Abstract
Algae and vascular plants are cysteine (Cys) prototrophs. They are able to import, reduce, and assimilate sulfate into Cys, methionine, and other organic sulfur-containing compounds. Characterization of genes encoding the enzymes required for Cys biosynthesis from the unicellular green alga Chlamydomonas reinhardtii reveals that transcriptional and posttranscriptional mechanisms regulate the pathway. The derived amino acid sequences of the C. reinhardtii genes encoding 5′-adenylylsulfate (APS) reductase and serine (Ser) acetyltransferase are orthologous to sequences from vascular plants. The Cys biosynthetic pathway of C. reinhardtii is regulated by sulfate availability. The steady-state level of transcripts and activity of ATP sulfurylase, APS reductase, Ser acetyltransferase, and O-acetyl-Ser (thiol) lyase increase when cells are deprived of sulfate. The sac1 mutation, which impairs C. reinhardtii ability to acclimate to sulfur-deficient conditions, prevents the increase in accumulation of the transcripts encoding these enzymes and also prevents the increase in activity of all the enzymes except APS reductase. The sac2 mutation, which does not affect accumulation of APS reductase transcripts, blocks the increase in APS reductase activity. These results suggest that APS reductase activity is regulated posttranscriptionally in a SAC2-dependent process.
Plants and algae are primary producers. They absorb sunlight and through photosynthesis, convert it into chemical energy stored as carbohydrate. They also import inorganic nutrients from their environment and convert them into biologically active compounds. Photosynthesis and nutrient acquisition are coordinated with growth.
Photosynthetic organisms acclimate to nutrient-deficient conditions through a suite of physiological changes that can be classified as general and specific (Harder and Dijkhuizen, 1983; Davies and Grossman, 1998). General responses, which occur when an organism is deprived of any essential nutrient, include a decrease in the rate of photosynthesis, a decrease in or cessation of cell division, and an accumulation of starch or glycogen. Specific changes occur in response to loss of a specific nutrient and are different for each nutrient. Specific responses are those that enable the organism to scavenge the limiting nutrient from internal or external sources, and those that increase the efficiency of nutrient assimilation.
The ability to sense and respond to a nutrient-limiting environments is necessary for an organism to successfully compete in natural environments where nutrients are often limiting. However, the mechanisms used to sense nutrient availability and control physiological changes in response to nutrient limitation are largely unknown. We are using the unicellular green alga Chlamydomonas reinhardtii as a model system to study sulfur metabolism and the response to sulfur-deficient conditions.
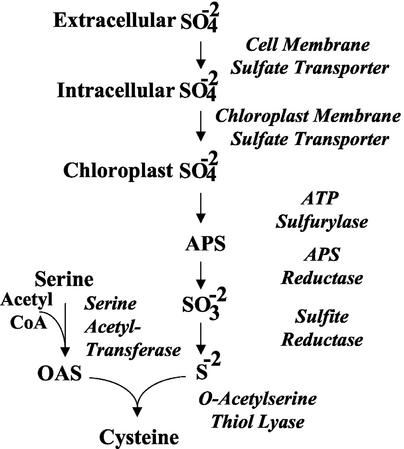
Sulfur is considered a macronutrient because it is required at relatively high levels. Sulfur is a constituent of proteins, lipids, carbohydrates, electron transport systems, and many other cellular constituents and intermediate metabolites (Leustek and Saito, 1999; Leustek et al., 2000). The sulfur assimilation pathway is shown in Figure 1. Most sulfur is imported into cells as sulfate and then translocated into chloroplasts. Before sulfate is metabolized, it is adenylated by ATP sulfurylase to form 5′-adenylylsulfate, APS. APS is a branch point intermediate that can be phosphorylated by APS kinase and used in sulfation reactions, or used to synthesize Cys. To form Cys, APS is reduced by sequential reactions catalyzed by APS reductase and sulfite reductase to form sulfide. Sulfide is subsequently combined with O-acetyl-Ser to form Cys by O-acetyl-Ser (thiol) lyase. Cys is incorporated into proteins and is the precursor of Met and S-adenosyl-l-Met in one set of reactions, and glutathione and phytochelatins in another.
Figure 1.
The Cys biosynthetic pathway.
When placed in a sulfur-limiting environment, C. reinhardtii responds by inducing specific and general responses to sulfur deficiency. Specific responses include the induction of a set of periplasmic proteins including an arylsulfatase (Lien and Schreiner, 1975; de Hostos et al., 1988), an increase in sulfate transport activity (Yildiz et al., 1994), and elevated ATP sulfurylase (Yildiz et al., 1996) and O-acetyl-Ser (thiol) lyase expression (Ravina et al., 1999). General responses include a decline in photosynthetic activity and cell division (Davies et al., 1996) and an increase in starch accumulation (Ball et al., 1990). The sac mutants of C. reinhardtii are deficient in the response to sulfur-limiting conditions (Davies et al., 1994). The sac1 and sac2 mutants are deficient in inducing arylsulfatase, whereas sac3 constitutively expresses arylsulfatase in sulfate-replete medium. All three mutants are unable to increase sulfate transport to the same extent as wild-type cells when deprived of sulfate. In addition, sac1 but not sac2 or sac3, is unable to down-regulate photosynthesis in response to sulfur starvation (Davies et al., 1996). Thus, sac1 is deficient in the general and specific responses to sulfur starvation, whereas sac2 and sac3 appear to be deficient in only the specific responses.
To understand how C. reinhardtii regulates its response to sulfur limitation, the expression of the Cys biosynthetic pathway components and arylsulfatase was studied during sulfur starvation in wild-type and sac strains. For this purpose, genes encoding APS reductase, Ser acetyltransferase, and another arylsulfatase were isolated. Analysis of the expression of these genes indicates that sulfur starvation induces increases in transcripts encoding ATP sulfurylase, APS reductase, Ser acetyltransferase, and O-acetyl-Ser (thiol) lyase that are regulated by SAC1, but not by SAC2 or SAC3. However, the enzymatic activity of APS reductase appears to be posttranscriptionally regulated in a SAC2-dependent process.
