Abstract
Cryptogein is a 10-kD protein secreted by the oomycete Phytophthora cryptogea that induces a hypersensitive response on tobacco (Nicotiana tabacum var. Xanthi) plants and a systemic acquired resistance against various pathogens. The mode of action of this elicitor has been studied using tobacco cell suspensions. Our previous data indicated that within minutes, cryptogein signaling involves various events including changes in ion fluxes, protein phosphorylation, sugar metabolism, and, eventually, cell death. These results suggested that transport of sugars could be affected and, thus, involved in the complex relationships between plant and microorganisms via elicitors. This led us to investigate the effects of cryptogein on glucose (Glc) uptake and mitochondrial activity in tobacco cells. Cryptogein induces an immediate inhibition of Glc uptake, which is not attributable to plasma membrane (PM) depolarization. Conversely, cryptogein-induced valine uptake is because of PM depolarization. Inhibition of the PM Glc transporter(s) was shown to be mediated by a calcium-dependent phosphorylation process, and is independent of active oxygen species production. This inhibition was associated with a strong decrease in O2 uptake rate by cells and a large mitochondrial membrane depolarization. Thus, inhibition of Glc uptake accompanied by inhibition of phosphorylative oxidation may participate in hypersensitive cell death. These results are discussed in the context of competition between plants and microorganisms for apoplastic sugars.
Many incompatible plant-microorganism interactions are mediated by elicitors of defense responses. Studies on the mode of action of elicitors have revealed that they first activate plasma membrane (PM) proteins involved in recognition (Boller, 1995) and signal transduction. The latter phenomenon involves Ca2+ channels (Zimmermann et al., 1997; Lecourieux et al., 2002), anionic and K+ channels (Nürnberger et al., 1994; Wendehenne et al., 2002), NADPH oxidase (Keller et al., 1998), phospholipases (van der Luit et al., 2000), and probably other proteins that have not yet been identified. When activated, these proteins trigger within a few minutes a complex network of second messengers (free cytosolic calcium increase, cytosolic pH decrease, active oxygen species [AOS], PM depolarization, and changes in metabolism; Batz el al., 1998; Lebrun-Garcia et al., 1999), which in turn triggers defense reactions, as well as the systemic acquired resistance (SAR).
Little information is available concerning the exchange of organic solutes at the PM during plant/microorganism interactions. Both the plant and the phytopathogen compete for the solutes contained in the apoplast that separates them. This competition is particularly important in the case of sugars, which provide both a source of energy and carbohydrate skeletons. Thus, the relative capacity for plants and microorganisms to control the uptake of sugars and other nutrients from the apoplast may be a determinant in the final outcome of the interaction.
Several questions related to solute transport from host to fungus in biotrophic pathogens require clarification. These include the nature of the major transported solutes, the role of the various membranes present at the plant pathogen interface, and the mechanisms of solute transport (Clark and Hall, 1998) from the parasitized plant to the fungus, which is poorly known. For example, in the case of powdery mildews (Erysiphe graminis), although some data argue for Suc being the major form of reduced carbon absorbed by the haustoria of the fungus (Donaldson and Jorgensen, 1988; Manners, 1989), recent evidence indicates that Glc, rather than Suc, is taken up by the fungus (Sutton et al., 1999). Biotrophic fungi obtain nutrients from the host plant, and this may lead to a change in carbon partitioning within the plant. The pathogen forms an additional sink that either induces export from the infected sites of the plant or converts the infected tissue to a net sink (Farrar and Lewis, 1987; Ayres et al., 1996). The fungi may also secrete toxins and elicitors that affect the energy status of the plant cell membrane. The best studied toxins are helminthosporoside (Strobel, 1979), beticolins (Macri and Vianello, 1979; Macri et al., 1983; Blein et al., 1988), and fusicoccin (Marrè, 1979). These toxins, which impact directly or indirectly on the PM H+-ATPase, may have a general effect on the permeability of the membrane and on the activity of H+/solute (sugar, amino acids, and peptides) cotransporters and ion channels. Proteinaceous fungal elicitors have also been reported to increase (elicitor from Rynchosporium secalis, Wevelsiep et al., 1993; elicitor from Cladosporium fulvum, Vera-Estrella et al., 1994) or inhibit (Shiraishi et al., 1991; supprescines from Mycosphaerella pinodes, Kato et al., 1993) the activity of the PM H+-ATPase. Alternatively, the plant may reduce the uptake of hexose by fungi by synthesizing an extra set of hexose transporters that could be involved in the retrieval of hexoses leaked from infected cells. Thus, in Arabidopsis, the hexose transporter AtSTP4 is induced both by bacterial and fungal elicitors (Truernit et al., 1996) and by wounding.
Cryptogein is a 10-kD proteinaceous elicitor produced by Phytophthora cryptogea that induces the hypersensitive response (HR) in whole tobacco (Nicotiana tabacum var. Xanthi) plants and SAR against the tobacco pathogen Phytophthora parasitica var. nicotianae (Ricci, 1997). Using tobacco cell suspensions, it has been shown that cryptogein effects involved numerous PM proteins (for review, see Lebrun-Garcia et al., 1999). After its binding to a high-affinity PM N-glycoprotein (Bourque et al., 1999), cryptogein induces a large Ca2+ influx that depends on protein phosphorylation (Tavernier et al., 1995b). This Ca2+ influx initiates many different events, including phosphorylation of at least 18 polypeptides (Lecourieux-Ouaked et al., 2000), the activation of anionic and K+ channels leading to a large PM depolarization (Pugin et al., 1997; Wendehenne et al., 2002), the activation of mitogen-activated protein kinases (MAPKs; Lebrun-Garcia et al., 1998), NO production (Foissner et al., 2000), microtubule disruption (Binet et al., 2001), and the activation of an NADPH oxidase responsible for AOS production and cytosol acidification. NADPH oxidase activation also leads to a marked decrease in cytosolic Glc-6-phosphate and to the accumulation of glycolytic products (Pugin et al., 1997). These results indicate large modifications to sugar metabolism and suggest that cryptogein might induce changes in sugar fluxes across the PM.
The results reported here indicate that the elicitor cryptogein induces a rapid and total inhibition of Glc uptake in tobacco cells, probably because of the direct inhibition of the Glc transporter(s) by phosphorylation. This effect associated with marked reduction in O2 uptake by tobacco cells and with a large mitochondrial membrane depolarization could participate in hypersensitive cell death. These results are discussed in relation to the competition for nutrients between plant and fungus.
RESULTS
Inhibition of Glc Uptake in Cryptogein-Treated Tobacco Cells
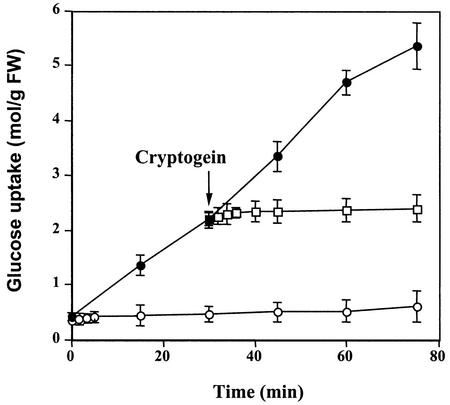
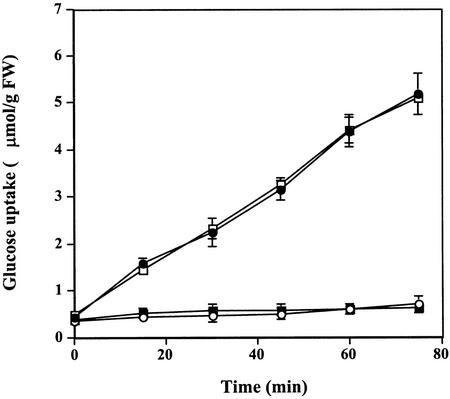
Glc uptake in control tobacco cells incubated with 2 mm 14C-Glc in a 2 mm HEPES (pH 5.75) medium was linear for at least 60 min; rates of uptake approached 4.6 μmol Glc g−1 fresh weight cells. The addition of 50 nm cryptogein to the cells totally repressed Glc uptake during the 75-min duration of the experiment. When added to control cells 35 min after incubation, cryptogein induced a quick inhibition of Glc uptake within 2 min (Fig. 1).
