Abstract
The seed oil derived from the tung (Aleurites fordii Hemsl.) tree contains approximately 80% α-eleostearic acid (18:3Δ9cis,11trans,13trans), an unusual conjugated fatty acid that imparts industrially important drying qualities to tung oil. Here, we describe the cloning and functional analysis of two closely related Δ12 oleate desaturase-like enzymes that constitute consecutive steps in the biosynthetic pathway of eleostearic acid. Polymerase chain reaction screening of a tung seed cDNA library using degenerate oligonucleotide primers resulted in identification of two desaturases, FAD2 and FADX, that shared 73% amino acid identity. Both enzymes were localized to the endoplasmic reticulum of tobacco (Nicotiana tabacum cv Bright-Yellow 2) cells, and reverse transcriptase-polymerase chain reaction revealed that FADX was expressed exclusively within developing tung seeds. Expression of the cDNAs encoding these enzymes in yeast (Saccharomyces cerevisiae) revealed that FAD2 converted oleic acid (18:1Δ9cis) into linoleic acid (18:2Δ9cis,12cis) and that FADX converted linoleic acid into α-eleostearic acid. Additional characterization revealed that FADX exhibited remarkable enzymatic plasticity, capable of generating a variety of alternative conjugated and Δ12-desaturated fatty acid products in yeast cells cultured in the presence of exogenously supplied fatty acid substrates. Unlike other desaturases reported to date, the double bond introduced by FADX during fatty acid desaturation was in the trans, rather than cis, configuration. Phylogenetic analysis revealed that tung FADX is grouped with Δ12 fatty acid desaturases and hydroxylases rather than conjugases, which is consistent with its desaturase activity. Comparison of FADX and other lipid-modifying enzymes (desaturase, hydroxylase, epoxygenase, acetylenase, and conjugase) revealed several amino acid positions near the active site that may be important determinants of enzymatic activity.
Conjugated fatty acids are naturally occurring compounds that have specialized uses in nutraceutical and industrial applications. For example, conjugated linoleic acid (CLA) is a potent anticancer compound present in foods derived from ruminant animals (Belury, 2002). This bioactive fatty acid (predominantly the 18:2Δ9cis,11trans isomer) is synthesized by rumen bacteria and then absorbed by the animal and concentrated in milk fat or adipose tissue. Rumen bacteria also synthesize 18:1Δ11trans, which can be absorbed and then desaturated by an animal stearoyl-CoA desaturase to produce CLA (Corl et al., 2001). Conjugated fatty acids such as α-eleostearic acid (18:3Δ9cis,11trans,13trans) have recently shown promise for anticancer applications (Igarashi and Miyazawa, 2000; Kohno et al., 2002), as well as serum lipid-lowering effects in mammals (Koba et al., 2002). Oils containing α-eleostearic acid may also be used for industrial drying applications. Tung oil, which is derived from seeds of the tung tree (Aleurites fordii Hemsl.), is commonly used in formulations of inks, dyes, coatings, and resins because of its unique ability to dry to a clear, hard finish (Sonntag, 1979). The polymer formed by tung oil results from oxidation of α-eleostearic acid, which accounts for approximately 80% of the total fatty acids in the oil.
At least three different mechanisms have been documented for the biosynthesis of conjugated fatty acids. In addition to the Δ9 desaturation of a Δ11 fatty acid substrate described above for CLA, conjugated fatty acids can also be synthesized by isomerization mechanisms. For instance, CLA can be produced in rumen bacteria by isomerization of linoleic acid (18:2Δ9cis,12cis; Griinari and Bauman, 1999), and isomerase reactions have been described for production of conjugated fatty acids in marine algae (Zheng et al., 2002). A third mechanism for generating conjugated fatty acids, which is typical of higher plants, involves fatty acid oxidation and bond rearrangement. For example, radiolabeling studies with developing bitter gourd seeds revealed that linoleic acid (18:2Δ9cis,12cis) was modified at the Δ12 position to produce α-eleostearic acid (18:3Δ9cis,11trans,13trans; Liu et al., 1997), whereas in marigold, the Δ9 position of linoleic acid was modified to produce calendic acid (18:3Δ8trans,10trans,12cis; Crombie and Holloway, 1985).
Several enzymes have recently been identified in a variety of higher plants that are capable of synthesizing conjugated fatty acids such as calendic, α-eleostearic, and α-parinaric acids (18:4Δ9cis,11trans,13trans,15cis; Cahoon et al., 1999, 2001; Qiu et al., 2001). Interestingly, these so-called “conjugase” enzymes are closely related in terms of their overall amino acid identity to the Δ12 oleate desaturase (FAD2) family of enzymes. It is now apparent that FAD2 has played an important role in the diversification of fatty acid structures, because several other FAD2-like enzymes have been identified in various higher plant seeds that are capable of synthesizing hydroxy, epoxy, and acetylenic fatty acids (for review, see Shanklin and Cahoon, 1998).
The availability of cDNAs encoding divergent FAD2-like enzymes enables the production of transgenic plants containing industrially and/or nutraceutically important fatty acids. However, emerging evidence indicates that generation of such modified plants, capable of accumulating high amounts of an exotic fatty acid in their seeds, may not be as simple as the ectopic expression of a single gene coding for an enzyme involved in the biosynthesis of an unusual fatty acid (Voelker and Kinney, 2001). Additional enzymes and cofactors from the endogenous plant species may be required for efficient production and accumulation of exotic fatty acids in transgenic organisms.
To begin to address this problem, we are attempting to identify all of the components involved in eleostearic acid biosynthesis and accumulation in tung seeds. Here, we report the identification and functional analysis of a tung Δ12 oleate desaturase (FAD2), which synthesizes linoleic acid, and a divergent tung FAD2 termed FADX, which converts linoleic acid into α-eleostearic acid. Detailed analysis of FADX substrate/product relationships indicated that the enzyme exhibits remarkable plasticity, capable of synthesizing a wide variety of unusual conjugated and desaturated fatty acid products. To our knowledge, this is the first plant enzyme described to date that exhibits both conjugase and desaturase activities. Furthermore, the desaturase activity of FADX is novel compared with other plant desaturases in that the double bond introduced by FADX is in the trans, rather than cis configuration. Amino acid sequence comparison of tung FADX with other conjugases and lipid-modifying enzymes provides insight to the evolution of enzyme structure/function relationships. The significance of these findings and the evolution of plant fatty acid diversity are discussed.
RESULTS
Sequence Features of Tung FAD2 and FADX Proteins
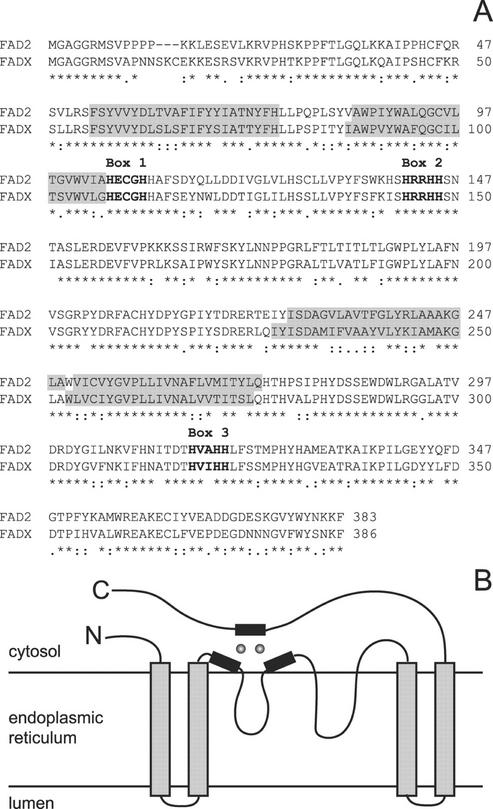
Two cDNAs were isolated from a tung seed cDNA library (for details, see “Materials and Methods”) that possessed extensive similarity to extant FAD2 enzymes identified from other plant species. The cDNAs encoded proteins of 383 and 386 amino acids that were 73% identical to each other and 78% and 69% identical to Arabidopsis FAD2, respectively. The cDNA with higher sequence identity to the FAD2 enzyme family was designated FAD2, whereas the slightly more divergent sequence was called FADX (Fig. 1A). Both FAD2 and FADX polypeptide sequences exhibited several features conserved among the desaturase family including several potential membrane-spanning domains and three highly conserved His-rich boxes. A model has been proposed in which these membrane-spanning segments function to anchor the enzyme in the endoplasmic reticulum (ER) membrane, with the majority of the protein, including the active site His boxes, exposed on the cytosolic side of the ER (Fig. 1B; Stukey et al., 1990; Shanklin et al., 1994; Dyer and Mullen, 2001).