RESULTS
Characterization of Genes Encoding APS Reductase and Ser Acetyltransferase
To investigate the regulation of the Cys biosynthetic pathway in C. reinhardtii, genes encoding the enzymes of this pathway have been isolated. Genes encoding ATP sulfurylase (Yildiz et al., 1996) and O-acetyl-Ser (thiol) lyase (Ravina et al., 1999) had previously been isolated and characterized. To isolate genes encoding APS reductase and Ser acetyl transferase (SAT) from C. reinhardtii, strains of Escherichia coli deficient in these enzymes were functionally complemented with a cDNA library made from RNA isolated from sulfur-deprived C. reinhardtii. A single clone of APS reductase, APR1, and SAT, SAT1, were isolated. The sequence of these clones has been deposited in GenBank (accession nos.: APR1, AF069951 and SAT1, AY095344). This functional complementation cloning strategy has been used to isolate cDNAs encoding APS reductase from Arabidopsis (Gutierrez-Marcos et al., 1996; Setya et al., 1996) and Enteromorpha intestinalis (Gao et al., 2000) and SAT cDNAs from watermelon (Citrullus vulgaris; Saito et al., 1995) and Arabidopsis (Ruffet et al., 1995; Howarth et al., 1997).
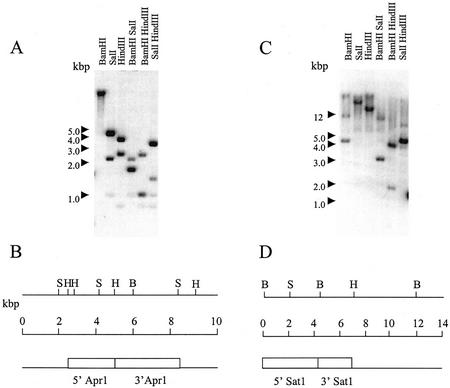
C. reinhardtii contains single genes encoding APS reductase and SAT. Figure 2 shows DNA gel blots using the APR1 and SATI cDNAs as probes detected simple banding patterns that indicated single genes. The maps in Figure 2, B and D, were derived from the DNA gel-blot analysis using 5′ and 3′ ends of the APR1 and SAT1 cDNA as probes, respectively (data not shown). In addition, all the cDNA clones of APR1 and SAT1 isolated by functionally complementing the E. coli mutants were derived from the same genes, and comparison of the APR1 and SAT1 protein sequences with the C. reinhardtii expressed sequence tag database containing over 100,000 sequences did not reveal the existence of paralogous genes.
Figure 2.
Molecular analysis of APR1 and SAT1. A, Genomic DNA was digested with BamHI, SalI, and HindIII singly and in combination, blotted onto a membrane, and hybridized with radioactively labeled APR1 cDNA. B, A restriction map of the genomic region containing the APR1 gene. The map was compiled from DNA gel blots using the 5′ and 3′ ends of the APR1 cDNA separately as hybridization probes. The 5′ probe contained cDNA sequences 5′ of the HindIII site designated in the map. The 3′ probe contained cDNA sequences 3′ of the HindIII site designated in the map. C, Genomic DNA was digested with BamHI, SalI, and HindIII singly and in combination, blotted onto a membrane, and hybridized with radioactively labeled SAT1 cDNA. D, A restriction map of the genomic region containing the SAT1 gene. The map was compiled from DNA gel blots using the 5′ and 3′ ends of the SAT1 cDNA separately as hybridization probes. The 5′ probe contained cDNA sequences 5′ of the BamHI site designated in the map. The 3′ probe contained cDNA sequences 3′ of the BamHI site designated in the map.
C. reinhardtii APS reductase has the three conserved domains characteristic of plant proteins. These include an amino terminal transit peptide responsible for targeting the protein to the chloroplast followed by a portion homologous to bacterial APS and PAPS reductases (Leustek et al., 2000), and at the carboxyl terminus, a domain homologous with thioredoxin and protein disulfide isomerase. The portion homologous to APS and PAPS reductase of bacteria (Leustek et al., 2000) shares 55% sequence identity with cysH from Pseudomonas aeruginosa. The C-terminal portion (amino acids 281–413) contains a region similar to protein disulfide isomerases (32% identical and 55% similar to a protein disulfide isomerase from Schistosoma mansoni). Similar to the analogous domain of vascular plant APS reductase, this region probably functions as a glutaredoxin (Setya et al., 1996; Bick et al., 1998).
The derived amino acid sequence of C. reinhardtii SAT1 is most similar to SAT genes of vascular plants showing 43% to 49% identity and 58% to 64% similarity over most of the protein. The sequence contains the putative acetyl-coenzyme A binding domain common to these proteins (Evans et al., 1991; Ruffet et al., 1995). The extreme C-terminal portion of the C. reinhardtii SAT protein is unlike the same portion of the plant proteins. The C. reinhardtii sequence shows no similarity to the vascular plant sequences after the Ile at position 368. The C. reinhardtii sequence specifically lacks the Gly, Met, and His residues shown to be involved in Cys-regulated feedback inhibition in the watermelon enzyme (Inoue et al., 1999).
SAT proteins have been identified in the chloroplast, cytoplasm, and mitochondria in vascular plants (Noji et al., 1998; Inoue et al., 2000). The coding sequence of the C. reinhardtii SAT1 cDNA has the features of a transit peptide, suggesting that the enzyme is targeted to the chloroplast.
C. reinhardtii Has Two Arylsulfatase Genes
When exposed to sulfur-limiting conditions, C. reinhardtii induces a periplasmically localized arylsulfatase that can cleave sulfate from extracellular aromatic sulfate esters (Lien and Schreiner, 1975; de Hostos et al., 1988). This arylsulfatase is part of a sulfur scavenging system that is used by C. reinhardtii when sulfate is not available. A cDNA (de Hostos et al., 1989) and genomic clone (Davies et al., 1992) encoding arylsulfatase were previously isolated, but sequence differences between them suggest that two arylsulfatase genes are present in C. reinhardtii. The cDNA library made from RNA isolated from sulfur-deprived cells was screened by hybridization with the previously isolated ARS cDNA. Two types of clones were isolated representing two different arylsulfatase genes designated ARS1 (the previously isolated cDNA clone) and ARS2 (corresponding with the previously isolated genomic clone). The sequence of ARS2 has been deposited in GenBank (accession no. AY095343).