Figure 1.
Effect of cryptogein on Glc uptake (μmol g−1 cell fresh weight) in tobacco cells. Cells were pre-incubated for 5 min in the presence of 14C-Glc (2 mm, 0.055 MBq g−1 fresh weight cells) before addition of 50 nm cryptogein. Aliquots of cell suspensions were withdrawn each 15 min and analyzed by liquid scintillation counting. ●, Control; ○, cryptogein added at time 0; □, cryptogein added at time 30 min. The data represent the means of three replicate experiments ±se.
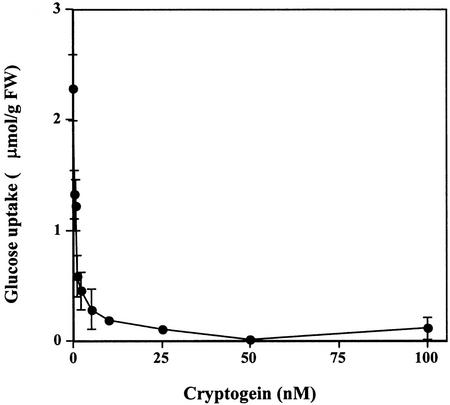
The extent to which Glc uptake was inhibited increased at higher concentrations of cryptogein (1–25 nm; Fig. 2). Glc uptake was totally suppressed with 25 nm cryptogein and the cryptogein concentration inducing 50% inhibition was about 2 nm.
Figure 2.
Effects of increasing cryptogein concentrations on Glc uptake (μmol g−1 cell fresh weight) in tobacco cells. 14C-Glc uptake was determined after a 45-min incubation with cryptogein (0–100 nm) as described in Figure 1. Results are expressed as means of three replicate experiments ±se.
Cryptogein (50 nm) induces the alkalinization of the culture medium (about 0.8 pH units within 10 min in the 2 mm HEPES [pH 5.75] equilibration medium; Bourque et al., 1998). Therefore, Glc uptake was also measured with tobacco cells equilibrated in a 50 mm HEPES medium buffered at pH 6.2 to avoid indirect effects of external pH changes on sugar uptake. Under those conditions, the rate of Glc uptake in control cells and the cryptogein-induced inhibition of Glc uptake were similar to the ones monitored in the 2 mm HEPES at pH 5.75 buffer (data not shown).
Inhibition of Glc Uptake Depends on Calcium Influx
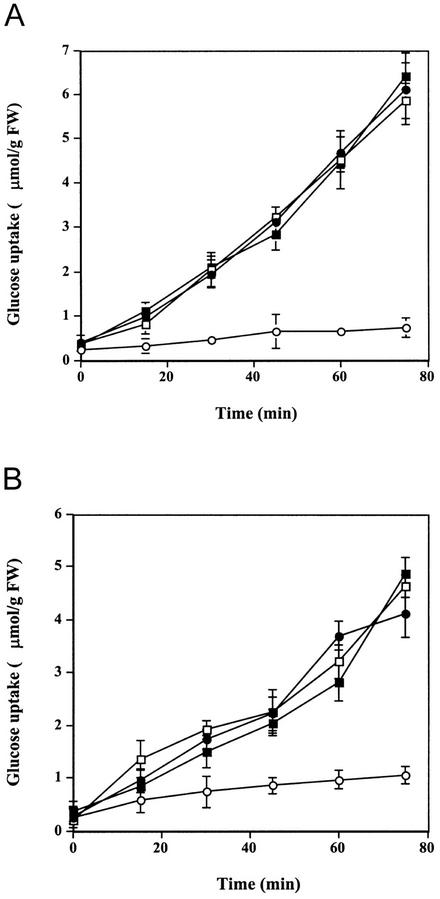
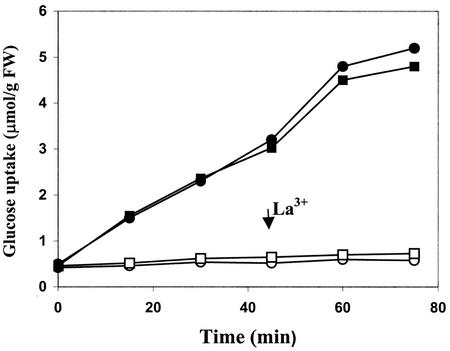
Calcium influx is a very early event in cryptogein signal transduction. It stimulates a cascade of subsequent events, including AOS production, MAPK activation, and cytosol acidification (Tavernier et al., 1995b; Pugin et al., 1997; Lebrun-Garcia et al., 1998). To determine whether cryptogein-induced inhibition of Glc uptake depends on calcium influx, cryptogein treatments were performed in the presence of La3+, which blocks both cryptogein-induced calcium influx and calcium-dependent effects (Tavernier et al., 1995b; Pugin et al., 1997). Five-minute pretreatment of tobacco cells with 1 mm La3+ prevented the inhibition of Glc uptake normally induced by 50 nm cryptogein (Fig. 3A). La3+ did not affect Glc uptake in control tobacco cells. Similar results were obtained with the calcium channel chelator EGTA (data not shown).
Figure 3.
Effects of the Ca2+ channel blocker La3+ and the Ca2+ ionophore A23187 on Glc uptake (μmol g−1 cell fresh weight) into tobacco cells. A, ●, Effects of La3+:control cells; □, control cells treated with 1 mm La3+; ○, cells treated with 50 nm cryptogein; ▪, cells treated with 50 nm cryptogein and 1 mm La3+. B, ●, Effects of A23187:control cells; □, control cells treated with 5 μm A23187; ▪, control cells treated with 10 μm A23187; ○, cells treated with 50 nm cryptogein. Results are expressed as means of three replicate experiments ±se.
Because cryptogein-induced Glc uptake inhibition depends on calcium influx, we tested whether the calcium ionophore A23187 could mimic cryptogein effects on Glc uptake. A23187 (5 and 10 μm), which induced a calcium influx of similar magnitude to that induced by cryptogein (Tavernier et al., 1995b), did not inhibit Glc uptake in tobacco cells in the absence of cryptogein (Fig. 3B). This result fits well with previous data showing that A23187 is unable to induce AOS production or extracellular medium alkalinization, two events depending on calcium influx. It also confirmed that calcium influx is necessary but not sufficient to activate the signal transduction cascade of cryptogein.
Cryptogein-Induced Inhibition of Glc Uptake Depends on Protein Phosphorylation
Protein phosphorylation is the first event detected in cryptogein signaling transduction. Inhibition of protein phosphorylation by staurosporine, an inhibitor of protein kinases, blocks all the responses observed so far, including Ca2+ influx, extracellular medium alkalinization, MAPK activation, and AOS production (Tavernier et al., 1995b; Pugin et al., 1997; Lebrun-Garcia et al., 1998). In contrast, calyculin A, an inhibitor of protein phosphatases 1 and 2A, mimics the effects of cryptogein in tobacco cells. For example, it induces Ca2+ influx, AOS production, and the phosphorylation of the same 18 polypeptides as cryptogein within the first 5 min of treatment (Lecourieux-Ouaked et al., 2000). As expected, staurosporine (5 μm) inhibited cryptogein's effect on Glc uptake (Table I). In control cells, staurosporine had no effect on Glc uptake (Table I). In contrast, the inhibition of Glc uptake induced by calyculin A (500 nm) in control cells exhibited a similar pattern (intensity and kinetics) to the one induced by cryptogein (Fig. 4). Under similar conditions, 10 nm to 1 μm okadaic acid, another protein phosphatase inhibitor, which does not induce the effects of cryptogein (Viard et al., 1994), did not inhibit Glc uptake (data not shown).