Figure 1.
Comparison of tung FAD2 and FADX polypeptide sequences and topological model of membrane-bound fatty acid desaturases. A, Sequence alignment of tung FAD2 and FADX showing the presence of predicted membrane-spanning domains (gray) and highly conserved His boxes (bold). B, Topological model of fatty acid desaturases in which the enzyme is anchored in the ER membrane by two pairs of closely spaced membrane-spanning domains, with the three His boxes oriented on the cytosolic side of the membrane for coordination of two iron atoms (gray circles) at the active site center.
Subcellular Localization of Tung FAD2 and FADX
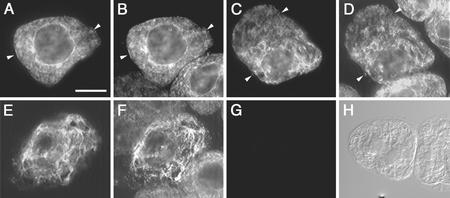
To determine whether tung FAD2 and FADX were localized in the ER, we examined the immunofluorescence staining patterns in tobacco (Nicotiana tabacum cv Bright-Yellow 2 [BY-2]) suspension-cultured cells transiently transformed with DNA constructs coding for either myc epitope-tagged FAD2 or FADX. Figure 2 shows that cells expressing either myc-FAD2 or myc-FADX exhibited reticular staining patterns identical to the staining patterns attributable to endogenous calreticulin, a bona fide ER marker protein (Fig. 2, A–D). However, in approximately 50% of the cells transformed with myc-FADX, the ER exhibited an altered, more punctate, and often clustered morphology that was not detected in neighboring untransformed cells or other myc-FADX-transformed cells (Fig. 2, compare E, F, and C). This morphology was also not observed in any of the cells transformed with myc-FAD2 nor in cells transformed with Arabidopsis FAD2 or Brassica sp. FAD3 (Dyer and Mullen, 2001). These data collectively indicated that both tung FAD2 and FADX were localized exclusively in the ER and that on occasion, transient overexpression of FADX caused a dramatic rearrangement of the ER.
Figure 2.
Immunofluorescence localizations of tung FAD2 and FADX transiently expressed in tobacco BY-2 suspension cultured cells. BY-2 cells were biolistically bombarded with DNA encoding either myc-tagged FAD2 or FADX cDNAs, allowed to recover for 20 to 24 h, and then formaldehyde-fixed and processed for indirect immunofluorescence microscopy. A and B, Colocalization of myc-FAD2 (A) and endogenous calreticulin (B) within the ER of a transiently transformed cell. Arrows denote examples of colocalization. Note the presence of endogenous calreticulin staining in neighboring untransformed cells (see B, D, and F). C and D, Colocalization of myc-FADX (C) and calreticulin (D) in the ER of a transformed cell. Arrows denote examples of colocalization. E and F, Altered ER morphology in a cell expressing FADX (E) showing punctate and reticular staining patterns of the ER, as evidenced by altered calreticulin staining in the same transformed cell (F). G, Control cells showing lack of fluorescence in myc-FAD2-bombarded cells when primary anti-myc antibodies were omitted. H, Differential interference contrast image of the cells present in G. Bar in A = 10 μm.
Tissue-Specific Expression of Tung FADX
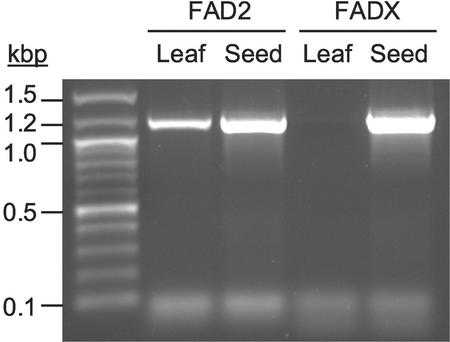
To investigate gene expression patterns of tung FAD2 and FADX, RNA was extracted from both tung seeds and leaves and used in reverse transcriptase (RT)-PCR reactions containing primers specific for each cDNA. As shown in Figure 3, tung FAD2 was expressed in both leaf and seed tissue, whereas FADX was expressed exclusively within developing seeds. Because α-eleostearic acid is present only in seed tissue, tung FADX may encode a divergent FAD2 enzyme responsible for eleostearic acid biosynthesis.
Figure 3.
Expression patterns of tung FAD2 and FADX genes in tung leaves or seeds. RNA was extracted from tung leaves or seeds and used in RT-PCR reactions programmed with primers specific for either FAD2 or FADX cDNAs. Expected product sizes, inclusive of the entire FAD2 or FADX ORFs, were approximately 1.2 kb.
Functional Analysis of FAD2 and FADX Expressed in Yeast (Saccharomyces cerevisiae)
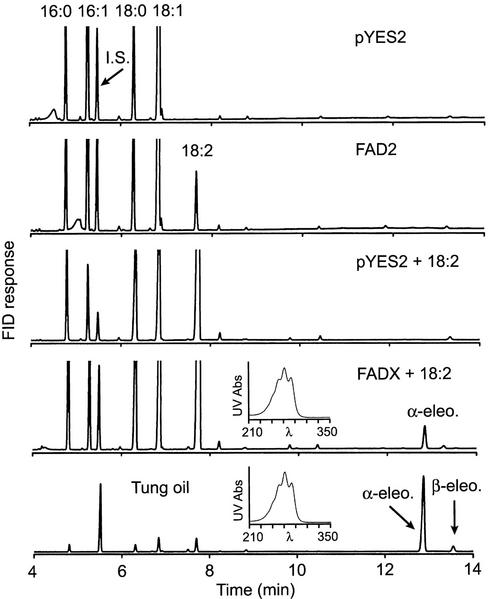
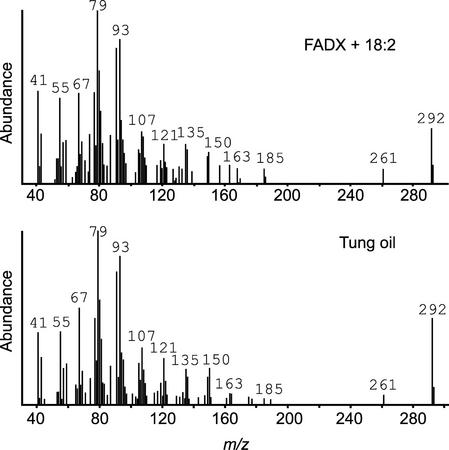
Yeast is an excellent model system for analyzing the function of plant lipid-modifying enzymes, because these yeast cells lack the polyunsaturated or exotic fatty acids typically found in plant oils. To determine the function of tung FAD2 and FADX, the respective cDNAs were expressed in yeast, lipids were extracted, and fatty acid composition was examined by gas chromatography (GC), GC/mass spectrometry (MS), and HPLC/photodiode array detector (PDA) analyses of fatty acid methyl esters (FAME). Expression of FAD2 in yeast cells resulted in the appearance of a new fatty acid whose methyl ester had an identical GC retention time to linoleic acid (18:2Δ9cis,12cis) methyl ester (Fig. 4, FAD2). The mass spectrum of the new FAME was characterized by an abundant molecular ion at m/z = 294 (data not shown), consistent with its identification as methyl linoleate. On the other hand, expression of FADX in yeast cells, cultivated in the presence of exogenously supplied linoleic acid, resulted in the appearance of a new FAME with an identical GC retention time (Fig. 4, FADX + 18:2) and mass spectrum (Fig. 5) compared with α-eleostearic acid (18:3Δ9cis,11trans,13trans) methyl ester. Additional support for identification was obtained by HPLC/PDA, which demonstrated that the new FAME derived from yeast cells had an identical HPLC retention time and UV spectrum compared with native α-eleostearic acid methyl ester derived from tung oil (Fig. 4, insets). Taken together, these data indicated that tung FAD2 synthesized linoleic acid (18:2Δ9cis,12cis) and that FADX converted linoleic acid to α-eleostearic acid (18:3Δ9cis,11trans,13trans).
Figure 4.
Functional analysis of tung FAD2 and FADX expressed in yeast. Yeast cells harboring a control plasmid (pYES2), high-copy plasmid bearing tung FAD2 (FAD2), or high-copy plasmid containing tung FADX (FADX) were cultured in the absence or presence of exogenous linoleic acid (18:2), and then cells were harvested, lipids were extracted, and fatty acid composition was determined by GC analysis of FAME. Specific plasmids and growth conditions are shown on each panel. Labeled peaks correspond to the methyl esters of palmitic (16:0), palmitoleic (16:1), stearic (18:0), oleic (18:1), and linoleic (18:2) acids. Methyl heptadecanoate (17:0) was included as an internal standard (I.S.). The GC chromatogram of FAME derived from tung oil is also shown (Tung oil) to illustrate the positions of α-eleostearic (18:3Δ9cis,11trans,13trans) and β-eleostearic (18:3Δ9trans,11trans,13trans) acid methyl esters. Insets, UV spectra derived from HPLC/PDA analyses of FAME, which illustrate the presence of α-eleostearic acid in each sample. Conjugated FAME were not detected in any of the other samples.