Sulfur-Regulated Expression of the Sulfate Assimilation and Sulfur Scavenging Genes in C. reinhardtii
Vascular plants increase expression of some sulfate assimilation genes in response to sulfate starvation (Leustek et al., 2000). To determine if the sulfur-assimilating enzymes of C. reinhardtii are regulated by sulfur availability, transcript accumulation and enzyme activity was monitored in C. reinhardtii cells grown in sulfur-replete and sulfur-deficient medium.
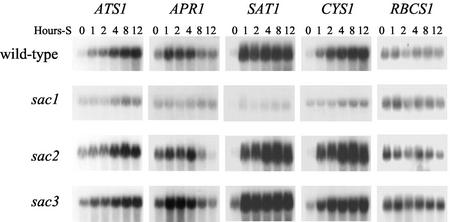
In C. reinhardtii, accumulation of transcripts encoding ATP sulfurylase and O-acetyl-Ser (thiol) lyase is regulated by sulfur availability (Yildiz et al., 1996; Ravina et al., 1999). To gain a more complete understanding of the regulation of sulfate assimilation in C. reinhardtii, the other components of the Cys biosynthetic pathway were examined. Figure 3 shows the accumulation of transcripts encoding ATP sulfurylase (ATS1), APS reductase (APR1), SAT (SAT1), and O-acetyl-Ser (thiol) lyase (CYS1) in wild-type cells deprived of sulfur. The transcripts encoding ATS1, SAT1, and CYS1 increase in abundance within 1 h of transfer to sulfur-free medium and continue to increase in abundance throughout the 12-h treatment. Accumulation of the APR1 transcript also increases in response to sulfur deprivation; it is most abundant 1 to 4 h after the initiation of sulfur deprivation, but then declines and reaches a level comparable with that in sulfur-sufficient cells within 8 h.
Figure 3.
Expression of ATS1, APR1, SAT1, and CYS1 in wild-type and sac mutants. Cells were grown to mid-log phase in sulfate-replete medium, washed twice with sulfur-deficient medium, and were then incubated for 0, 1, 2, 4, 8, or 12 h prior to harvesting RNA. One microgram of RNA from the indicated samples was separated by electrophoresis, blotted onto a membrane, and hybridized separately with the indicated cDNA clones. Hybridization with a genomic clone of RBCS1 was also performed as a control.
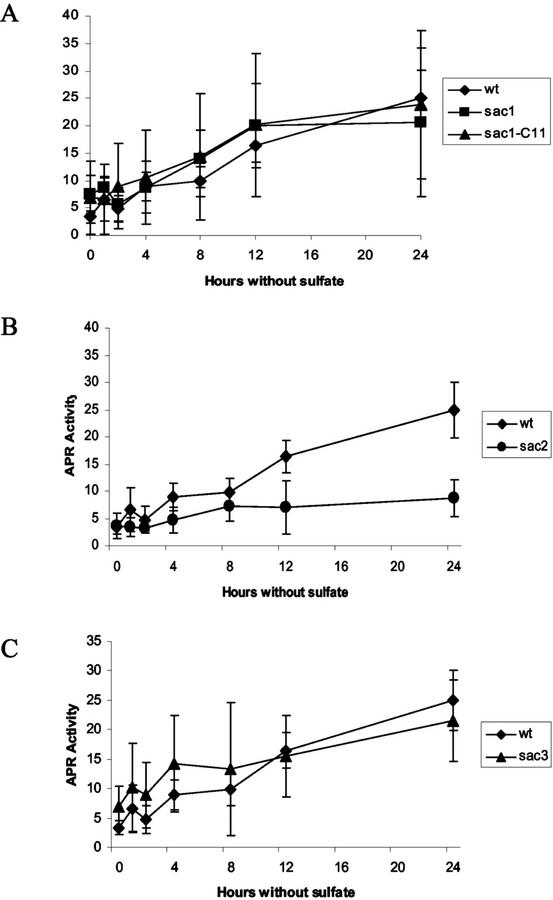
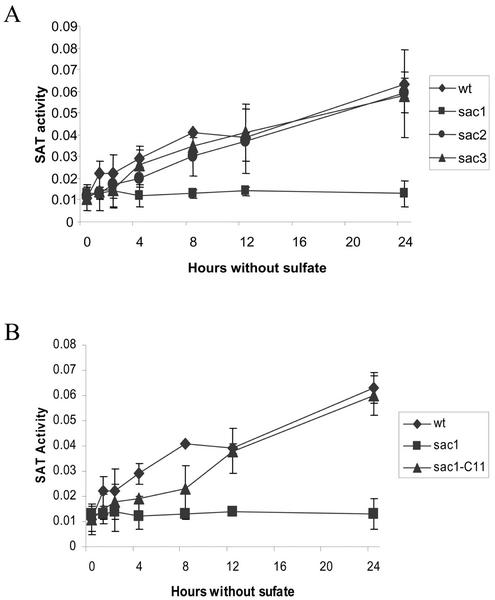
The activities of the Cys biosynthetic enzymes are also regulated by sulfate availability. APS reductase, SAT, and O-acetyl-Ser (thiol) lyase activities were measured in extracts of C. reinhardtii deprived of sulfate. Figures 4A, 5A, and 6A show that in wild-type cells, activities of all of these enzymes increase within 1 h of the initiation of sulfur starvation, and that the enzymatic activities increase progressively over 24 h. The continuous increase in APS reductase occurs despite the decrease in transcript abundance between 4 and 8 h of sulfur deficiency, suggesting that this enzyme is controlled, at least partly, by a posttranslational mechanism.
Figure 4.
APS reductase activity in wild-type and sac mutants. Cells were grown to mid-log phase in sulfate replete medium, washed twice with sulfur-deficient medium, and were then incubated for 0, 1, 2, 4, 8, 12, or 24 h prior to harvesting. APS reductase activity is expressed as nanomoles per minute per milligram of protein. A, APS reductase activity in wild-type (♦), sac1 (▪), and sac1-C11 (▴) strains. B, APS reductase activity in wild-type (♦) and sac2 (●) strains. C, APS reductase activity in wild-type (♦) and sac3 (▴) strains. Each value is the mean of at least three independent samples ± sd.