Table I.
Glc uptake (mmol g−1 cell fresh wt) in tobacco cells treated with 50 nm cryptogein in presence or absence of 5 mm staurosporine
| Tobacco Cells | Glc Uptake
|
||
|---|---|---|---|
| Control | +0.5% (w/v) DMSO | +5 mm Staurosporine | |
| mmol g−1 cell fresh wt | |||
| Control | 2.10 ± 0.24 | 2.07 ± 0.16 | 2.01 ± 0.11 |
| Cryptogein (50 nm) | 0.08 ± 0.05 | 0.12 ± 0.1 | 1.95 ± 0.15 |
14C-Glc concentration in the uptake medium was 2 mm. Staurosporine and dimethyl sulfoxide (DMSO) were added to control and cryptogein-treated tobacco cells 5 min prior to cryptogein addition (t = 0). After 45 min of treatment, the intracellular 14C-Glc content was determined by liquid scintillation counting. The values are the average of three replicate experiments ± se.
Figure 4.
Effects of the protein phosphatase inhibitor calyculin A on Glc uptake (μmol g−1 cell fresh weight) into tobacco cells. ●, Control cells; ○, cells treated with 50 nm cryptogein; □, cells treated with 500 nm calyculin A. Results were expressed as means of two replicate experiments ±se.
To test the possibility that calyculin-induced inhibition of Glc uptake might be mediated by calcium influx, tobacco cells were treated simultaneously with calyculin A and La3+, which suppresses calcium influx. Under these conditions, Glc uptake was inhibited, indicating that the calyculin effect was not mediated by calcium movement (data not shown).
Cryptogein-Induced Inhibition of Glc Uptake Does Not Depend on AOS Production
Previous data have shown that diphenyleneiodonium (DPI), an inhibitor of the neutrophil PM NADPH oxidase, inhibits the cryptogein-induced AOS production (Pugin et al., 1997), which depends on both protein phosphorylation and calcium influx. To check whether Glc uptake inhibition depended or not on AOS production, tobacco cells were treated with 50 μm DPI 5 min before the addition of cryptogein. However, DPI did not affect Glc uptake in both the control and the cryptogein-treated cells (Fig. 5), indicating that Glc uptake inhibition is independent of AOS production.
Figure 5.
Effects of the PM NADPH oxidase inhibitor DPI on the inhibition of Glc uptake (μmol g−1 cell fresh weight) induced by cryptogein into tobacco cells. ●, Control cells; □, cells treated with 50 μm DPI; ○, cells treated with 50 nm cryptogein; ▪, cells treated with 50 nm cryptogein and 50 μm DPI. Results are expressed as means of three replicate experiments ±se.
Origin of the Inhibition of Glc Uptake
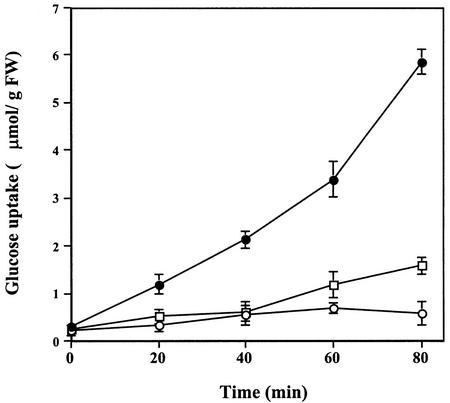
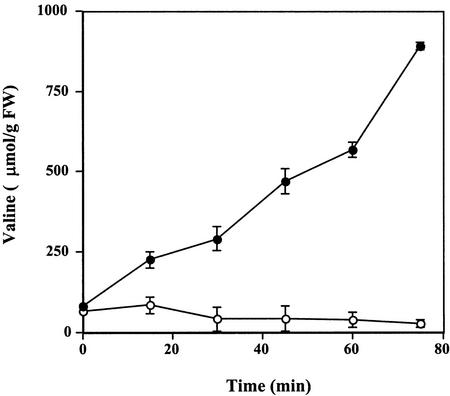
Within the first 5 min, cryptogein induces an anion efflux and calcium influx, which give rise to fast and large PM depolarization from −160 to −50 mV (Pugin et al., 1997; Wendehenne et al., 2002). This PM depolarization might explain the inhibition of Glc uptake, which is coupled with H+ entry and, thus, depends on the transmembrane electrochemical potential difference (ΔΨ; for review, see Delrot et al., 2000). Our data indicated that cryptogein also induced a rapid and marked inhibition of Val uptake (Fig. 6), comparable with that of Glc. This result is consistent with PM depolarization; amino acid transporters, such as Val, are also proton symporters (Despeghel and Delrot, 1983; Li and Bush, 1990).
Figure 6.
Time course of 3H-Val uptake (μmol g−1 cell fresh weight) in tobacco cells treated with cryptogein. Cells were pre-incubated for 5 min in the presence of 3H-Val (2 mm, 0.055 MBq g−1 fresh weight cell) before addition of 50 nm cryptogein. Aliquots were withdrawn at the times indicated and analyzed by liquid scintillation counting. ●, Control cells; ○, cells treated with cryptogein. The data represent the means of three replicate experiments ±se.
The existence of a Glc/H+ symport(s) in tobacco cell suspension was assessed by measuring Glc uptake after treatment with either carbonylcyanide-m-chlorophenylhydrazone (CCCP), a protonophore collapsing the proton gradient or valinomycin (in presence of K+), a specific K+ ionophore collapsing the membrane potential. The data (not shown) indicate that after a 15-min treatment, 5 μm CCCP induced an inhibition of Glc uptake of about 80% in control cells and 98% in cryptogein-treated cells. Valinomycin (5 μm) induced comparable effects, inhibiting Glc uptake by about 80% irrespective of the extracellular K+ concentration (from 1–10 mm) in the absence of cryptogein. This inhibition approached 95% in cryptogein-treated cells (data not shown).
Taken together, these results would support the idea that Glc uptake inhibition is a secondary event requiring PM depolarization. However, the lag times for PM depolarization and Glc uptake inhibition are not consistent with this hypothesis. Glc uptake inhibition occurred in the first 2 min after cryptogein treatment (Fig. 1), whereas PM depolarization occurred after 7 min (Pugin et al., 1997). Thus, further experiments were performed to investigate more precisely the relationship between PM depolarization and Glc uptake inhibition. Previous observations had shown that by preventing calcium entry, La3+ suppressed PM depolarization when added before cryptogein (Pugin et al., 1997). Furthermore, La3+ allowed the reestablishment of the membrane potential when it was added after the elicitor to the largely depolarized cells. This result indicated that a sustained calcium influx was necessary to maintain the PM depolarization. Thus, similar assays were performed by measuring Glc uptake in cell suspensions where La3+ was added before cryptogein or 45 min after cryptogein. As shown in Figure 3, when added before cryptogein, La3+ suppressed the inhibition of Glc uptake. When added 45 min after cryptogein, however, La3+ was ineffective at restoring Glc uptake (Fig. 7). Taken together, these results suggest that inhibition of Glc uptake by cryptogein is not a result of PM depolarization.
Figure 7.