Figure 5.
Mass spectra of α-eleostearic acid methyl ester derived from yeast cells expressing FADX (top) or tung oil (bottom).
Co-Expression of the Tung Enzymes Reveals Novel Desaturase Functions of FADX
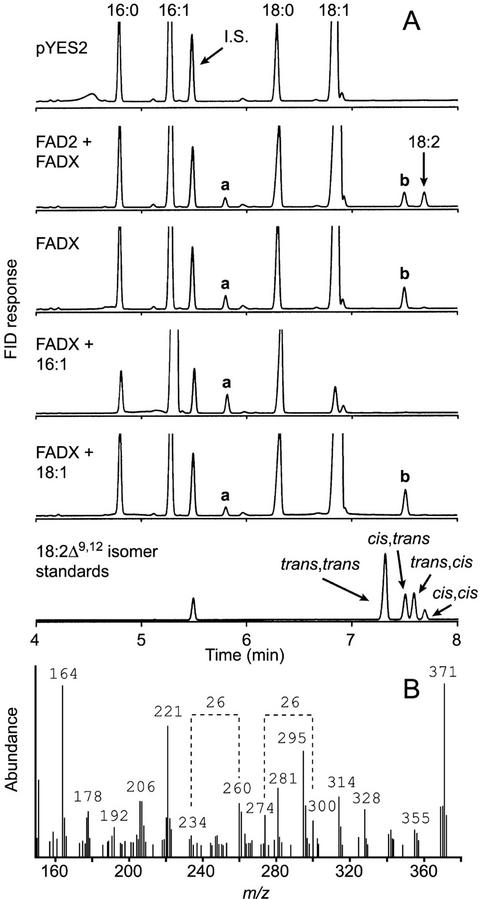
Co-expression of tung FAD2 and FADX in yeast cells resulted in the appearance of both linoleic and α-eleostearic acids (Fig. 6A, FAD2 + FADX; the eleostearic acid methyl ester peak is not shown to allow expansion of the x axis in the region of newly identified peaks a and b). The amount of α-eleostearic acid synthesized during co-expression of the enzymes, however, was much lower (0.3% [w/w] total fatty acids) than when cells containing FADX alone were cultivated in the presence of exogenously supplied linoleic acid (2.1% [w/w]). These data suggested that substrate availability was a limiting factor for α-eleostearic acid biosynthesis by FADX.
Figure 6.
FADX-dependent synthesis of unusual desaturated fatty acids in yeast. A, Yeast cells containing a control plasmid (pYES2), FAD2 and FADX plasmids (FAD2 + FADX), or the FADX plasmid alone (FADX) were cultured in the absence or presence of palmitoleic (16:1) or oleic (18:1) acid. Cells were harvested, lipids were extracted, and fatty acid composition was determined by GC analysis of FAME. Specific introduced plasmids and growth conditions are shown on each panel. The GC chromatogram of a mixture of the four isomers of linoleic acid methyl esters is included. Peaks a and b were identified as 16:2Δ9cis,12trans and 18:2Δ9cis,12trans, respectively (for additional details, see “Results”). B, Mass spectrum of the picolinyl ester derivative of fatty acid corresponding to peak b. Mass loss of 26, represented by fragments of 234 and 260 as well as 274 and 300, is diagnostic of double bonds in the Δ9 and Δ12 positions of the 18:2 picolinyl ester.
Two additional fatty acids (corresponding to peaks a and b in Fig. 6A) were surprisingly detected in yeast lipids that were not observed in previous experiments presented in Figure 4. We suspected that because the amount of endogenous linoleic acid in these yeast cells was low, the additional peaks were attributable to the activity of FADX on other abundant fatty acids present in yeast cells. Evidence in support of this hypothesis was obtained by expression of FADX in the absence of FAD2 or exogenously supplied linoleic acid. GC results indicated that peaks a and b were still present, whereas no α-linoleic acid or α-eleostearic acid was detected (Fig. 6A, FADX).
Because the two most abundant fatty acids in wild-type yeast cells were oleic (18:1Δ9cis) and palmitoleic acids (16:1Δ9cis), we next investigated whether these fatty acids might serve as substrates for the synthesis of unknowns a and b by modulating their relative intracellular concentrations. Incubation of yeast cells expressing FADX with exogenously supplied palmitoleic acid led to substantial incorporation of this fatty acid into yeast cells, accounting for approximately 79% of total fatty acids (Fig. 6A, FADX + 16:1). Oleic acid was reduced to only 3% under these growth conditions, likely because of both massive incorporation of exogenous palmitoleic acid and suppression of endogenous yeast stearoyl-CoA desaturase activity (Bossie and Martin, 1989). Alteration of fatty acid composition in favor of palmitoleic acid resulted in an increase of unknown peak a and disappearance of peak b (Fig. 6A, FADX + 16:1). On the other hand, incubation of yeast cells expressing FADX with exogenously supplied oleic acid resulted in an increase in oleic acid content and increase in the relative proportion of unknown fatty acid b (Fig. 6A, FADX + 18:1). Taken together, these results indicated that unknown peaks a and b were attributable to activity of FADX on endogenous palmitoleic and oleic acids, respectively.
The mass spectrum of the FAME corresponding to peak a exhibited a prominent molecular ion at m/z = 266, characteristic of a 16:2 methyl ester, whereas the spectrum of unknown b had a molecular ion at m/z = 294, indicative of an 18:2 methyl ester (data not shown). GC/MS analysis of the picolinyl derivatives of a (data not shown) and b (Fig. 6B) indicated that the double bonds were located at the Δ9 and Δ12 positions. Comparison of the GC retention time of unknown b to the retention times of the four possible 18:2Δ9,12 isomers (Fig. 6A) revealed that unknown b was identical to 18:2Δ9cis,12trans. These data collectively identify unknown b as the 18:2Δ9cis,12trans isomer of linoleic acid. By inference, we postulate that unknown peak a represents 16:2Δ9cis,12trans.
These data indicate that tung FADX can desaturate both palmitoleic and oleic acids to produce stereoisomers of 16:2 and 18:2 fatty acids, respectively. The amount of 18:2Δ9cis,12trans produced by FADX in yeast cells was surprisingly similar to the amount of α-eleostearic acid (Table I), indicating that tung FADX is a bifunctional enzyme with robust desaturase/conjugase activity.
Table I.
Conversion of fatty acids in yeast cells expressing tung FAD2 or FADX
| Enzyme Substrate Total Product Total Detection Fatty Acids Fatty Acids | |||||
|---|---|---|---|---|---|
| wt % | wt % | ||||
See “Materials and Methods” for details regarding cell culturing and lipid extraction. Fatty acid percentages (w/w of total cellular fatty acids) are reported as the average and sd of at least three independent experiments.
GC/FID.
GC/MS.
HPLC/PDA.
GC/MS of picolinyl derivatives.
Tentative identification.
Values obtained by analyzing yeast cells expressing FADX and cultured in the absence of any exogenous fatty acids (see Fig. 6A, panel labeled FADX).