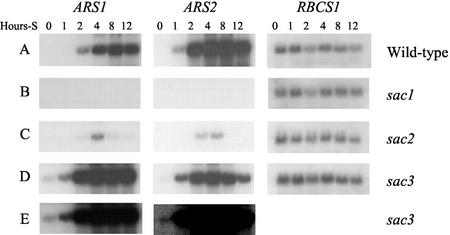
It is well documented that arylsulfatase activity in C. reinhardtii is regulated by sulfur availability and that ARS transcripts accumulate when cells are deprived of sulfur. Because two very similar ARS-encoding genes are present in C. reinhardtii, their expression was compared. To determine if both ARS genes are expressed in C. reinhardtii, gene-specific probes were isolated from the 3′ portion of genomic sequences that hybridize specifically to ARS1 and ARS2, respectively (data not shown). The RNA gel-blot experiment in Figure 7 shows that ARS1 and ARS2 are expressed in cells deprived of sulfur and not in cells grown in sulfur-sufficient medium. Furthermore, transcripts from both genes accumulate with similar kinetics.
Figure 7.
Expression of ARS1 and ARS2 in wild-type and sac mutants. Wild-type (A), sac1 (B), sac2 (C), and sac3 (D) were grown to mid-log phase in sulfate-replete medium, washed twice with sulfur-deficient medium, and were then incubated for 0, 1, 2, 4, 8, or 12 h prior to harvesting RNA. One microgram of RNA was separated by electrophoresis, blotted onto a membrane, and hybridized separately with cDNA clones of ARS1 and ARS2. Hybridization with a genomic clone of RbcS1 was also performed as a control. E, Overexposure of the blots shown in D shows that ARS1 and ARS2 are expressed in sac3 cells grown in sulfur-replete medium.
Regulation of Sulfate Assimilation and Sulfur Scavenging Genes in C. reinhardtii sac Mutants
The sac1, sac2, and sac3 mutants were isolated based on their inability to properly regulate arylsulfatase activity (Davies et al., 1994). Arylsulfatase expression is deficient in sac1 and sac2 strains, whereas sac3 constitutively expresses ARS in sulfate-replete medium. All three mutants are unable to increase sulfate transport in response to sulfur starvation (Davies et al., 1994). Transcripts encoding ATP sulfurylase (Yildiz et al., 1996), a cell wall protein (Takahashi et al., 2001), and several proteins of the periplasmic space, which normally accumulate in response to sulfur starvation, do not accumulate in the sac1 strain (de Hostos et al., 1988; Davies et al., 1994). sac1 also blocks the ability to decrease photosynthetic electron transport during sulfur starvation (Davies et al., 1996; Wykoff et al., 1998). The sac2 and sac3 mutants are not defective in regulating the accumulation of the sulfur starvation-induced periplasmic proteins or photosynthetic electron transport (Davies et al., 1994, 1996).
To determine how the sac mutations affect the regulation of the sulfate assimilation enzymes, transcript accumulation and enzymatic activities of specific components of the Cys biosynthetic pathway were measured. Sulfur regulated induction of the Cys biosynthetic pathway transcripts were strongly inhibited by sac1, but not by sac2 or sac3. Figure 3 shows blots of RNA isolated from wild-type, sac1, sac2, and sac3 strains deprived of sulfur and hybridized with probes for transcripts of ATP sulfurylase (ATS1), APS reductase (APR1), SAT (SAT1), and O-acetyl-Ser (thiol) lyase (CYS1). The abundance of these transcripts in the sac1 strain increases only slightly, whereas in sac2 and sac3, they increase with kinetics similar to that in the wild-type strain.
The activities of SAT and O-acetyl-Ser (thiol) lyase are also blocked in sac1, but not in sac2 or sac3 (Figs. 5A and 6A). In wild-type, sac2 and sac3 cells, the activities of SAT and O-acetyl-Ser (thiol) lyase increase within a1 h of sulfur deprivation and continue to increase for at least 24 h. However, in sac1 cells, the activities of these enzymes do not increase at all during the period of sulfur starvation. The defect is specifically attributed to the sac1 mutation because the induction of enzyme activities is fully restored in a C. reinhardtii strain, sac1-C11, in which sac1 has been complemented with the wild-type allele (Davies et al., 1996; Figs. 5B and 6B).
Figure 5.
SAT activity in wild-type and sac mutants. Cells were grown to mid-log phase in sulfate-replete medium, washed twice with sulfur-deficient medium, and were then incubated for 0, 1, 2, 4, 8, 12, or 24 h prior to harvesting. SAT activity is expressed as micromoles of Cys produced per minute per milligram of protein. A, SAT activity in wild-type (♦), sac1 (▪), sac2 (●), and sac3 (▴) strains. B, SAT activity in wild-type (♦), sac1 (▪), and the complemented sac1 strain, sac1-C11 (▴). Each value is the mean of at least three independent samples ± sd.
Figure 6.
O-Acetyl-Ser (thiol) lyase activity in wild-type and sac mutants. Cells were grown to mid-log phase in sulfate-replete medium, washed twice with sulfur-deficient medium, and were then incubated for 0, 1, 2, 4, 8, 12, or 24 h prior to harvesting. O-Acetyl-Ser (thiol) lyase activity is expressed as micromoles Cys produced per minute per milligram of protein. A, O-Acetyl-Ser (thiol) lyase activity in wild-type (♦), sac1 (▪), sac2 (●), and sac3 (♦) strains. B, O-Acetyl-Ser (thiol) lyase activity in wild-type (♦), sac1 (▪), and the complemented sac1 strain, sac1-C11 (▴). Each value is the mean of at least three independent samples ± sd. The standard deviations of these measurements were less than 4% of the values.
APS reductase is regulated differently. In wild-type, sac1, sac3, and sac1-C11 strains, APS reductase activity increases with similar kinetics during sulfur deprivation (Fig. 4, A and C). The increase in APS reductase activity in the sac1 strain occurs despite almost negligible increase in APR1 transcript accumulation in this strain (Fig. 3). However, in sulfur-deprived sac2 cells, APS reductase activity is less than in wild-type cells (Fig. 4B), whereas APR1 transcript accumulation is similar in these strains (Fig. 3). Arylsulfatase activity was measured in these samples to confirm the identity of the strains (data not shown). The results strongly suggest that APS reductase activity is regulated posttranscriptionally and that the SAC2 gene affects this regulation.