Effects of La3+ on Glc uptake inhibition in tobacco cells (μmol g−1 cell fresh weight) during cryptogein treatment. After a 45-min treatment with 50 nm cryptogein, 2 mm La3+ (arrow) was added to tobacco cells. ●, Control cells; ○, cells treated with 50 nm cryptogein; ▪, control tobacco cells treated with 2 mm La3+ at time 45 min; ♦, 50 nm treated tobacco cells added with 2 mm La3+ at time 45 min.
Another approach used to study the relationship between PM depolarization and cryptogein-induced inhibition of Glc uptake consisted of monitoring these parameters in purified PM vesicles energized by an artificial proton motive force. In this experimental system, the uptake activity of proton symporters is energized by an imposed pH and electrical gradient. Therefore, provided that the passive electrical permeability of the membrane is not affected, the activity of the proton symporters does not depend on proton pumping by ATPase, but rather reflects the intrinsic activity of the transporters (Lemoine and Delrot, 1989; Bush, 1990). PMs were prepared either from control tobacco cells or from cells treated for 30 min with cryptogein. The integrity of both PM vesicle preparations was first tested by monitoring ATP-dependent proton transport. Neither the rate of ATPase activity (1.2 μmol Pi h−1 mg−1 proteins) nor the rate of proton pumping (0.76 fluorescence units min−1 mg−1 proteins) were significantly affected by cryptogein treatment, indicating that cryptogein does not induce passive proton leakage in PM vesicles. The proton motive force-dependent uptakes of Glc or Val were also measured in the PM vesicle preparations. Table II clearly shows a strong inhibition (80%) of proton motive force-dependent Glc uptake in PM vesicles from cryptogein-treated cells when compared with control PM vesicles. In the same experimental conditions, Val uptake was not affected in PM vesicles from cryptogein-treated cells (Table II). Taken together, these results indicate that Val uptake inhibition is probably because of PM depolarization, whereas the inhibition of the Glc transporter(s) is because of posttranslational modifications. Cryptogein has no direct effect on Glc uptake or Val transporters. The addition of cryptogein to vesicle preparations did not affect Glc or Val transport (data not shown).
Table II.
Glc and Val uptakes in PM vesicles prepared from both control and 30-min cryptogein-treated tobacco cells
| Cells | Glc Uptake | Val Uptake |
|---|---|---|
| nmol mg−1 PM proteins | ||
| Control | 2.15 ± 0.08 | 136.8 ± 6.4 |
| Cryptogein (50 nm) | 0.43 ± 0.05 | 152.7 ± 7.8 |
Glc and Val contents in vesicles are measured after 2 min uptake as described in “Materials and Methods.” The values are the average of three independent experiments (±se).
Inhibition of O2 Uptake Rate
The strong inhibition of Glc uptake induced by cryptogein was expected to induce a dramatic shortage of sugars that could be oxidized during mitochondrial respiration, thus depriving the cell in energy and leading to subsequent cell death. This led us to monitor the effects of cryptogein on O2 uptake and the polarization status of the mitochondria, both events reflecting the mitochondria activity.
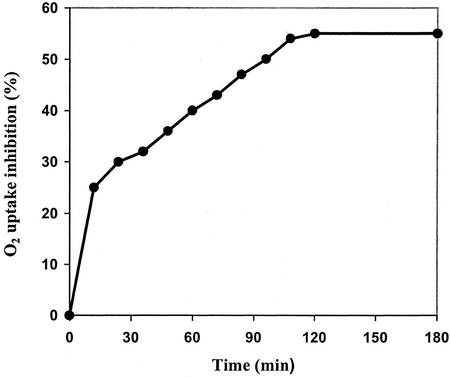
Cell suspensions in both the absence or presence of catalase had constant rates of O2 uptake for at least 3 h (between 4.0 and 5.5 nmol O2 min−1/0.1 g fresh weight depending on the assay). The addition of 50 nm cryptogein, in the presence of catalase, induced a sudden decrease in O2 uptake rate in the first 12 min of treatment (25% inhibition). Thereafter, O2 uptake decreased linearly and inhibition reached an optimum value of 52% at 108 min that remained constant until the 3-h treatment (Fig. 8). We were not able to monitor cryptogein effects for longer periods of treatment because O2 uptake rate in control cells decreased after 3 h. Catalase was added to restore O2 consumed during H2O2 production because of NADPH oxidase activation in cryptogein-treated cells (Pugin et al., 1997). In the absence of catalase in cryptogein-treated cell suspensions, the decrease in O2 would correspond to the sum of mitochondrial respiration and the oxidative burst. As expected, catalase decreased the apparent O2 uptake rate in the first 20 min of treatment (about 20%), whereas the enzyme did not affect O2 uptake rate in control cells (data not shown).
Figure 8.
Inhibition of O2 uptake rate in cryptogein-treated cells. Tobacco cell suspensions (0.1 g fresh weight mL−1) were treated with 50 nm cryptogein and catalase (1,800 units). Control cell suspensions contain only catalase. Catalase was added to restore O2 consumed during hydrogen peroxide (H2O2) production (oxidative burst). Under these conditions, O2 uptake rate corresponds to the mitochondrial respiration. The figure shows one representative assay from a sample of eight.
Depolarization of the Mitochondrial Membrane
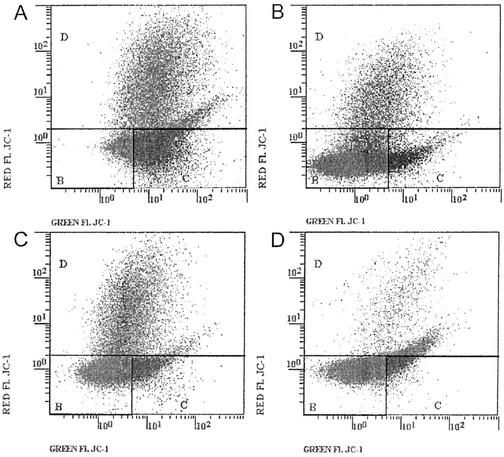
5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimoidazolcarboxyanine iodide (JC-1) is a cationic dye that exhibits potential-dependent accumulation in mitochondria, indicated by a fluorescence emission shift from green (525 nm) to red (590 nm). This potential-sensitive color shift is because of concentration-dependent formation of red fluorescent aggregates. JC-1 is more specific for mitochondrial than for PM potentials, and is more consistent in its response to membrane depolarization than other cationic dyes. The most widely documented application of JC-1 has been for detection of mitochondrial depolarization occurring in the early stage of apoptosis (Cossarizza et al., 1993). Mitochondrial depolarization is indicated by a decrease in the red fluorescence. For our control tobacco cells, flow cytometry indicated three distinct cell populations in which mitochondria were more or less polarized (Fig. 9A). A first population representing about 10% of the cells did not stain and could be non-polarized; however, these cells were not dead, as indicated by red neutral analysis (less than 2% cell death in control suspensions). A second population representing about 48% of cells was weakly polarized (green fluorescence), and a third population (42%) was highly polarized (green and red fluorescence; Table III). These differences in mitochondrial membrane potential could correspond to cells in different physiological states in non-synchronized cell suspensions. The sensitivity of the dye, using tobacco cell suspensions, was verified by the response to K+/valinomycin-induced depolarization (Fig. 9B; Table III). As expected, valinomycin rapidly induced a large decrease in the intensity of red and green fluorescence observed on profiles of flow cytometry. The proportion of depolarized cells reached 82% after 12 min of treatment (Table III). Under similar conditions, the time course of cryptogein effects on mitochondrial potential showed a comparable decrease in the intensity of red and green fluorescence. After 12 min of cryptogein treatment, about 63% of cells contained depolarized mitochondria (Fig. 9C; Table III), and after 1 h of treatment, the ratio of cells with high mitochondrial membrane potential fell to 3% (Fig. 9D; Table III).
Figure 9.