Alternative Conjugase Activities of Tung FADX
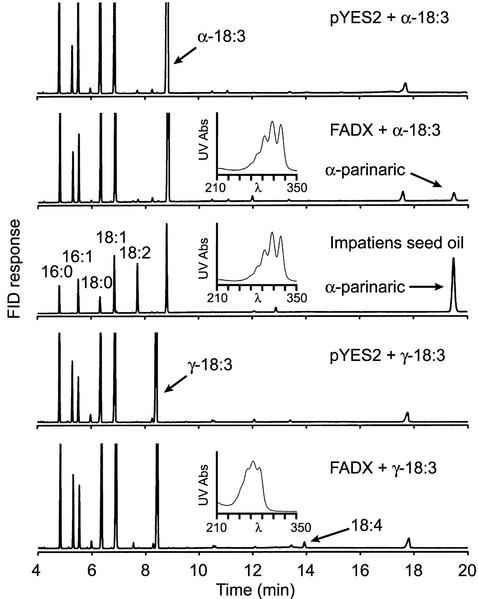
On the basis of the catalytic plasticity of FADX, we next determined whether the enzyme could also synthesize other types of conjugated fatty acids from substrates containing an existing double bond at the Δ12 position. Incubation of yeast cells with α-linolenic acid (18:3Δ9cis,12cis,15cis) resulted in the appearance of a new fatty acid whose methyl ester had an identical GC retention time (Fig. 7, FADX + α-18:3) and mass spectrum (data not shown) compared with α-parinaric acid (18:4Δ9cis,11trans,13trans,15cis) methyl ester. The FAME also had an identical HPLC retention time and UV spectrum compared with α-parinaric acid methyl ester derived from I. balsamina seed oil (Fig. 7, insets). The amount of α-parinaric acid synthesized by FADX was indistinguishable from the amount of α-eleostearic acid (Table I). Cultivation of yeast cells with γ-linolenic acid (18:3Δ6cis,9cis,12cis) resulted in the appearance of a new peak in the GC chromatogram that did not match the retention time of any of our standards (Fig. 7, FADX + γ-18:3). GC/MS indicated that this compound was an 18:4 FAME, and the mass spectrum was similar but not identical to α-parinaric acid methyl ester (data not shown). HPLC/PDA analysis revealed that this FAME contained a trienoic conjugated bond system (Fig. 7, inset), but the λmax of each of the three major peaks was shifted by 1.2 nm compared with α-eleostearic acid. On the basis of these data, we tentatively identified this fatty acid as 18:4Δ6cis,9cis,11trans,13trans. The amount of this fatty acid synthesized by FADX was significantly lower than either α-eleostearic or α-parinaric acids (Table I).
Figure 7.
Synthesis of alternative conjugated fatty acids in yeast cells expressing tung FADX. Yeast cells containing either a control plasmid (pYES2) or FADX plasmid were cultured in the presence of α- or γ-linolenic acid, and then cells were harvested, lipids were extracted, and fatty acid composition was determined by GC analysis of FAME. Specific plasmids and growth conditions are shown on each panel. The GC chromatogram of FAME derived from Impatiens balsamina seed oil is shown to illustrate the position of α-parinaric acid methyl ester. Insets, UV spectra derived from HPLC/PDA analyses of FAME, which illustrate the presence of α-parinaric acid (FADX + α-18:3 and I. balsamina seed oil) or an unknown FAME that contains a trienoic conjugated bond system (tentative assignment 18:4Δ6cis,9cis,11trans,13trans; for additional details, see “Results”). Conjugated FAME were not detected in any of the other samples.
Alternative Fatty Acid Products Are Present in Developing Tung Seeds
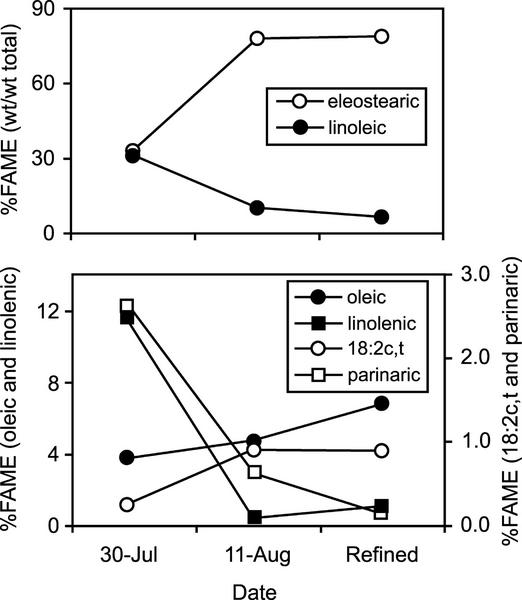
To determine whether any of the alternative conjugated or desaturated fatty acids synthesized by FADX in yeast cells were also present in developing tung seeds, we examined the fatty acid composition of refined tung oil and oil extracted from tung seeds at early and middle stages of seed development. As shown in Figure 8, top panel, there was an inverse relationship between linoleic and α-eleostearic acid content throughout seed development, as expected for efficient conversion of linoleic acid into α-eleostearic acid. Two of the alternative fatty acids, α-parinaric acid and 18:2Δ9cis,12trans, were also detected in tung lipids, and their abundance correlated with the relative content of α-linolenic and oleic acids, respectively (Fig. 8, bottom panel). Developing tung seeds do not contain palmitoleic acid (Fig. 4), and notably, the 16:2Δ9cis,12trans fatty acid synthesized by FADX in yeast cells was not detected in tung tissue.
Figure 8.
Detection of unusual desaturated and conjugated fatty acids in developing tung seeds. Lipids were extracted from tung seeds harvested at early (July 30; approximately 16 weeks after flowering) and mid (August 11; approximately 18 weeks after flowering) stages of seed development (Sell et al., 1948), and then fatty acid composition was determined using GC and compared with the composition of refined tung oil.
Evolutionary Relationships among Fatty Acid-Modifying Enzymes
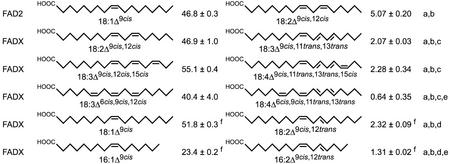
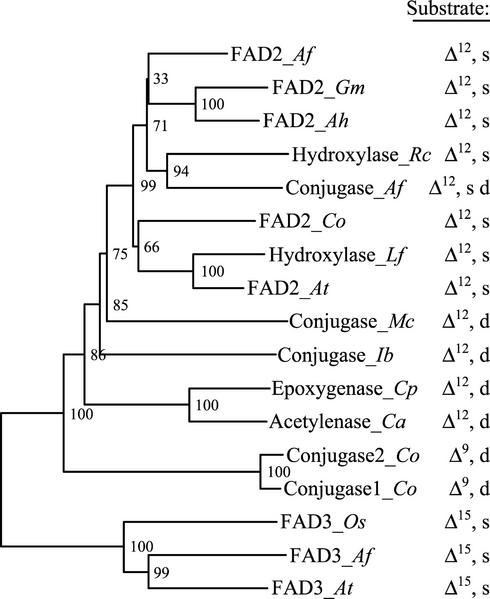
To investigate the evolutionary relationships between tung FADX and other fatty acid-modifying enzymes, we performed a phylogenetic analysis of FAD2s, diverged FAD2s, and FAD3 enzymes from a variety of different plant species (Fig. 9). The results demonstrated that diverged FAD2 enzymes with similar functionality (e.g. hydroxylases and conjugases) do not form separate clades, indicating that these enzymes arose independently several times during evolution. As noted previously (Sperling et al., 2001), it is apparent that the enzymes are grouped primarily by regioselectivity (position of fatty acid modification) and bond status at the position modified by the respective enzymes (Fig. 9). For example, the FAD2 and hydroxylase enzymes each modify the Δ12 position of fatty acids that contain a single bond between C12 and C13, whereas more distantly related enzymes modify the fatty acids that contain a double bond between these same carbon atoms. Although conjugases typically act upon fatty acid substrates containing an existing double bond (Fig. 9), tung FADX was grouped with enzymes that act upon fatty acids containing a saturated, rather than unsaturated, bond at the Δ12 position. This grouping, however, is consistent with the Δ12 desaturase activity of tung FADX described above.
Figure 9.
Dendrogram showing evolutionary relationships of FAD2, divergent FAD2, and FAD3 polypeptide sequences. The dendrogram represents results from neighbor-joining analysis of amino acid sequences obtained using the ClustalX algorithm. Bootstrap values are shown in percent at nodes. The properties of fatty acid substrates acted upon by each enzyme are shown to the right. Δn signifies the carbon position modified by the enzyme, whereas “s” or “d” designates an existing single or double bond within the fatty acid substrate between carbons Δn and Δn+1. The enzymes and GenBank accession numbers used for the analysis were: FAD2 from tung (FAD2_Af), AF525534; FAD2 from soybean (Glycine max; FAD2_Gm), L43920; FAD2 from peanut (Arachis hypogaea; FAD2_Ah), AF030319; Hydroxylase from castor bean (Ricinus communis; Hydroxylase_Rc), U22378; Conjugase from tung (Conjugase_Af), AF525535; FAD2 from Calendula officinalis (FAD2_Co), AF343065; Hydroxylase from Lesquerella fendleri (Hydroxylase_Lf), AF016103; FAD2 from Arabidopsis (FAD2_At), L26296; Conjugase from Momordica charantia (Conjugase_Mc), AF182521; Conjugase from I. balsamina, (Conjugase_Ib), AF182520; Epoxygenase from Crepis palaestina (Epoxygenase_Cp), Y16283; Acetylenase from Crepis alpina (Acetylenase_Ca), Y16285; Conjugase 2 from C. officinalis (Conjugase2_Co), AF310156; Conjugase 1 from C. officinalis (Conjugase1_Co), AF310155; FAD3 from rice (Oryza sativa; FAD3_Os), D78506; FAD3 from tung (FAD3_Af), AF047172; and FAD3 from Arabidopsis (FAD3_At), D26508.