RNA gel-blot analysis was carried out to determine whether the sac mutations differentially affect the expression of the two ARS genes. As shown in Figure 7, accumulation of transcripts encoding ARS1 and ARS2 are affected similarly by sac1, sac2, and sac3. The sulfur-starvation induced accumulation of transcripts for the arylsulfatase genes is completely blocked in sac1, and is inhibited in sac2. sac3 mutants constitutively express both genes during growth on sulfur-replete medium (Fig. 7E) and increase accumulation of both in response to sulfur limitation (Fig. 7D).
DISCUSSION
The genes encoding enzymes of the Cys biosynthetic pathway of C. reinhardtii are very similar to those found in vascular plants. Here, the isolation and characterization of C. reinhardtii genes encoding APS reductase and SAT is presented. Genes encoding ATP sulfurylase (Yildiz et al., 1996) and O-acetyl-Ser (thiol) lyase (Ravina et al., 1999) have previously been described. In C. reinhardtii, each of these enzymes appears to be encoded by single gene that is similar to the orthologous genes in vascular plants. Vascular plants have multiple copies of these genes. Arabidopsis contains at least three genes encoding each of these proteins (Leustek and Saito, 1999; Leustek et al., 2000).
Cys biosynthetic enzymes appear to be localized in the chloroplast of C. reinhardtii. All the enzymes contain N-terminal extensions that resemble chloroplast transit peptides. Localization of the same enzymes in vascular plants is more complex. Sulfate reduction is thought to occur primarily in the plastids of vascular plants because all APS reductases (Gutierrez-Marcos et al., 1996; Setya et al., 1996; Rotte and Leustek, 2000) and sulfite reductase (Bork et al., 1998) proteins have chloroplast transit peptides and are localized in this compartment. However, ATP sulfurylase activity also exists in the cytosol (Rotte and Leustek, 2000), and SAT and O-acetyl-Ser (thiol) lyase activities have been detected in the cytosolic, mitochondrial, and plastid fractions of plant cells (Noji et al., 1998; Jost et al., 2000). The product of the ATP sulfurylase reaction, APS, is used throughout the cell in many different sulfation reactions (Leustek, 2002). The subcellular distribution of SAT and O-acetyl-Ser (thiol) lyase suggests that Cys is synthesized in several locations in vascular plant cells. By contrast, in C. reinhardtii, Cys appears to the synthesized only in chloroplasts.
Phylogenetic comparison of the ATP sulfurylase, APS reductase, SAT, and O-acetyl-Ser (thiol) lyase sequences from C. reinhardtii and Arabidopsis suggests that the algal and plant sequences diverged prior to the gene amplification events that gave rise to the gene families in vascular plants. Therefore, the appearance of these enzymes in multiple cellular locations is a relatively recent event and is correlated with the cellular specialization associated with evolution of multicellular organisms.
In contrast to the single genes encoding Cys biosynthetic enzymes, C. reinhardtii contains two genes encoding arylsulfatases. The presence of two ARS genes appears to have been caused by a very recent duplication. The two cDNAs and proteins are 91% and 98% identical, respectively. A phylogenetic comparison of the two C. reinhardtii ARS genes with the Volvox cateri ARS sequence (Hallmann and Sumper, 1994) (GenBank accession no. S43229; not shown) suggests that the C. reinhardtii sequences were duplicated after these algae diverged from one another. The regulation of the two C. reinhardtii ARS genes appears to be identical. Transcripts of both genes accumulate with similar kinetics when cells are deprived of sulfate. In addition, expression of both genes is similarly affected by the sac1, sac2, and sac3 mutations.
Regulation of the Cys Biosynthetic Pathway
The Cys biosynthetic pathway of C. reinhardtii is controlled by sulfate availability. Transcripts of genes encoding ATP sulfurylase (Yildiz et al., 1996), APS reductase, SAT, and O-acetyl-Ser (thiol) lyase (Ravina et al., 1999) are more abundant in cells placed in sulfate-deficient medium than in cells grown in sulfate-sufficient medium. The activity of Cys biosynthetic enzymes is also greater in sulfate-limited cells (Ravina et al., 1999 and this study). The increase in activity of these enzymes may enable C. reinhardtii to more efficiently assimilate sulfate when its availability is low. The increase in Cys biosynthesis in response to limiting sulfate is common among sulfate-reducing organisms. It has been observed in bacteria, fungi, vascular plants, and algae (Kredich, 1996; Marzluf, 1997; Thomas and Surdin-Kerjan, 1997; Leustek and Saito, 1999; Leustek et al., 2000).
Sulfate regulated gene expression in C. reinhardtii is known to be controlled by at least three genes. The SAC1, SAC2, and SAC3 genes were identified in mutants defective in regulating expression of arylsulfatase activity (Davies et al., 1994). Arylsulfatase is normally induced only when cells are deprived of sulfate (de Hostos et al., 1988). The sac1 and sac2 mutants are unable to induce arylsulfatase activity to the same extent as wild-type cells, and the sac3 mutant constitutively expresses arylsulfatase in sulfate-replete medium. The SAC1 gene encodes an integral membrane protein with similarity to sodium dicarboxylate transporters of other organisms (Davies et al., 1996), and SAC3 encodes an SNF1-like Ser threonine kinase (Davies et al., 1999). The SAC2 gene has not been cloned.
SAC1 is essential for many of the responses to sulfate limitation. The sac1 strain is unable to increase sulfate transport and ATP sulfurylase transcript accumulation or to down-regulate photosynthesis, all of which normally occur when cells are deprived of sulfate (Davies et al., 1994, 1996; Wykoff et al., 1998). This work demonstrates that SAC1 is also required for the increase in accumulation of transcripts encoding APS reductase, SAT, and O-acetyl-Ser (thiol) lyase. The increase in enzymatic activity of SAT and O-acetyl-Ser (thiol) lyase is also blocked by the sac1 mutation. However, the increase in APS reductase is not. The sulfate-deprived sac2 mutant accumulates APS reductase transcripts, but not APS reductase activity. These results suggest that SAC2 is needed for posttranscriptional control of APS reductase activity in cells deprived of sulfate. The sac3 mutation has no apparent effect on transcript accumulation of any of the Cys biosynthetic pathway genes or on their enzymatic activity.