Mitochondrial membrane potential decrease in tobacco cell suspensions in response to valinomycin or cryptogein, monitored using flow cytometry and JC-1 as a membrane potential probe. The fluorescence associated to JC-1 was measured as described in experimental procedure on at least 30,000 cells per assay and expressed on a logarithmic scale. For each histogram, three regions were delimited corresponding to cells with high membrane potential (d), cells with low membrane potential (b), and cells not stained (c), respectively. A, Control cells; B, cells treated with 1 μm valinomycin for 12 min; C and D, cells treated with 50 nm cryptogein during 12 min and 1 h, respectively. The figure is one representative assay from a sample of five.
Table III.
Ratio (%) of tobacco cells with high, low, and undetectable mitochondrial membrane potential in control cells and after treatments with 1 mm valinomycin (12 min) or 50 nm cryptogein (12 min and 1 h)
| Membrane Potential | Undetectable | Low | High |
|---|---|---|---|
| Control | 10 | 48 | 42 |
| Valinomycin/K+ (t = 12 min) | 82 | 8 | 10 |
| Cryptogein (t = 12 min) | 63 | 15 | 22 |
| Cryptogein (t = 1 h) | 82 | 15 | 3 |
The values were obtained from flow cytometry histograms (Fig. 9).
DISCUSSION
Previous studies using tobacco cell suspensions clearly demonstrated that cryptogein activates a set of PM proteins, including high-affinity binding sites (Bourque et al., 1999), calcium channels (Tavernier et al., 1995b; Lecourieux et al., 2002), potassium and anionic channels (Wendehenne et al., 2002), and an NADPH oxidase responsible for the production of AOS. Channel activation is responsible for a large PM depolarization, and NADPH oxidation leads to the activation of the pentose phosphate pathway, to a decrease in the Glc-6-phosphate content, and to an increase in glycolytic products (Pugin et al., 1997), indicating changes to the plant carbohydrate metabolism. All the events induced upon binding of cryptogein on the PM are blocked by staurosporine, an inhibitor of protein kinases, and mimicked by calyculin A, an inhibitor of protein phosphatases (Lecourieux-Ouaked et al., 2000). Given that sugar transport at the PM level depends on the proton motive force (Delrot et al., 2000) and because Suc uptake may be regulated by phosphorylation (Roblin et al., 1998), our previous results led us to investigate a possible effect of cryptogein on sugar uptake. The interest of such study is further strengthened by a possible role of sugars in signaling and gene expression (Jang and Sheen, 1994; Smeekens and Rook, 1997; Gibson, 2000). The model cryptogein/tobacco cells offered a unique opportunity to study sugar transport during an elicitation process.
Our time course and concentration dependence studies (Figs. 1 and 2) showed that cryptogein inhibited Glc uptake within 1 to 2 min. The efficient elicitor concentrations correspond to those which trigger other well known cryptogein-induced responses: Ca2+ influx, extracellular medium alkalinization, or AOS production (Bourque et al., 1998). The concentration of cryptogein required for 50% inhibition was about 2 nm, a value that is consistent with the Kd value (2 nm) obtained for the binding of cryptogein on high-affinity binding sites (Wendehenne et al., 1995).
Because Glc uptake occurs with proton symport, it depends on the transmembrane pH gradient and on the transmembrane potential difference, which in turn are controlled by the proton pump ATPase and the ionic permeability of the PM. Because cryptogein induced an acidification of the cytosol, an alkalinization of the culture medium, and a strong PM depolarization from −160 to −60 mV (Pugin et al., 1997), Glc uptake inhibition by cryptogein could be a consequence of the decrease of proton motive force. This would also account for the inhibitory effect of cryptogein on Val uptake, which also depends on proton motrice force (Fig. 6). However, further experiments negated this hypothesis, suggesting instead a more direct effect on the Glc transporter(s).
Direct inhibition of Glc transporter(s) was demonstrated by measuring Glc uptake in PM vesicles prepared from both control and cryptogein-treated tobacco cells. This experimental system, in which the proton motive force is imposed artificially and no longer depends on the proton pumping ATPase, allows, under certain conditions, direct monitoring of the activity of the Glc transporter(s). PM integrity was first checked in both vesicle preparations (control and cryptogein-treated cells). Although cryptogein did not affect passive ionic permeability of the PM vesicles, Glc uptake was inhibited by about 80% in vesicle preparations from cryptogein-treated cells compared with PM vesicles from control cells (Table II). In the same experimental conditions, Val uptake was not affected in PM vesicles from cryptogein-treated cells, demonstrating that Val uptake inhibition was because of PM depolarization, whereas Glc transport inhibition resulted because of an inhibition of Glc transporter(s). Our previous data (Pugin et al., 1997) showing that cryptogein-induced PM depolarization occurs later (7 min) than Glc uptake (2 min; present assays) are also in favor of a Glc uptake inhibition independent of PM depolarization. Other assays support the regulation of Glc transport by a phosphorylation/dephosphorylation process. On the one hand, cryptogein-induced inhibition of Glc uptake in tobacco cells was suppressed by a calcium channel blocker (La3+) or a protein kinase inhibitor (staurosporine). On the other hand, Glc uptake in control cells was inhibited by the protein phosphatase (type 1 and 2A) inhibitor calyculin A in a calcium-independent way. Taken together, these results indicate that: (a) in cryptogein-treated cells, the Glc transporter(s) may be inhibited by a calcium-dependent phosphorylation process (direct or indirect calcium-dependent activation of a protein kinase or inhibition of a protein phosphatase); and (b) in control cells, the inhibition of Glc uptake by calyculin A may be because of a direct inhibition of the same protein phosphatase that, in concert with a protein kinase, controls the Glc transport activity. As previously reported (Roblin et al., 1998), the Suc transporter(s) is active when dephosphorylated and inactive when phosphorylated.
The inhibition of Glc uptake should be—at least partially—responsible for the rapid and expansive inhibition of O2 uptake in cryptogein-treated cells, which reached about 50% after 90 min of treatment (Fig. 8). This effect reflects an inhibition of the mitochondrial respiration as indicated by the sudden decrease in mitochondrial potential (Fig. 9; Table III). Thus, the inhibition of the Glc uptake in elicitor-treated cells leads to a decrease in energy production and probably participates with calcium influx in the cell death (HR) that occurs later. Moreover, mitochondria depolarization could lead to the release of programmed cell death proteins such as cytochrome c and apoptosis-inducing factor (Susin et al., 1999). A previous analysis of the kinetics of cell death in cryptogein-treated cell suspensions (Binet et al., 2001) indicated that cell death appeared after 6 h of treatment and that about 75% of cells were dead after 24 h of treatment. Nevertheless, Glc uptake inhibition might not be the sole process responsible for O2 uptake decrease. Other cryptogein-induced events, particularly NO production (Foissner et al., 2000), could inhibit mitochondrial respiration (Wendehenne et al., 2001) as recently reported (Zottini et al., 2002).
The results described here for the effects of cryptogein on the tobacco Glc transporter(s) can be compared with those reported for the sugar beet (Beta vulgaris) Suc transporter. Roblin et al. (1998) showed that proton-driven Suc uptake is inhibited in PM vesicles prepared from sugar beet leaves infiltrated with the phosphatase inhibitor okadaic acid. It was also concluded that the Suc transporter is inhibited by phosphorylation (or derepressed by dephosphorylation). Regulation of sugar transporter activity by phosphorylation, therefore, may be a possible common mechanism for Suc and hexose transporters. However, the phosphatases involved in dephosphorylation are possibly different because okadaic acid is active toward the sugar beet Suc transporter (Roblin et al., 1998), but inactive toward the tobacco Glc transporter(s) (this report). The monosaccharide transporter NtMST1 from tobacco contains several potential phosphorylation sites, some of which are highly conserved in hexose transporters from Arabidopsis (AtSTP1 and AtSTP4) and in Chlorella kessleri (CkHUP1), but it is not known whether NtMST1 is responsible for Glc uptake in our tobacco cell suspensions. Therefore, these potential phosphorylation sites would be good candidates for regulation by phosphorylation. For example, amino acid residues T48, T104, and T376 (NtMST1) are located in cytoplasmic phosphorylation sequences conserved in AtSTP1, AtSTP4, and CkHUP1. S226 and S263 (NtMST1) are also in cytoplasmic phosphorylation consensus sites, but they are conserved only in Arabidopsis. Our data indicate that the activity of hexose transport is affected by phosphorylation. However, they do not prove yet that the hexose transporter(s) themselves are phosphorylated. Further experiments are necessary to clarify this point and to determine which sites would be involved in the inhibition described here.