DISCUSSION
Here, we describe the functional analysis of two fatty acid-modifying enzymes from developing tung seeds that represent consecutive steps in the metabolic pathway of tung oil biosynthesis: a Δ12 oleate desaturase (FAD2) that converts oleic acid (18:1Δ9cis) into linoleic acid (18:2Δ9cis,12cis) and a divergent FAD2 enzyme (FADX) that modifies the Δ12 double bond of linoleic acid to produce eleostearic acid (18:3Δ9cis,11trans,13trans). Consistent with their roles in fatty acid modification, both of these enzymes were localized to the ER of tobacco BY-2 cells. However, the expression of FADX surprisingly was occasionally associated with dramatic changes in morphology of the ER (see Fig. 2, E and F). It is currently unknown whether these alterations in ER were induced by some physical aspect of the FADX protein structure or by the possible presence of large amounts of α-eleostearic acid in ER membranes.
In addition to converting linoleic acid into eleostearic acid, the FADX enzyme exhibited a remarkable array of enzymatic activities when expressed in yeast. Perhaps most surprising was the robust fatty acid desaturase activity of the enzyme, introducing a double bond at the Δ12 position of both palmitoleic (16:1Δ9cis) and oleic (18:1Δ9cis) acids. The expression of other so-called “normal” plant FAD2 enzymes in yeast is often associated with Δ12 desaturation of palmitoleic and oleic acids to produce hexadecadienoic (16:2Δ9cis,12cis) and linoleic (18:2Δ9cis,12cis) acids, respectively (Covello and Reed, 1996). Tung FADX has apparently retained the ability to act upon these same fatty acid substrates. However, unlike other “normal” FAD2 enzymes, the double bond introduced by FADX at the Δ12 position is in the trans, rather than cis, configuration. Although Sperling et al. (1998) previously reported a sphingolipid desaturase that was capable of synthesizing double bonds in both the cis and trans orientations, tung FADX is the first enzyme reported that stereoselectively introduces a trans double bond into fatty acid structures. It is noteworthy to mention that the 18:2Δ9cis,12trans stereoisomer of linoleic acid occurs at low levels (1%–3%) in the seed oils of tung (Fig. 8), Dimorphotheca sinuata, and Crepis rubra (Morris and Marshall, 1966). Each of these plants accumulates exotic fatty acids, and our results suggest that a divergent FAD2 is likely responsible for the synthesis of the 18:2Δ9cis,12trans present in the oils.
Although no other conjugase described to date has been reported to display both desaturase and conjugase activities, several other divergent FAD2 enzymes have demonstrated bifunctionality (Broun et al., 1998a; Sperling et al., 2000). For example, the fatty acid hydroxylase from L. fendleri exhibits both hydroxylase and desaturase activity, introducing either a double bond or hydroxyl group at the Δ12 position of oleic acid (Broun et al., 1998a). The bifunctionality of tung FADX, however, is quite surprising in light of the proposed differences in the mechanism of fatty acid desaturation and conjugated bond formation. In a typical desaturation reaction, single hydrogen atoms are removed from adjacent carbon atoms (e.g. C12 and C13), and the hydrogens are transferred to an oxygen atom held at the reaction center (along with two electrons obtained from the microsomal electron transport chain) to form water as a by-product (Shanklin and Cahoon, 1998). The mechanism of conjugated double bond formation features removal of hydrogen atoms from nonadjacent carbon atoms that are separated by an existing double bond (Crombie and Holloway, 1985; Hamberg, 1992; Rodriguez et al., 2002). Migration of the existing double bond results in the formation of the two conjugated double bonds (Fritsche et al., 1999). It will now be interesting to determine whether FADX uses a single-hydrogen abstraction method to produce both desaturated and conjugated fatty acid products or whether the enzyme has enough flexibility to employ either method of hydrogen abstraction, depending on the type of fatty acid substrate it encounters.
Amino Acid Properties Near the Active Site Pocket of FADX
When considering the functional properties of tung FADX and the phylogenetic data presented in Figure 9, it is apparent that FADX is a relatively newly evolved fatty acid conjugase and that there has not yet been sufficient selective pressure to eliminate the fatty acid desaturase activity of FADX. Therefore, tung FADX may be an excellent enzyme to study the structure/function relationships associated with various enzyme activities. It is clear that the FADX active site pocket contains structural features that allow both desaturation and conjugation reactions to occur.
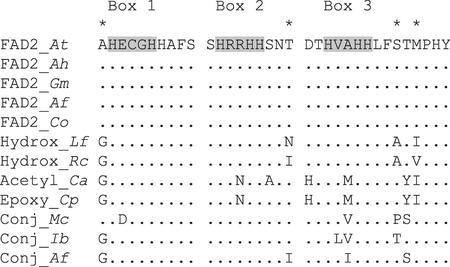
In a previous study, Somerville's group compared the polypeptide sequences of fatty acid desaturase and hydroxylase enzymes and identified seven amino acid positions that were important determinants of enzyme activity (Broun et al., 1998b). Four of these amino acid positions were notably in the immediate vicinity of the His boxes thought to coordinate the two iron atoms at the active site center of the enzyme (positions marked by asterisks in Fig. 10). The contribution of these four positions to catalytic specificity was explored in greater detail by Broadwater et al. (2002), who showed that replacement of FAD2 amino acids with their hydroxylase counterparts at these amino acid positions conferred robust hydroxylase activity to FAD2. To determine whether we could identify specific changes in amino acid sequence that might define fatty acid conjugase activity, the sequences of plant enzymes in the immediate vicinity of the three His active site boxes were compared (Fig. 10). Only FAD2-related enzymes that modified the Δ12 position of fatty acids were analyzed to exclude amino acid differences that might be associated with relative positioning of fatty acids within the active site, rather than type of chemistry performed by the enzymes.
Figure 10.
Comparison of His boxes from various classes of enzymes. The amino acid sequence of Arabidopsis FAD2 is shown at the top, with His boxes shaded gray. Amino acids identical to Arabidopsis FAD2 are represented by single dots. The enzymes are grouped according to functionality rather than evolutionary relationships, and each enzyme modifies the Δ12 position of fatty acid substrates. Amino acid positions previously shown to be important determinants of desaturase or hydroxylase activity (Broun et al., 1998b) are marked by asterisks. Gene designations and GenBank accession numbers are provided in the legend of Figure 9.
These types of comparisons revealed two amino acid positions, represented by Ala residues in the first and third His boxes of FAD2, as possible determinants of enzyme activity. The first Ala near box 1 of FAD2 is replaced by a smaller Gly residue in most diverged FAD2s, with the exception of the M. charantia conjugase. This enzyme, however, contains an Asp rather than Glu in box 1, resulting in a similar reduction in amino acid side chain volume in the immediate vicinity of the first His residue. The second Ala position of FAD2, in box 3, is replaced by larger hydrophobic amino acids in the majority of diverged FAD2s. The conjugases each contain a branched β-carbon amino acid at this position (Ile or Val), whereas the epoxygenase and acetylenase contain a Met, and the hydroxylases retain the original Ala residue. It is not currently known whether the increase in side chain volume at the second Ala position compensates for the decrease in hydrophobic volume at the first position, a condition often encountered in the hydrophobic core of closely related proteins (Richardson and Richardson, 1989). However, the close apposition of the His boxes in existing models of desaturase enzyme structures (Fig. 1B) suggests that these two Ala positions might physically interact (Fig. 1B). Subtle changes in the local environment because of amino acid substitutions at these positions could potentially alter substrate/product relationships.
A second motif located just after box 3, consisting of Ser-Thr-Met, is perfectly conserved among the FAD2 enzymes and altered to varying degrees in the divergent FAD2 enzymes. The tung conjugase, which is the only conjugase described to date that retains fatty acid desaturase activity, is also the only conjugase that contains both the Ser and Met residues that were previously shown to be important determinants of FAD2 desaturase activity. These data suggest specific amino acid positions that might collectively determine the substrate product relationships of the lipid-modifying enzymes.