Vascular plants also respond to limiting sulfate levels by inducing the Cys biosynthetic pathway. This regulation occurs by increases in transcript accumulation and enzymatic activity, as well as posttranslational processes. The accumulation of transcripts encoding several sulfate transporters and APS reductase genes is increased when plants are deprived of sulfate (Takahashi et al., 1997). O-Acetyl-Ser, Cys, and glutathione appear to modulate the expression of proteins for sulfate transport and assimilation (Neuenschwander et al., 1991; Smith et al., 1997). Synthesis of Cys is modulated posttranslationally by accumulation of O-acetyl-Ser. O-Acetyl-Ser disrupts the SAT/O-acetyl-Ser (thiol) lyase complex and stimulates sulfide production by activating the sulfate reduction pathway (Bogdanova and Hell, 1997; Droux et al., 1998). Sulfide promotes formation of this complex and thereby stimulates O-acetyl-Ser synthesis (Leustek et al., 2000). Plant APS reductase may also be regulated posttranslationally in response to oxidative stress (Bick et al., 2001). Oxidative stress is known to stimulate glutathione and Cys synthesis. Glutathione is an antioxidant used to combat damaging reactive oxygen. The simultaneous operation of positive and negative signals maintains the rate of Cys synthesis in proportion to the plants demand for Cys and glutathione.
Genes involved in signaling and coordinating the response to sulfur deficiency in vascular plants are unknown. Analysis of the sac mutants of C. reinhardtii clearly demonstrates that the SAC genes are involved in regulating this alga's response to sulfur stress. Genes encoding SAC1 and SAC3 have been identified. SAC1 encodes a putative integral membrane protein that is similar to genes found in Synechocystis PCC6803 and several other bacteria. The proteins encoded by these genes share low level of similarity with the sodium dicarboxylate family of transporters in vertebrates. The function of these bacterial genes is unknown at this time. No SAC1 homolog has been found in vascular plants (Davies et al., 1996; Leustek et al., 2000). SAC3 is a type 2 SNF1-related kinase (SnRK2) and is similar to family kinases found in vascular plants. Several of these SnRK2-encoding transcripts increase in abundance in response to environmental stresses (Anderberg and Walker-Simmons, 1992; Park et al., 1993; Yoon et al., 1997), but the function of most of the SnRK2 proteins found in plants have yet to be elucidated. Arabidopsis contains at least 12 SnRK2 proteins that have significant similarity (49% amino acid identity and 60% amino acid similarity over most of the protein) with SAC3. Some of these proteins may be involved in regulating the plant's response to sulfur deficiency.
MATERIALS AND METHODS
Cell Growth and DNA and RNA Gel Blots
Cells were grown in nutrient-replete or sulfate-deficient Tris acetate phosphate medium as described previously (Davies et al., 1994). DNA and RNA gel blots were performed as described previously (Davies et al., 1994, 1996).
Cloning the APR1, SAT1, and ARS2 cDNAs
To clone APR1, a Chlamydomonas reinhardtii cDNA library was excised as plasmids (Davies et al., 1996), electroporated into the APS reductase-deficient Escherichia coli strain JM96 [thr-1 leuB6(Am) fhuA2 lacY1 glnV44(AS) gal-6 LAM- trp-1 hisG1(Fs) cysH56 rpsL9 malT1(LamR) xylA7 mtlA2 argH1(del) thi-1], and Cys prototrophs were selected. Fourteen colonies were isolated from screening 1.0 × 106 transformants. Plasmids from the complemented strains were purified and reintroduced into JM96 to confirm their ability to complement the mutant phenotype.
To clone SAT1, the E. coli strain JM70 [thr-1 leuB6(Am) fhuA2 lacY1 glnV44(AS) gal-6 LAM-trp-1 hisG1(Fs) rpsL99(strR) malT1(LamR) xylA7 cysE52 mtlA2 argH1(del) thi-1] was transformed with the plasmid cDNA library, and Cys prototrophs were selected. Cys prototrophs were selected on M9 medium supplemented with amino acids (excluding Met and Cys) and thiamine. Four colonies were isolated from a total of 1 × 106 transformants. Plasmids were isolated and reintroduced to confirm their ability to complement the mutant phenotype. Preliminary sequence analysis indicated that all four clones contained identical sequences. The Cys auxotrophy of these cells was complemented by transforming these cells with plasmids from an excised cDNA made from RNA extracted from sulfur-deprived C. reinhardtii cells (Davies et al., 1996). Because none of the clones isolated by functional complementation appeared to be full length, a cDNA library was screened by hybridization with one of the isolated cDNA clones. A clone containing a 2,194-bp insert was isolated from the library and was sequenced.
The ARS2 cDNA was isolated by hybridization (Maniatis et al., 1982) of the ARS1 cDNA to the cDNA library in (Davies et al., 1996).
Molecular Techniques
DNA and RNA gel-blot analysis was performed as described previously (Davies et al., 1994, 1996). For RNA gel-blot analysis, the 579-bp SalI fragment of p2.02 (Goldschmidt-Clermont and Rahire, 1986), which encodes a portion of the RBCS1 gene, was used as a loading control. The abundance of this transcript does not change relative to the rRNA in the sample over the time course of sulfur deprivation.
Determination of APS Reductase, SAT, and O-Acetyl-Ser (Thiol) Lyase (OASTL) Activities
C. reinhardtii cells were lysed by freezing in liquid nitrogen and thawing in 25 mm potassium phosphate, pH 7.5, for SAT and OASTL measurement, and 100 mm Tris-HCl, pH 8.5, 100 mm Na2SO4 for APS reductase measurement. The homogenates were centrifuged at 15,800g, and the resulting supernatants were used in the enzyme assays. APS reductase was measured using dithiothreitol as an electron donor (Bick et al., 2001). SAT activity was determined by measuring its capacity to promote Cys synthesis in a coupled enzyme assay using the OASTL activity present in the crude extracts. OASTL activity was measured as previously described (Ravina et al., 1999).
Footnotes
This work was funded in part by the United States National Science Foundation (grant no. IBN–9817594 to T.L.), by the U.S. Department of Agriculture (grant no. 9900622 to J.P.D.), and by funds from the Iowa State University Office of Biotechnology.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.012484.