In Arabidopsis, various elicitors induce the expression of the hexose transporter AtSTP4 (Truernit et al., 1996). Whether a similar effect is induced in tobacco by cryptogein remains to be investigated. The different effects reported on sugar transport after fungal infection (Truernit et al., 1996; Clark and Hall, 1998; Sutton et al., 1999; present report) underline that the control of carbon availability is a major issue of the plant/fungus interaction. In addition to carbon availability, this may also affect gene expression through the carbohydrate status of the apoplast, the protoplasm, and sugar sensing.
In fungus-infected leaves undergoing an HR, elicitor-induced localized cell death is instrumental in activating defense reactions in the surrounding tissues to restrict further pathogen growth. The rapid action of cryptogein on tobacco cells indicates that an early step in this process is a paralysis of their ability to retrieve hexoses from the apoplast. Although this carbon supply would be freely available to the fungus, the inhibition of Glc uptake would benefit the plant by accelerating cell death-mediated defense reactions. The plant Glc transporter(s), therefore, could be a crossroad tool for both pathogen and plant strategies attempting to secure their propagation or survival. Elicitor-induced inhibition of Glc transport and dependent HR participate in the circumvention of the fungal virulence and the expression of resistance.
MATERIALS AND METHODS
Plant Material and Chemicals
Tobacco cells (Nicotiana tabacum var. Xanthi) were cultivated in Chandler's medium on a rotary shaker (150 rpm, 25°C, photon flux rate of 30–40 μmol m−2 s−1) as previously described (Bourque et al., 1998). Cells were maintained in the exponential phase and subcultured 1 d before utilization. Cryptogein was purified according to Bonnet et al. (1996).
Glc {d-[14C(U)] (0.48 GBq mmol−1)} and Val {l-[3,4-3H] (1.7 TBq mmol−)} were from NEN Life Science Products (Boston). Staurosporine, calyculin A, valinomycin, CCCP, DPI, and A23187 were from Sigma (St. Louis) and were added to cell suspensions from concentrated stock solutions in DMSO. Equivalent DMSO volumes were added to controls.
Glc and Val Uptake by Tobacco Cells
Cells were collected during the exponential growth phase and washed by filtration in a suspension buffer containing 175 mm mannitol, 0.5 mm CaCl2, 0.5 mm K2SO4, and 2 mm HEPES or 50 mm HEPES adjusted with KOH to pH 5.75 or 6.2, respectively. Cells were resuspended at 0.1 g fresh weight mL−1 with suspension buffer and equilibrated for 2 h on a rotary shaker (150 rpm, 24°C).
Glc and Val uptake were measured after addition of 2 mm 14C-Glc (0.055 MBq g−1 fresh weight cells) or 2 mm 3H-Val (0.055 MBq g−1 fresh weight cells) 5 min before the treatment with 50 nm cryptogein. After different periods of treatment (0–75 min), duplicate 2-mL aliquots of cell suspensions were collected and filtered under vacuum on GF/A glass fiber filters (Whatman International Ltd., Maidstone, UK), washed once for 1 min with 10 mL of cell suspension buffer medium, and then washed twice with 5 mL of the suspension buffer for 20 s. Cells remaining on filters were collected, weighed, and placed in scintillation vials with 10 mL of Ready-Safe cocktail (Beckman Instruments, Fullerton, CA). The radioactivity of the vials was then counted by liquid scintillation (Packard 2000, Hewlett-Packard, Palo Alto, CA).
Other chemicals (LaCl3, A23187, staurosporine, calyculin A, DPI, and CCCP) were added when indicated in the results at different concentrations and for various times. Control tobacco cells were incubated under the same conditions without cryptogein.
Glc and Val Uptakes in PM Vesicle Preparations
Tobacco PMs from both control and cryptogein-treated tobacco cells were prepared by two-phase partitioning as previously described (Bourque et al., 1999) with minor modifications listed below. After two-phase partitioning, PM vesicles were diluted in equilibration buffer (300 mm sorbitol, 0.5 mm CaCl2, 0.25 mm MgCl2, 0.5 mm dithiothreitol, and 50 mm KH2PO4/K2HPO4 [pH 7.5]). They were then centrifuged (100,000g for 1 h), and the final pellets were suspended in this buffer at about 10 mg mL−1 proteins before storage at −80°C.
Glc uptake in tobacco PM vesicles was studied as described previously (Lemoine and Delrot, 1989). In brief, at time 0, 20 μg of PM proteins stored in equilibration buffer was mixed with 400 μL of uptake medium: 300 mm sorbitol, 1 mm 14C-Glc (0.005 MBq mL−1), 0.5 mm CaCl2, 0.25 mm MgCl2, 50 μm valinomycin, and either 50 mm NaH2PO4/Na2HPO4 (pH 5.5) or 50 mm KH2PO4/K2HPO4 (pH 7.5) for measurement of Glc uptake either dependent on or independent of the proton motive force, respectively. Val uptake was measured in the same conditions using 1 mm 3H-Val (0.005 MBq mL−1) in the uptake medium. After 1 or 2 min of incubation, the reaction was stopped by addition of 2 mL of uptake medium (pH 7.5) containing 0.5 mm HgCl2. The mixture was then filtered through nitrocellulose filters (HAWP, Millipore, Bedford, MA) and washed once with 2 mL of the same medium. The filters were placed in scintillation vials with 10 mL of Ready-Safe cocktail for counting. The mean value obtained for each condition corresponds to six measurements.
PM Vesicle Integrity
ATP-dependent H+ transport and ability of vesicles to maintain a pH gradient were measured by monitoring the fluorescence quenching of acridine orange with inside-out PM vesicles prepared from control tobacco cells and cells treated for 30 min with 25 nm cryptogein (Fraichard et al., 1991; Noubahni et al., 1996). ATP hydrolysis was measured as described by Magnin et al. (1995).
O2 Uptake Rate
Tobacco cells were prepared as previously described for Glc and Val uptake assays. O2 uptake rate by tobacco cells (0.1g fresh weight mL−1) was measured at 25°C in 1 mL of medium containing 2 mm HEPES, 0.5 mm CaCl2, 0.5 mm K2SO4, and 10 mm Glc (pH 6.6). Cell suspensions were treated with 50 nm cryptogein and catalase (1,800 units); control cell suspensions contained catalase (1,800 units) only. Catalase was added to restore O2 consumed during H2O2 production (oxidative burst). O2 uptake rate was measured for at least 3 h with a Clark-type oxygen electrode system purchased from Hansatech Instrument Ltd. (Norfolk, UK). The O2 concentration in air-saturated medium was taken as 240 μm.