Implications for Plant Fatty Acid Diversification
Our results demonstrate that a single divergent enzyme, tung FADX, can act upon each of the common unsaturated fatty acids in plants (oleic, linoleic, and linolenic acids) to produce three different unusual fatty acids (18:2Δ9cis,12trans, α-eleostearic, and α-parinaric acids, respectively). Each of these unusual products is known to accumulate in the seed oils of various plant species (Smith, 1970). In yeast cells, the particular unusual fatty acid synthesized by FADX was influenced primarily by substrate availability, suggesting that FADX might generate completely different fatty acid products depending upon the metabolic context in which the enzyme evolves and operates. The fact that a single enzyme can synthesize different types of fatty acids suggests a mechanism for accelerated evolution of plant fatty acid diversity, because production of various fatty acids is dependent on metabolic context of the enzyme, rather than a one to one relationship between the enzyme and a specific product. We are currently testing this hypothesis by expressing tung FADX in a variety of plant species that differ in endogenous fatty acid composition. Understanding the relationships between substrate availability, product formation, and the involvement of downstream acyltransferase enzymes will greatly assist in the development of transgenic organisms tailored for the production of desirable lipid compounds.
MATERIALS AND METHODS
Cloning and Vector Construction
Tung (Aleurites fordii Hemsl.) seeds were collected from the orchards of the American Tung Oil Corporation near Lumberton, Mississippi. RNA was extracted from developing tung seeds using the method of Bugos et al. (1995) and used for construction of a λTriplEx cDNA library (BD Biosciences Clontech, Palo Alto, CA). To identify FAD2-like cDNAs, PCR reactions were carried out using the tung seed cDNA library as template and degenerate primers encoding conserved regions of FAD2-like enzymes. The primer set was F2Xtop, 5′-AARAARGCNATHCCICCICAYTGYTT-3′, and F2Xbot, 5′-TGRTARTCIGARAAIGCRTGRTGNCC-3′, which corresponded to peptides KKAIPPHCF and CGHHAFSDYQ of Arabidopsis FAD2, respectively. Products of expected size (approximately 250 bp) were cloned into pCR 2.1-TOPO (Invitrogen, Carlsbad, CA) and DNA sequences of several clones were determined using an ABI Prism 377 DNA Sequencer (Applied Biosystems, Foster City, CA). Two similar but nonidentical fragments were identified that encoded FAD2-like sequences. The fragments were arbitrarily designated tung FAD2–1 and FAD2–2. Each fragment was used to design additional specific primers to obtain the 5′ and 3′ sequences of each cDNA. Each PCR reaction contained one specific FAD2 primer and a second primer that bound to the λ-phage DNA sequence immediately adjacent to the cDNA insert. Specific primers included FAD2–1 forward (5′-GTCCTCACTGGCGTTTGGGTTATAGCA-3′), FAD2–1 reverse (5′-AATTGGCCAGGCCACATAAGA GAGAGGTTG-3′), FAD2–2 forward (5′-CTCCGATAACCTACATCGCTTGGCCT-3′), and FAD2–2 reverse (5′-GACAGGCCAAGCGATGTAGGTTATCGGAG-3′). λ-Primers included the 5′ (5′-CTCGGGAAGCGCGCCATTGTGTTGGT-3′) and 3′ (5′-ATACGACTCACTATAGGGCGAATTGGCC-3′) LD-insert screening amplimers (BD Biosciences Clontech). Identification of the 5′ and 3′ regions of each cDNA permitted assembly of the full cDNA sequences. BLASTP analyses revealed that FAD2–1 was more similar to normal FAD2 enzymes, whereas FAD2–2 was more divergent. Thus, the FAD2–1 cDNA was designated tung FAD2 (GenBank accession no. AF525534), whereas FAD2–2 was designated tung FADX (GenBank accession no. AF525535). The cDNAs of tung FAD2 and FADX shared identity with partial coding sequences proposed to encode tung desaturase and conjugase enzymes, respectively (GenBank accession nos. AY071832 and AY071833). The complete open reading frame (ORF) of FAD2 was amplified from the tung cDNA library using primers FD2-forward (5′-ggaattcgctagcATGGGTGCTGGTGGCAGAATGTCA-3′) and FD2-reverse (5′-tggatccgaattcCCAGAACTTCCAAGCCCTTCACTTTTGC-3′). The FD2-forward primer included EcoRI and NheI sites (lowercase letters) upstream of the start codon, whereas FD2-reverse included EcoRI and BamHI sites. The entire FADX ORF was amplified from the tung cDNA library using primers FDX-forward (5′-gaagcttgtctagaATGGGAGCTGGTGGCCGAATGTCT-3′) and FDX-reverse (5′-aaggatccACTCCATATCTCGTAACAAGGTCAAACCTC-3′). The FDX-forward primer included HindIII and XbaI sites upstream of the start codon, and the FDX-reverse primer contained a 3′ BamHI site. PCR products were subcloned into pCR2.1 and then confirmed by DNA sequencing.
For inducible expression of tung FADX in yeast, the FADX ORF was excised from pCR2.1 by digestion with HindIII/BamHI and then subcloned into similarly digested pYES2, a high-copy yeast shuttle vector (2 micron, URA) containing a Gal-inducible promoter (Invitrogen). To construct a second Gal-inducible expression vector for subcloning tung FAD2, the GAL promoter, polylinker, and CYC terminator cassette were transferred from vector pYES2 to yeast shuttle vector pRS-423 (Christianson et al., 1992). The promoter/terminator cassette was initially amplified from pYES2 using PCR and primers Galcyctop (5′-gggcgcgcGGCCGCAAATTAAAGCCT-3′) and Galcycbot (5′-gggcgcgcCGGATTAGAAGCCGCCGAG-3′). PCR products were cloned into pCR2.1 and verified by automated sequencing. The promoter/terminator cassette was then excised using BssHII, gel purified, and subcloned into similarly prepared pRS-423. The final expression vector was termed pYES2-HIS (2 micron, HIS). The tung FAD2 was subcloned into this vector as an EcoRI fragment, and orientation was determined by restriction mapping.
For immunofluorescence localization of tung FAD2 and FADX in tobacco BY-2 cells (see below), the ORFs were transferred to a plant transformation vector (pRTL2-myc) that contains the CMV 35S promoter, NOS terminator, and an initiator Met codon followed by sequences encoding the myc epitope tag (EQKLISEEDL). The 3′ end of the epitope tag sequence contains a NheI site for the fusion in-frame of passenger sequences. Tung FAD2 and FADX ORFs were fused in-frame to the 3′ end of the epitope tag by subcloning NheI/BamHI and XbaI/BamHI prepared fragments, respectively, into the NheI/BamHI sites of pRTL2-myc. The final plasmids were called pRTL2-mycTF2 and pRTL2-mycTFADX.
Immunofluorescence Microscopy
Tobacco (Nicotiana tabacum cv BY-2) suspension culture cells were transiently transformed with DNA encoding either tung FAD2 or FADX, and then cells were processed for indirect immunofluorescence microscopy as described previously (Dyer and Mullen, 2001). In brief, BY-2 cells were biolistically bombarded with plasmid DNA (pRTL2-mycTF2 or pRTL2-mycFADX), and then cells were allowed to recover for 20 to 24 h at 26°C in the dark. Cells were fixed in 4% (w/v) formaldehyde, washed several times in phosphate-buffered saline, pH 7.4, and then incubated in 0.1% (w/v) pectolyase Y-23 (Seishin Pharmaceutical Co., Tokyo) to facilitate disruption of cell walls. After several washes in phosphate-buffered saline, cells were permeabilized using 0.3% (v/v) Triton X-100 (Sigma-Aldrich, St. Louis), and then primary and secondary antibodies were applied. Primary antibody sources and concentrations used were as follows: mouse anti-myc epitope affinity-purified (Protein A Sepharose) IgGs (1:500; clone 9E10; Covance Research Products, Berkeley, CA); rabbit anti-castor bean calreticulin (1:500; kindly provided by Sean Coughlan [DuPont, Wilmington, DE]; Coughlan et al., 1997). Fluorescent dye-conjugated secondary antibodies included goat anti-mouse Alexa Fluor 488 (1:1,000; Cedar Lane Laboratories Ltd., Ontario, Canada) and goat anti-rabbit rhodamine red-X (1:500; Jackson ImmunoResearch Laboratories Inc., West Grove, PA). Labeled cells were viewed using an Axioskop 2 MOT epifluorescence microscope (Carl Zeiss, Thornwood, PA) with a 63X Plan Apochromat oil immersion objective (Carl Zeiss) and a Retiga 1300 CCD camera (Qimaging, British Columbia, Canada). All images shown were deconvolved and adjusted for brightness and contrast using northern Eclipse 5.0 software (Empix Imaging Inc., Ontario, Canada), and then composed into figures using Adobe Photoshop 5.5 (Adobe Systems, Klamath Falls, OR).
