of 20 Hz. This difference is due to the restraint imposed on the demonstration of potentiation by maximal or near maximal motor responses since reduction of the mechanical response at 20 Hz by either phentolamine (post-synaptic block) or guanethidine (pre-synaptic block) resulted in a great increase in potentiation of the response at this frequency. 2 TEA in concentrations up to 1 mM similarly potentites the response to inhibitory nerve stimulation and again the greatest effect is at low frequencies. Higher concentrations (5-20 mM) progressively depress the inhibitory response. It is suggested that TEA may specifically antagonize the post-synaptic action of the inhibitory transmitter and that at higher concentrations of TEA this effect dominates the pre-synaptic action in increasing transmitter release. 3 TEA has no effect on the motor response to tyramine. 4 TEA (5-20 mM) causes a maintained rise in muscle tone. Part of this is abolished by phentolamine but part is resistant. A similar muscle stimulant action of TEA is observed in muscles from rats previously treated with 6-hydroxydopamine in which indirect sympathomimetic drugs and field stimulation could no longer produce a motor response. These results suggest that part of the motor effect of TEA is due to an increased spontaneous release of noradrenaline and part to a direct action on the muscle. 5 TEA 0.125 mM to 20 mM antagonize the stimulant action of carbachol. Dose-response curves show a parallel shift to the right with no change in the maximum response suggesting a competitive atropine-like action. Such an effect has previously been reported in amphibian tissue but not so far as we can determine in mammalian preparations. 6 The possible mode of action of TEA is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG C. M., BINSTOCK L. ANOMALOUS RECTIFICATION IN THE SQUID GIANT AXON INJECTED WITH TETRAETHYLAMMONIUM CHLORIDE. J Gen Physiol. 1965 May;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEVAN J. A. Action of tetraethylammonium chloride on the sympathetic ganglia-pulmonary artery preparation. J Pharmacol Exp Ther. 1963 May;140:193–198. [PubMed] [Google Scholar]
- Beaulieu G., Frank G. B., Inoue F. Tetraethylammonium-induced contractions of frog's skeletal muscle. II. Effects on intramuscular nerve endings. Can J Physiol Pharmacol. 1967 Sep;45(5):833–844. doi: 10.1139/y67-098. [DOI] [PubMed] [Google Scholar]
- Beaulieu G., Frank G. B. Tetraethylammonium-induced contractions of frog's skeletal muscle. 3. Mechanism of action by calcium release. Can J Physiol Pharmacol. 1967 Sep;45(5):845–855. doi: 10.1139/y67-099. [DOI] [PubMed] [Google Scholar]
- Beleslin D. B., Rakić M. M. Stimulant action of tetraethylammonium on the peristaltic reflex of the guinea-pig isolated ileum. Br J Pharmacol. 1969 Sep;37(1):245–250. doi: 10.1111/j.1476-5381.1969.tb09541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLIER B., EXLEY K. A. MECHANISM OF THE ANTAGONISM BY TETRAETHYLAMMONIUM OF NEUROMUSCULAR BLOCK DUE TO D-TUBOCURARINE OR CALCIUM DEFICIENCY. Nature. 1963 Aug 17;199:702–703. doi: 10.1038/199702a0. [DOI] [PubMed] [Google Scholar]
- Creed K. E., Gillespie J. S., Muir T. C. The electrical basis of excitation and inhibition in the rat anoccygeus muscle. J Physiol. 1975 Feb;245(1):33–47. doi: 10.1113/jphysiol.1975.sp010833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. The electrical properties of crustacean muscle fibres. J Physiol. 1953 Apr 28;120(1-2):171–204. doi: 10.1113/jphysiol.1953.sp004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S. The rat anococcygeus muscle and its response to nerve stimulation and to some drugs. Br J Pharmacol. 1972 Jul;45(3):404–416. doi: 10.1111/j.1476-5381.1972.tb08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Tilmisany A. K. Proceedings: The effect of tetraethylammonium (TEA) on the anococcygeus muscle and on its response to motor and to inhibitory nerve stimulation. Br J Pharmacol. 1974 May;51(1):141P–142P. [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., WATANABE A. The effect of tetraethylammonium chloride on the muscle membrane examined with an intracellular microelectrode. J Physiol. 1955 Sep 28;129(3):513–527. doi: 10.1113/jphysiol.1955.sp005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Kuriyama H., Sakamoto Y. Effects of tetraethylammonium chloride on the membrane activity of guinea-pig stomach smooth muscle. J Physiol. 1970 Dec;211(2):445–460. doi: 10.1113/jphysiol.1970.sp009286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENSLER C. J. The anticurare activity of tetraethylammonium ion in the cat. Br J Pharmacol Chemother. 1950 Jun;5(2):204–209. doi: 10.1111/j.1476-5381.1950.tb01008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOKETSU K. Action of tetraethylammonium chloride on neuromuscular transmission in frogs. Am J Physiol. 1958 Apr;193(1):213–218. doi: 10.1152/ajplegacy.1958.193.1.213. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C. T. Excitation and contraction in bovine tracheal smooth muscle. J Physiol. 1975 Jan;244(2):263–281. doi: 10.1113/jphysiol.1975.sp010796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Prat J. C., Puig M., Wakade A. R. Modification of the evoked release of noradrenaline from the perfused cat spleen by various ions and agents. J Physiol. 1972 Mar;221(3):601–615. doi: 10.1113/jphysiol.1972.sp009770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhaus A. L., Prichard J. W. Calcium dependent action potentials produced in leech Retzius cells by tetraethylammonium chloride. J Physiol. 1975 Mar;246(2):351–369. doi: 10.1113/jphysiol.1975.sp010894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H., Stämpfli R. Die Wirkung von Tetraäthylammoniumchlorid auf den einzelnen Ranvierschen Schnürring. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;287(4):311–325. [PubMed] [Google Scholar]
- Stjärne L. Michaelis-Menten kinetics of calcium dependence of sympathetic neurotransmitter secretion in guinea-pig vas deferens: comparison between effects of phentolamine and of tetraethylammonium. Acta Physiol Scand. 1973 Sep;89(1):142–144. doi: 10.1111/j.1748-1716.1973.tb05505.x. [DOI] [PubMed] [Google Scholar]
- TASAKI I., HAGIWAR A. S. Demonstration of two stable potential states in the squid giant axon under tetraethylammonium chloride. J Gen Physiol. 1957 Jul 20;40(6):859–885. doi: 10.1085/jgp.40.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H., Haefely W., Staehelin H. Potentiation by tetraethylammonium of the response of the cat spleen to postganglionic sympathetic nerve stimulation. J Pharmacol Exp Ther. 1967 Sep;157(3):532–540. [PubMed] [Google Scholar]