Mitochondrial Membrane Potential
Tobacco cells were prepared as described for O2 uptake (0.1g fresh weight mL−1) in a medium containing 50 mm HEPES, 0.5 mm CaCl2, 0.5 mm K2SO4, and 10 mm Glc (pH 7.0). Before treatment, cells were first stained with the mitochondrial membrane potential probe JC-1 by incubating 2 mL of cell suspensions for 15 min (24°C in the dark) with 2 μg mL−1 JC-1 (3 μm). JC-1 from Molecular Probes Inc. (Eugene, OR) was dissolved and stored according to the manufacturer's instructions. Then, cells were treated with 100 nm or 1 μm valinomycin (Sigma) a drug known to affect mitochondrial membrane potential or 50 nm cryptogein. Cells without prior washing were subjected to flow analysis using a Coulter Epics Elite Flow Cytometer, ESP Cell Sorter (Beckman Instruments, Fullerton, CA). An air-cooled argon laser operating at 20 mW was used for excitation at 488 nm. Fluorescence signals were collected using a bandpass filter centered at 525 and 575 nm. A minimum of 30.104 events per sample was acquired in list mode and analyzed with Expo 2 software (Beckman Instruments).
ACKNOWLEDGMENTS
We wish to thank Dr. Michel Ponchet (Institut National de la Recherche Agronomique, Antibes, France) for the generous gift of cryptogein and Annick Chiltz (INRA, Dijon, France) for technical assistance. We are grateful to Prof. Kevin Gould (University of Auckland, New Zealand) for reviewing the English manuscript and to Prof. Leendert C. van Loon (Graduate School Experimental Plant Science, Utrecht, The Netherlands) for helpful discussion.
Footnotes
This work was supported by the Ministère de l'Education Nationale, de la Recherche, et de la Technologie, by the Institut National de la Recherche Agronomique, and by the Conseil Régional de Bourgogne.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.009449.
LITERATURE CITED
- Ayres PG, Press MC, Spencer-Phillips PTN. Photoassimilate distribution. In: Zamski E, Schaffer AA, editors. Plants and Crops. New York: Marcel Dekker; 1996. pp. 479–499. [Google Scholar]
- Batz O, Logemann E, Reinold S, Hahlbrock K. Extensive reprogramming of primary and secondary metabolism by fungal elicitor or infection in parsley cells. J Biol Chem. 1998;379:1127–1135. doi: 10.1515/bchm.1998.379.8-9.1127. [DOI] [PubMed] [Google Scholar]
- Binet MN, Humbert C, Lecourieux D, Vantard M, Pugin A. Disruption of microtubular cytoskeleton induced by cryptogein, an elicitor of hypersensitive response in tobacco cells. Plant Physiol. 2001;125:564–572. doi: 10.1104/pp.125.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blein JP, Bourdil I, Rossignol M, Scalla R. Cercospora beticola toxin inhibits vanadate-sensitive H+ transport in corn roots membrane vesicles. Plant Physiol. 1988;88:429–434. doi: 10.1104/pp.88.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T. Chemoperception of microbial signals in plant cells. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:189–214. [Google Scholar]
- Bonnet P, Bourdon E, Ponchet M, Blein JP, Ricci P. Acquired resistance triggered by elicitins in tobacco and other plants. Eur J Plant Pathol. 1996;102:181–192. [Google Scholar]
- Bourque S, Binet MN, Ponchet M, Pugin A, Lebrun-Garcia A. Characterization of the cryptogein binding sites on plant plasma membranes. J Biol Chem. 1999;274:34699–34705. doi: 10.1074/jbc.274.49.34699. [DOI] [PubMed] [Google Scholar]
- Bourque S, Ponchet M, Binet MN, Ricci P, Pugin A, Lebrun-Garcia A. Comparison of binding properties and early biological effects of elicitins in tobacco cells. Plant Physiol. 1998;118:1317–1326. doi: 10.1104/pp.118.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush DR. Electrogenicity, pH-dependence, and stoichiometry of the sucrose proton symport. Plant Physiol. 1990;93:1590–1596. doi: 10.1104/pp.93.4.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JIM, Hall JL. Solute transport into healthy and powdery mildew-infected leaves of pea and uptake by powdery mildew mycelium. New Phytol. 1998;140:261–269. doi: 10.1046/j.1469-8137.1998.00263.x. [DOI] [PubMed] [Google Scholar]
- Cossarizza A, Baccarani-Contri A, Kalashnikova G, Franceschi C. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimoidazolcarboxyanine iodide (JC-1) Biochem Biophys Res Commun. 1993;197:40–45. doi: 10.1006/bbrc.1993.2438. [DOI] [PubMed] [Google Scholar]
- Delrot S, Atanassova R, Maurousset L. Regulation of sugar, amino acid and peptide plant membrane transporters. Biochim Biophys Acta. 2000;1465:281–306. doi: 10.1016/s0005-2736(00)00145-0. [DOI] [PubMed] [Google Scholar]
- Despeghel JP, Delrot S. Energetics of amino acid uptake by Vicia faba leaf tissue. Plant Physiol. 1983;71:1–6. doi: 10.1104/pp.71.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson IA, Jorgensen JH. Barley powdery mildew “invertase” is an α-glucosidase. Carlsberg Res Commun. 1988;53:421–430. [Google Scholar]
- Farrar JF, Lewis DH. In: Fungal Infection of Plants. Pegg GF, Ayres PG, editors. Cambridge, UK: Cambridge University Press; 1987. pp. 92–132. [Google Scholar]
- Foissner I, Wendehenne D, Langebartels C, Durner J. Technical advance: in vivo imaging of an elicitor-induced nitric oxide burst in tobacco. Plant J. 2000;23:817–824. doi: 10.1046/j.1365-313x.2000.00835.x. [DOI] [PubMed] [Google Scholar]
- Fraichard A, Magnin T, Trossat C, Pugin A. Properties of the proton pumping pyrophosphatase in tonoplast vesicles of Acer pseudoplatanus. Functional molecular mass and polypeptide composition. Plant Physiol Biochem. 1991;31:349–359. [Google Scholar]
- Gibson SI. Plant sugar-response pathways. Part of a complex regulatory web. Plant Physiol. 2000;124:1532–1539. doi: 10.1104/pp.124.4.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JC, Sheen J. Sugar sensing in higher plants. Plant Cell. 1984;9:5–19. doi: 10.1105/tpc.6.11.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Shiraishi T, Toyoda K, Saitoh K, Satoh Y, Tahara M, Yamada T, Oku H. Inhibition of the ATPase activity in pea plasma membranes by fungal suppressors from Mycosphaerella pinodes and their peptide moieties. Plant Cell Physiol. 1993;34:439–445. [PubMed] [Google Scholar]
- Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C. A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell. 1998;10:255–266. doi: 10.1105/tpc.10.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun-Garcia A, Ouaked F, Chiltz A, Pugin A. Activation of MAPK homologues by elicitors in tobacco cells. Plant J. 1998;15:773–781. doi: 10.1046/j.1365-313x.1998.00269.x. [DOI] [PubMed] [Google Scholar]
- Lebrun-Garcia A, Bourque S, Binet MN, Ouaked F, Wendehenne D, Chiltz A, Schaffner A, Pugin A. Involvement of plasma membrane proteins in plant defense responses. Analysis of the cryptogein signal transduction in tobacco. Biochimie. 1999;81:663–668. doi: 10.1016/s0300-9084(99)80123-0. [DOI] [PubMed] [Google Scholar]
- Lecourieux D, Mazars C, Pauly N, Ranjeva R, Pugin A. Analysis and effects of cytosolic free calcium increases in response to elicitors in Nicotiana plumbaginifolia cells. Plant Cell. 2002;14:2627–2641. doi: 10.1105/tpc.005579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourieux-Ouaked F, Pugin A, Lebrun-Garcia A. Phosphoproteins involved in the signal transduction of cryptogein, an elicitor of defense reactions in tobacco. Mol Plant-Microbe Interact. 2000;13:821–829. doi: 10.1094/MPMI.2000.13.8.821. [DOI] [PubMed] [Google Scholar]