RNA Extraction and RT-PCR Analysis
RNA was extracted from tung leaves using the method of Bugos et al. (1995). RNA was extracted from tung seeds using the Trizol reagent as described by the manufacturer (Invitrogen). RT-PCR was carried out using the Advantage RT-for-PCR kit from BD Biosciences Clontech. In brief, 1 mg of RNA was reverse transcribed, and then the cDNA was used in PCR reactions with FAD2- or FADX-specific primers. The FAD2 primers were TF2for2 (5′-GATGGGTGCTGGTGGCAGAATGTCA-3′) and TF2rev2 (5′-CCAGAACTTCCAAGCCCTTCACTTTTGC-3′), and the FADX primers were FDXfor2 (5′-AATGGGAGCTGGTGGCCGAATGTCT-3′) and FDXrev2 (5′-ACTCCATATCTCGTAACAAGGTCAAACCTC-3′). PCR was conducted for 30 cycles, with a primer-annealing temperature of 70°C. PCR products were analyzed by gel electrophoresis.
Yeast (Saccharomyces cerevisiae) Strains and Culturing Conditions
Yeast strain MMYO11α (McCammon et al., 1990) was used in all studies. Untransformed yeast cells were maintained on yeast peptone dextrose medium (1% [w/v] yeast extract, 2% [w/v] peptone, and 2% [w/v] dextrose) solidified with 2% agar. Plasmids were transfected into yeast cells using the lithium acetate method of Gietz and Woods (1994), and transformants were maintained on synthetic dextrose (2% [w/v] dextrose and 0.67% [w/v] yeast nitrogen base without amino acids) plates containing appropriate auxotrophic supplements. Yeast cells were inoculated into 10 mL of synthetic dextrose medium and grown overnight in an incubator/shaker at 30°C, 300 rpm. The next day, a volume representing 12.5 OD600 units of cells was subjected to centrifugation, the supernatant was removed, and the cells were resuspended in 50 mL of S-Gal medium (2% [w/v] Gal and 0.67% [w/v] yeast nitrogen base without amino acids, appropriate auxotrophic supplements). Free fatty acids, when included, were added to the Gal medium at a final concentration of 0.1% (v/v). Cells were grown for 40 to 42 h at 20°C, 300 rpm using a Forma Scientific model 4580 refrigerated console incubator/shaker (Thermo Forma, Marietta, OH).
Extraction and Analysis of Lipids
Yeast cells were harvested by centrifugation, washed three times with water, and then converted to spheroplasts by enzymatic digestion of cells walls as described (Dyer et al., 1996). Lipids were extracted from spheroplasts using the chloroform/methanol method of Bligh and Dyer (1959). Butylated hydroxytoluene was included as an antioxidant in all organic solvents at a final concentration of 0.01% (w/v). Tung fruits were harvested at early and mid stages of seed development, and oil was extracted using the method of Bligh and Dyer (1959). Seeds of Impatiens balsamina were purchased from Ferry Morse Seed Company (Fulton, KY), and seed oil was extracted by grinding seeds in the presence of anhydrous sodium sulfate and petroleum ether. The volume of petroleum ether was reduced using a rotary evaporator. FAME were prepared using sodium methoxide transesterification. Reactions were carried out in anaerobic and low light environments when preparing FAME of conjugated fatty acids to minimize oxidation/polymerization reactions. FAME reactions were terminated by the addition of saturated sodium chloride, and then FAME were extracted using hexane. FAME were passed over a sodium sulfate column, and then hexane volume was reduced under a gentle stream of argon. Methyl heptadecanoate was included as an internal standard.
FAME were separated, quantitated, and identified using GC/flame ionization detector (FID) and GC/MS. FAME-containing conjugated fatty acids were also characterized using HPLC/PDA. For GC/FID and GC/MS, FAME were analyzed using a GC/FID/MSD system (Hewlett Packard, Palo Alto, CA) with ChemStation software (Agilent, Palo Alto, CA). The GC (model 5890 series II) was equipped with a FID and a mass selective detector (MSD; model 5971A). The GC/FID and GC/MSD were fitted with two identical SP-2380 capillary columns (30 m × 0.25 mm, 0.2 μm film thickness; Supelco, Bellefonte, PA). The operating conditions for GC/FID analysis were set as follows: injector, 220°C; FID, 220°C; flow rate of helium gas as a carrier-linear velocity, 31 cm s−1; split ratio, 70:1; flow rate of air, 385 mL min−1; hydrogen, 32 mL min−1; auxiliary gas (helium), 30 mL min−1. No hold at initial column temperature of 160°C, program rate was 4°C min−1, and final temperature was 200°C and held 5 min at final temperature. For samples containing parinaric acid methyl ester, the final hold step was 15 min. The GC/MSD settings were as follows: injector, 220°C; GC/MS interface, 250°C; carrier gas-linear velocity. 29 cm s−1; split ratio, 50:1. The column temperature was programmed as described for GC/FID. GC/MS analysis of picolinyl ester derivatives was performed as described (Destaillats and Angers, 2002). For HPLC/PDA analysis, FAME were separated on an HPLC system (Waters Corp., Milford, MA) equipped with a multisolvent delivery system (model 600E), autosampler (model 712), tunable absorbance detector (UV; model 486), and PDA (model 996). The UV and PDA detectors were installed parallel to each other and an automated switching valve was equipped to direct the flow to the detector. The separation was performed on a Waters Nova-Pak C18 column (300 × 3.9 mm, 60 Å, 4 μm). The mobile phase was acetonitrile/isopropanol (70:30), and the flow rate was 1.0 mL min−1. The solvents were sparged with helium at the flow rate of 30 mL min−1.
ACKNOWLEDGMENTS
We thank Blake Hanson (American Tung Oil Corporation) for access to tung orchards, Pamela Harris (U.S. Department of Agriculture-Agricultural Research Service, Southern Regional Research Center) for assistance with DNA sequencing, and John Rayapati (Archer Daniels Midland) and Edgar Cahoon (Donald Danforth Plant Science Center) for critical reading of the manuscript.
Footnotes
This work was supported by the U.S. Department of Agriculture-Agricultural Research Service (Current Research Information System project no. 6435–41000–049–00D), by the Natural Sciences and Engineering Research Council of Canada (grant no. 217291), and by the Ontario Premier's Research in Excellence Award (to R.T.M.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.010835.