LITERATURE CITED
- Anderberg RJ, Walker-Simmons MK. Isolation of a wheat cDNA clone for an abscisic acid-inducible transcript with homology to protein kinases. Proc Natl Acad Sci USA. 1992;89:10183–10187. doi: 10.1073/pnas.89.21.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball S, Dirick L, Decq A, Martiat J-C, Matagne R. Physiology of starch storage in the monocellular alga Chlamydomonas reinhardtii. Plant Sci. 1990;66:1–9. [Google Scholar]
- Bick JA, Aslund F, Chen Y, Leustek T. Glutaredoxin function for the carboxyl-terminal domain of the plant-type. Proc Natl Acad Sci USA. 1998;95:8404–8409. doi: 10.1073/pnas.95.14.8404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick JA, Setterdahl AT, Knaff DB, Chen Y, Pitcher LH, Zilinskas BA, Leustek T. Regulation of the plant-type 5′-adenylyl sulfate reductase by oxidative stress. Biochemistry. 2001;40:9040–9048. doi: 10.1021/bi010518v. [DOI] [PubMed] [Google Scholar]
- Bogdanova N, Hell R. Cysteine synthesis in plants: protein-protein interactions of serine acetyltransferase from Arabidopsis thaliana. Plant J. 1997;11:251–262. doi: 10.1046/j.1365-313x.1997.11020251.x. [DOI] [PubMed] [Google Scholar]
- Bork C, Schwenn JD, Hell R. Isolation and characterization of a gene for assimilatory sulfite reductase from Arabidopsis thaliana. Gene. 1998;212:147–153. doi: 10.1016/s0378-1119(98)00155-3. [DOI] [PubMed] [Google Scholar]
- Davies JP, Grossman AR. Survival during macronutrient limitation. In: Rochaix J-D, Goldschmidt-Clermont M, Merchant S, editors. The Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 613–635. [Google Scholar]
- Davies JP, Weeks DP, Grossman AR. Expression of the arylsulfatase gene from the β2-tubulin promoter in Chlamydomonas reinhardtii. Nucleic Acids Res. 1992;20:2959–2965. doi: 10.1093/nar/20.12.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JP, Yildiz FH, Grossman AR. Mutants of Chlamydomonas with aberrant responses to sulfur deprivation. Plant Cell. 1994;6:53–63. doi: 10.1105/tpc.6.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JP, Yildiz FH, Grossman A. Sac1, a putative regulator that is critical for survival of Chlamydomonas reinhardtii during sulfur deprivation. EMBO J. 1996;15:2150–2159. [PMC free article] [PubMed] [Google Scholar]
- Davies JP, Yildiz FH, Grossman AR. Sac3, an Snf1-like serine/threonine kinase that positively and negatively regulates the responses of Chlamydomonas reinhardtii to sulfur limitation. Plant Cell. 1999;11:1179–1190. doi: 10.1105/tpc.11.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hostos EL, Schilling J, Grossman AR. Structure and expression of the gene encoding the periplasmic arylsulfatase of Chlamydomonas reinhardtii. Mol Gen Genet. 1989;218:229–239. doi: 10.1007/BF00331273. [DOI] [PubMed] [Google Scholar]
- de Hostos EL, Togasaki RK, Grossman A. Purification and biosynthesis of a derepressible periplasmic arylsulfatase from Chlamydomonas reinhardtii. J Cell Biol. 1988;106:29–37. doi: 10.1083/jcb.106.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droux M, Ruffet ML, Douce R, Job D. Interactions between serine acetyltransferase and O-acetylserine (thiol) lyase in higher plants: structural and kinetic properties of the free and bound enzymes. Eur J Biochem. 1998;255:235–245. doi: 10.1046/j.1432-1327.1998.2550235.x. [DOI] [PubMed] [Google Scholar]
- Evans DJ, Jones R, Woodley PR, Wilborn JR, Robson RL. Nucleotide sequence and genetic analysis of the Azotobacter chroococcum nifUSVWZM gene cluster, including a new gene (nifP) which encodes a serine acetyltransferase. J Bacteriol. 1991;173:5457–5469. doi: 10.1128/jb.173.17.5457-5469.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Schofield OM, Leustek T. Characterization of sulfate assimilation in marine algae focusing on the. Plant Physiol. 2000;123:1087–1096. doi: 10.1104/pp.123.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M, Rahire M. Sequence, evolution and differential expression of the two genes encoding variant small subunits of ribulose bisphosphate carboxylase/oxygenase in Chlamydomonas reinhardtii. J Mol Biol. 1986;191:421–432. doi: 10.1016/0022-2836(86)90137-3. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Marcos JF, Roberts MA, Campbell EI, Wray JL. Three members of a novel small gene-family from Arabidopsis thaliana able to complement functionally an Escherichia coli mutant defective in PAPS reductase activity encode proteins with a thioredoxin-like domain and “APS reductase” activity. Proc Natl Acad Sci USA. 1996;93:13377–13382. doi: 10.1073/pnas.93.23.13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmann A, Sumper M. An inducible arylsulfatase of Volvox carteri with properties suitable for a reporter-gene system: purification, characterization and molecular cloning. Eur J Biochem. 1994;221:143–150. doi: 10.1111/j.1432-1033.1994.tb18723.x. [DOI] [PubMed] [Google Scholar]
- Harder W, Dijkhuizen L. Physiological responses to nutrient limitation. Annu Rev Microbiol. 1983;37:1–23. doi: 10.1146/annurev.mi.37.100183.000245. [DOI] [PubMed] [Google Scholar]
- Howarth JR, Roberts MA, Wray JL. Cysteine biosynthesis in higher plants: a new member of the Arabidopsis. Biochim Biophys Acta. 1997;1350:123–127. doi: 10.1016/s0167-4781(96)00213-8. [DOI] [PubMed] [Google Scholar]
- Inoue K, Noji M, Katagiri T, Shinozaki K, Saito K. Subcellular localization and feedback inhibition of serine acetyltransferase. In: Brunold C, Rennenberg H, De Kok LJ, Stulen I, Davidan J-C, editors. Sulfur Nutrition and Sulfur Assimilation in Higher Plants. Bern, Switzerland: Paul Haupt; 2000. pp. 327–329. [Google Scholar]