- Lemoine R, Delrot S. Proton motive force driven sucrose uptake in sugar beet plasma membrane vesicles. FEBS Lett. 1989;249:129–133. [Google Scholar]
- Li ZC, Bush DR. pH-dependent amino acid transport into plasma membrane vesicles isolated from sugar beet leaves: I. Evidence for carrier-mediated, electrogenic flux through multiple transport systems. Plant Physiol. 1990;94:268–277. doi: 10.1104/pp.94.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macri F, Dell'Antone P, Vianello A. ATP-dependent proton uptake inhibited by Cercospora beticola toxin in pea stem microsomal vesicles. Plant Cell Environ. 1983;6:555–558. [Google Scholar]
- Macri F, Vianello A. Inhibition of K+ uptake, H+ extrusion and K+-activated ATPase, and depolarization of transmembrane potential in plant tissues treated with Cercospora beticola toxin. Physiol Plant Pathol. 1979;15:161–170. [Google Scholar]
- Magnin T, Fraichard A, Trossat C, Pugin A. The tonoplast H+-ATPase of Acer pseudoplatanus is a vacuolar-type ATPase that operates with a phosphoenzyme intermediate. Plant Physiol. 1995;109:285–292. doi: 10.1104/pp.109.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manners JM. The host-haustorium interface in powdery mildews. Aust J Plant Physiol. 1989;16:45–52. [Google Scholar]
- Marrè E. Fusicoccin: a tool in plant physiology. Annu Rev Plant Physiol. 1979;30:273–288. [Google Scholar]
- Noubahni AM, Sakr S, Denis MH, Delrot S. Transcriptional and post-translational control of the plant plasma membrane H(+)-ATPase by mechanical treatments. Biochem Biophys Acta. 1996;1281:213–219. doi: 10.1016/0005-2736(96)00017-x. [DOI] [PubMed] [Google Scholar]
- Nürnberger T, Nennstiel D, Jabs T, Sacks WR, Hahlbrock K, Scheel D. High affinity binding of a fungal oligopeptide elicitor to parsley plasma membranes triggers multiple defense responses. Cell. 1994;78:449–460. doi: 10.1016/0092-8674(94)90423-5. [DOI] [PubMed] [Google Scholar]
- Pugin A, Frachisse JM, Tavernier E, Bligny R, Gout E, Douce R, Guern J. Early events induced by the elicitor cryptogein in tobacco cells: involvement of a plasma membrane NADPH oxidase and activation of glycolysis and the pentose phosphate pathway. Plant Cell. 1997;9:2077–2091. doi: 10.1105/tpc.9.11.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci P. Induction of the hypersensitive response and systemic acquired resistance by fungal proteins: the case of elicitins. In: Stacey G, Keen NT, editors. Plant-Microbe Interactions. Vol. 3. New York: Chapman and Hall; 1997. pp. 53–75. [Google Scholar]
- Roblin G, Sakr S, Bonmort J, Delrot S. Regulation of a plant plasma membrane sucrose transporter by phosphorylation. FEBS Lett. 1998;424:165–168. doi: 10.1016/s0014-5793(98)00165-3. [DOI] [PubMed] [Google Scholar]
- Shiraishi T, Araki M, Yoshioka H, Kobayashi I, Yamada T, Ichinose Y, Kuno H, Oku H. Inhibition of ATPase activity in pea plasma membranes in situ by a suppressor from a pea pathogen, Mycosphaerella pinodes. Plant Cell Physiol. 1991;32:1067–1075. [Google Scholar]
- Smeekens S, Rook F. Sugar sensing and sugar-mediated signal transduction in plants. Plant Physiol. 1997;115:7–13. doi: 10.1104/pp.115.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel GA. The relationship between membrane ATPase activity in sugarcane and heat-induced resistance to helminthosporoside. Biochim Biophys Acta. 1979;554:460–468. doi: 10.1016/0005-2736(79)90384-5. [DOI] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brother GM, Mangion J, Jacotot E, Costantini P, Loeffler M et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Sutton PN, Henry MJ, Hall JL. Glucose, and not sucrose, is transported from wheat to wheat powdery mildew. Planta. 1999;208:426–430. [Google Scholar]
- Tavernier E, Stallaert V, Blein JP, Pugin A. Changes in lipid composition in tobacco cells treated with cryptogein, an elicitor from Phytophtora cryptogea. Plant Sci. 1995a;104:117–125. [Google Scholar]
- Tavernier E, Wendehenne D, Blein JP, Pugin A. Involvement of free calcium in action of cryptogein, a proteinaceous elicitor of hypersensitive reaction in tobacco cells. Plant Physiol. 1995b;109:1025–1031. doi: 10.1104/pp.109.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truernit E, Schmid J, Epple P, Illig J, Sauer N. The sink-specific and stress-regulated Arabidopsis STP4 gene: enhanced expression of a gene encoding a monosaccharide transporter by wounding, elicitors, and pathogen challenge. Plant Cell. 1996;8:2169–2182. doi: 10.1105/tpc.8.12.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Luit AH, Piatti T, van Doorn A, Musgrave A, Felix G, Boller T, Munnik T. Elicitation of suspension-cultured tomato cells triggers the formation of phosphatidic acid and diacylglycerol pyrophosphate. Plant Physiol. 2000;123:1507–1516. doi: 10.1104/pp.123.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Estrella R, Barkla BJ, Higgins VJ, Blumwald E. Plant defenseresponse to fungal pathogens: activation of host plasma membrane H+-ATPase by elicitor-induced enzyme dephosphorylation. Plant Physiol. 1994;104:209–215. doi: 10.1104/pp.104.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard MP, Martin F, Pugin A, Ricci P, Blein JP. Protein phosphorylation is induced in tobacco cells by the elicitor cryptogein. Plant Physiol. 1994;104:1245–1249. doi: 10.1104/pp.104.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendehenne D, Binet MN, Blein JP, Ricci P, Pugin A. Evidence for specific, high-affinity binding sites for a proteinaceous elicitor in tobacco plasma membrane. FEBS Lett. 1995;374:203–207. doi: 10.1016/0014-5793(95)01108-q. [DOI] [PubMed] [Google Scholar]
- Wendehenne D, Lamotte O, Frachisse J-M, Barbier-Brygoo H, Pugin A. Nitrate efflux is an essential component of the cryptogein signaling pathway leading to defense responses and hypersensitive cell death in tobacco. Plant Cell. 2002;14:1937–1951. doi: 10.1105/tpc.002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendehenne D, Pugin A, Klessig DF, Durner J. Nitric oxide: comparative synthesis and signaling in animal and plant cells. Trends Plant Sci. 2001;6:177–183. doi: 10.1016/s1360-1385(01)01893-3. [DOI] [PubMed] [Google Scholar]
- Wevelsiep L, Rüpping E, Knogge W. Stimulation of barley plasmalemma H+-ATPase by phytotoxic peptides from the fungal pathogen Rhynchosporium secalis. Plant Physiol. 1993;101:297–301. doi: 10.1104/pp.101.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S, Nürnberger T, Frachisse JM, Wirtz W, Guern J, Hedrich R, Scheel D. Receptor-mediated activation of a plant Ca(2+)-permeable ion channel involved in pathogen defense. Proc Natl Acad Sci USA. 1997;94:2751–2755. doi: 10.1073/pnas.94.6.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zottini M, Formentin E, Scattolin M, Carimi F, Lo Schiavo F, Terzi M. Nitric oxide affects mitochondrial functionality in vivo. FEBS Lett. 2002;515:75–78. doi: 10.1016/s0014-5793(02)02438-9. [DOI] [PubMed] [Google Scholar]