LITERATURE CITED
- Belury MA. Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action. Annu Rev Nutr. 2002;22:505–531. doi: 10.1146/annurev.nutr.22.021302.121842. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bossie MA, Martin CE. Nutritional regulation of yeast Δ-9 fatty acid desaturase activity. J Bacteriol. 1989;171:6409–6413. doi: 10.1128/jb.171.12.6409-6413.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater JA, Whittle E, Shanklin J. Desaturation and hydroxylation: Residues 148 and 324 of Arabidopsis FAD2, in addition to substrate chain length, exert a major influence in partitioning of catalytic specificity. J Biol Chem. 2002;277:15613–15620. doi: 10.1074/jbc.M200231200. [DOI] [PubMed] [Google Scholar]
- Broun P, Boddupalli S, Somerville C. A bifunctional oleate 12-hydroxylase: desaturase from Lesquerella fendleri. Plant J. 1998a;13:201–210. doi: 10.1046/j.1365-313x.1998.00023.x. [DOI] [PubMed] [Google Scholar]
- Broun P, Shanklin J, Whittle E, Somerville C. Catalytic plasticity of fatty acid modification enzymes underlying chemical diversity of plant lipids. Science. 1998b;282:1315–1317. doi: 10.1126/science.282.5392.1315. [DOI] [PubMed] [Google Scholar]
- Bugos RC, Chiang VL, Zhang XH, Campbell ER, Podila GK, Campbell WH. RNA isolation from plant tissues recalcitrant to extraction in guanidine. BioTechniques. 1995;19:734–737. [PubMed] [Google Scholar]
- Cahoon EB, Carlson TJ, Ripp KG, Schweiger BJ, Cook GA, Hall SE, Kinney AJ. Biosynthetic origin of conjugated double bonds: production of fatty acid components of high-value drying oils in transgenic soybean embryos. Proc Natl Acad Sci USA. 1999;96:12935–12940. doi: 10.1073/pnas.96.22.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoon EB, Ripp KG, Hall SE, Kinney AJ. Formation of conjugated delta8, delta10 double bonds by delta12-oleic acid desaturase related enzymes: biosynthetic origin of calendic acid. J Biol Chem. 2001;276:2637–2643. doi: 10.1074/jbc.M009188200. [DOI] [PubMed] [Google Scholar]
- Christianson TW, Sikorski RS, Dante M, Shero JH, Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- Corl BA, Baumgard LH, Dwyer DA, Griinari JM, Phillips BS, Bauman DE. The role of delta(9)-desaturase in the production of cis-9, trans-11 CLA. J Nutr Biochem. 2001;12:622–630. doi: 10.1016/s0955-2863(01)00180-2. [DOI] [PubMed] [Google Scholar]
- Coughlan SJ, Hastings C, Winfrey R., Jr Cloning and characterization of the calreticulin gene from Ricinus communis L. Plant Mol Biol. 1997;34:897–911. doi: 10.1023/a:1005822327479. [DOI] [PubMed] [Google Scholar]
- Covello PS, Reed DW. Functional expression of the extraplastidial Arabidopsis thaliana oleate desaturase gene (FAD2) in Saccharomyces cerevisiae. Plant Physiol. 1996;111:223–226. doi: 10.1104/pp.111.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombie L, Holloway SJ. J Chem Soc Perkin Trans 2425–2434. 1985. The biosynthesis of calendic acid, octadeca-(8E, 10E, 12Z)-trienoic acid, by developing marigold seeds: origins of (E,E,Z) and (Z,E,Z) conjugated triene acids in higher plants. [Google Scholar]
- Destaillats F, Angers P. One-step methodology for the synthesis of FA picolinyl esters from intact lipids. J Am Oil Chem Soc. 2002;79:253–256. [Google Scholar]
- Dyer JM, McNew JA, Goodman JM. The sorting sequence of the peroxisomal integral membrane protein PMP47 is contained within a short hydrophilic loop. J Cell Biol. 1996;133:269–280. doi: 10.1083/jcb.133.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer JM, Mullen RT. Immunocytological localization of two plant fatty acid desaturases in the endoplasmic reticulum. FEBS Lett. 2001;494:44–47. doi: 10.1016/s0014-5793(01)02315-8. [DOI] [PubMed] [Google Scholar]
- Fritsche K, Hornung E, Peitzsch N, Renz A, Feussner I. Isolation and characterization of a calendic acid producing (8, 11)-linoleoyl desaturase. FEBS Lett. 1999;462:249–253. doi: 10.1016/s0014-5793(99)01541-0. [DOI] [PubMed] [Google Scholar]
- Gietz RD, Woods RA. High efficiency transformation in yeast. In: Johnston JA, editor. Molecular Genetics of Yeast: Practical Approaches. New York: Oxford University Press; 1994. pp. 121–134. [Google Scholar]
- Griinari JM, Bauman DE. Biosynthesis of conjugated linoleic acid and its incorporation in meat and milk in ruminants. In: Yurawecz MP, Mossoba MM, Kramer JKG, Pariza MW, Nelson GJ, editors. Advances in Conjugated Linoleic Acid Research. Champaign, IL: AOCS Press; 1999. pp. 180–200. [Google Scholar]
- Hamberg M. Metabolism of 6,9,12-octadecatrienoic acid in the red alga Lithothamnion corallioides: mechanism of formation of a conjugated tetraene fatty acid. Biochem Biophys Res Commun. 1992;188:1220–1227. doi: 10.1016/0006-291x(92)91361-s. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Miyazawa T. Newly recognized cytotoxic effect of conjugated trienoic fatty acids on cultured human tumor cells. Cancer Lett. 2000;148:173–179. doi: 10.1016/s0304-3835(99)00332-8. [DOI] [PubMed] [Google Scholar]
- Koba K, Akahoshi A, Yamasaki M, Tanaka K, Yamada K, Iwata T, Kamegai T, Tsutsumi K, Sagano M. Dietary conjugated linolenic acid in relation to CLA differently modifies body fat mass and serum and liver lipid levels in rats. Lipids. 2002;37:343–350. doi: 10.1007/s11745-002-0901-7. [DOI] [PubMed] [Google Scholar]
- Kohno H, Suzuki R, Noguchi R, Hosokawa M, Miyashita K, Tanaka T. Dietary conjugated linolenic acid inhibits azoxymethane-induced colonic aberrant crypt foci in rats. Jpn J Cancer Res. 2002;93:133–142. doi: 10.1111/j.1349-7006.2002.tb01251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Hammond EG, Nikolau BJ. In vivo studies of the biosynthesis of α-eleostearic acid in the seed of Momordica charantia L. Plant Physiol. 1997;113:1343–1349. doi: 10.1104/pp.113.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCammon MT, Veenhuis M, Trapp SB, Goodman JM. Association of glyoxylate and beta-oxidation enzymes with peroxisomes of Saccharomyces cerevisiae. J Bacteriol. 1990;172:5816–5827. doi: 10.1128/jb.172.10.5816-5827.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris LJ, Marshall MO. Occurrence of cis,trans-linoleic acid in seed oils. Chem Ind. 1966;August 27:1493–1494. [PubMed] [Google Scholar]
- Qiu X, Reed DW, Hong H, MacKenzie SL, Covello PS. Identification and analysis of a gene from Calendula officinalis encoding a fatty acid conjugase. Plant Physiol. 2001;125:847–855. doi: 10.1104/pp.125.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JS, Richardson DC. Principles and patterns of protein conformation. In: Fasman GB, editor. Prediction of Protein Structure and the Principles of Protein Conformation. New York: Plenum; 1989. pp. 1–91. [Google Scholar]
- Rodriguez S, Clapes P, Camps F, Fabrias G. Stereospecificity of an enzymatic monoene 1,4-dehydrogenation reaction: conversion of (Z)-11-tetradecenoic acid into (E,E)-10,12-tetradecadienoic acid. J Org Chem. 2002;67:2228–2233. doi: 10.1021/jo0109927. [DOI] [PubMed] [Google Scholar]
- Sell HM, Best AH, Reuther W, Drosdoff M. Changes in chemical composition and biological activity of developing tung fruit with reference to oil synthesis. Plant Physiol. 1948;23:359–372. doi: 10.1104/pp.23.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanklin J, Cahoon EB. Desaturation and related modifications of fatty acids. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:611–641. doi: 10.1146/annurev.arplant.49.1.611. [DOI] [PubMed] [Google Scholar]
- Shanklin J, Whittle E, Fox BG. Eight histidine residues are catalytically essential in a membrane-associated iron enzyme, stearoyl-CoA desaturase, and are conserved in alkane hydroxylase and xylene monooxygenase. Biochemistry. 1994;33:12787–12794. doi: 10.1021/bi00209a009. [DOI] [PubMed] [Google Scholar]
- Smith CRJ. Occurrence of unusual fatty acids in plants. Prog Chem Fats Other Lipids. 1970;11:139–177. [Google Scholar]
- Sonntag NOV. Composition and characteristics of individual fats and oils. In: Swern D, editor. Bailey's Industrial Oil and Fat Products. New York: John Wiley & Sons; 1979. pp. 289–477. [Google Scholar]
- Sperling P, Lee M, Girke T, Zähringer U, Stymne S, Heinz E. A bifunctional Δ6-fatty acyl acetylenase/desaturase from the moss Ceratodon purpureus. Eur J Biochem. 2000;267:3801–3811. doi: 10.1046/j.1432-1327.2000.01418.x. [DOI] [PubMed] [Google Scholar]
- Sperling P, Ternes P, Moll H, Franke S, Zähringer U, Heinz E. Functional characterization of sphingolipid C4-hydroxylase genes from Arabidopsis thaliana. FEBS Lett. 2001;494:90–94. doi: 10.1016/s0014-5793(01)02332-8. [DOI] [PubMed] [Google Scholar]
- Sperling P, Zähringer U, Heinz E. A sphingolipid desaturase from higher plants. J Biol Chem. 1998;273:28590–28596. doi: 10.1074/jbc.273.44.28590. [DOI] [PubMed] [Google Scholar]
- Stukey JE, McDonough VM, Martin CE. The OLE1 gene of Saccharomyces cerevisiae encodes the Δ9 fatty acid desaturase and can be functionally replaced by the rat stearoyl-CoA desaturase gene. J Biol Chem. 1990;265:20144–20149. [PubMed] [Google Scholar]
- Voelker T, Kinney AJ. Variations in the biosynthesis of seed-storage lipids. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:335–361. doi: 10.1146/annurev.arplant.52.1.335. [DOI] [PubMed] [Google Scholar]
- Zheng W, Wise M, Wyrick A, Metz J, Yuan L, Gerwick W. Polyenoic fatty acid isomerase from the marine alga Ptilota filicina: protein characterization and functional expression of the cloned cDNA. Arch Biochem Biophys. 2002;401:11–20. doi: 10.1016/S0003-9861(02)00002-4. [DOI] [PubMed] [Google Scholar]