- Inoue K, Noji M, Saito K. Determination of the sites required for the allosteric inhibition of serine acetyltransferase by l-cysteine in plants. Eur J Biochem. 1999;266:220–227. doi: 10.1046/j.1432-1327.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Jost R, Berkowitz O, Wirtz M, Hopkins L, Hawkesford MJ, Hell R. Genomic and functional characterization of the oas gene family encoding O-acetylserine (thiol) lyases, enzymes catalyzing the final step in cysteine biosynthesis in Arabidopsis thaliana. Gene. 2000;253:237–247. doi: 10.1016/s0378-1119(00)00261-4. [DOI] [PubMed] [Google Scholar]
- Kredich N. Biosynthesis of Cysteine. Washington, DC: American Society of Microbiology; 1996. [Google Scholar]
- Leustek T. Sulfate metabolism. In: Meyerowitz EM, editor. The Arabidopsis Book. Rockville, MD: American Society of Plant Biologists; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leustek T, Martin MN, Bick J-A, Davies JP. Pathways and regulation of sulfur metabolism revealed through molecular genetic studies. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:141–166. doi: 10.1146/annurev.arplant.51.1.141. [DOI] [PubMed] [Google Scholar]
- Leustek T, Saito K. Sulfate transport and assimilation in plants. Plant Physiol. 1999;120:637–644. doi: 10.1104/pp.120.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien T, Schreiner O. Purification of a derepressible arylsulfatase from Chlamydomonas reinhardtii. Biochem Biophys Acta. 1975;384:168–179. doi: 10.1016/0005-2744(75)90106-0. [DOI] [PubMed] [Google Scholar]
- Maniatis T, Fritsch E, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- Marzluf GA. Molecular genetics of sulfur assimilation in filamentous fungi and yeast. Annu Rev Microbiol. 1997;51:73–96. doi: 10.1146/annurev.micro.51.1.73. [DOI] [PubMed] [Google Scholar]
- Neuenschwander U, Suter M, Brunold C. Regulation of sulfate assimilation by light and O-acetyl-l-serine in Lemna minor L. Plant Physiol. 1991;97:253–258. doi: 10.1104/pp.97.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noji M, Inoue K, Kimura N, Gouda A, Saito K. Isoform-dependent differences in feedback regulation and subcellular. J Biol Chem. 1998;273:32739–32745. doi: 10.1074/jbc.273.49.32739. [DOI] [PubMed] [Google Scholar]
- Park YS, Hong SW, Oh SA, Kwak JM, Lee HH, Nam HG. Two putative protein kinases from Arabidopsis thaliana contain highly acidic domains. Plant Mol Biol. 1993;22:615–624. doi: 10.1007/BF00047402. [DOI] [PubMed] [Google Scholar]
- Ravina CG, Barroso C, Vega JM, Gotor C. Cysteine biosynthesis in Chlamydomonas reinhardtii: molecular cloning. Eur J Biochem. 1999;264:848–853. doi: 10.1046/j.1432-1327.1999.00676.x. [DOI] [PubMed] [Google Scholar]
- Rotte C, Leustek T. Differential subcellular localization and expression of ATP sulfurylase and 5′-adenylylsulfate reductase during ontogenesis of Arabidopsis leaves indicates that cytosolic and plastid forms of ATP sulfurylase may have specialized functions. Plant Physiol. 2000;124:715–724. doi: 10.1104/pp.124.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffet ML, Lebrun M, Droux M, Douce R. Subcellular distribution of serine acetyltransferase from Pisum sativum and characterization of an Arabidopsis thaliana putative cytosolic isoform. Eur J Biochem. 1995;227:500–509. doi: 10.1111/j.1432-1033.1995.tb20416.x. [DOI] [PubMed] [Google Scholar]
- Saito K, Yokoyama H, Noji M, Murakoshi I. Molecular cloning and characterization of a plant serine acetyltransferase playing a regulatory role in cysteine biosynthesis from watermelon. J Biol Chem. 1995;270:16321–16326. doi: 10.1074/jbc.270.27.16321. [DOI] [PubMed] [Google Scholar]
- Setya A, Murillo M, Leustek T. Sulfate reduction in higher plants: molecular evidence for a novel 5′-adenylylsulfate reductase. Proc Natl Acad Sci USA. 1996;93:13383–13388. doi: 10.1073/pnas.93.23.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FW, Hawkesford MJ, Ealing PM, Clarkson DT, Van den Berg PJ, Belcher AR, Warrilow AG. Regulation of expression of a cDNA from barley roots encoding a high affinity sulphate transporter. Plant J. 1997;12:875–884. doi: 10.1046/j.1365-313x.1997.12040875.x. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Braby CE, Grossman AR. Sulfur economy and cell wall biosynthesis during sulfur limitation of Chlamydomonas reinhardtii. Plant Physiol. 2001;127:665–673. [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Yamazaki M, Sasakura N, Watanabe A, Leustek T, Engler JA, Engler G, Van Montagu M, Saito K. Regulation of sulfur assimilation in higher plants: a sulfate transporter induced in sulfate-starved roots plays a central role in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1997;94:11102–11107. doi: 10.1073/pnas.94.20.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Surdin-Kerjan Y. Metabolism of sulfur amino acids in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1997;61:503–532. doi: 10.1128/mmbr.61.4.503-532.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykoff DD, Davies JP, Melis A, Grossman AR. The regulation of photosynthetic electron transport during nutrient deprivation of Chlamydomonas reinhardtii. Plant Physiol. 1998;117:129–139. doi: 10.1104/pp.117.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz FH, Davies JP, Grossman AR. Characterization of sulfate transport in Chlamydomonas reinhardtii during sulfur-limited and sulfur-sufficient growth. Plant Physiol. 1994;104:981–987. doi: 10.1104/pp.104.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz FH, Davies JP, Grossman A. Sulfur availability and the SAC1 gene control adenosine triphosphate sulfurylase gene expression in Chlamydomonas reinhardtii. Plant Physiol. 1996;112:669–675. doi: 10.1104/pp.112.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HW, Kim MC, Shin PG, Kim JS, Kim CY, Lee SY, Hwang I, Bahk JD, Hong JC, Han C et al. Differential expression of two functional serine/threonine protein kinases from soybean that have an unusual acidic domain at the carboxy terminus. Mol Gen Genet. 1997;255:359–371. doi: 10.1007/s004380050507. [DOI] [PubMed] [Google Scholar]