Abstract
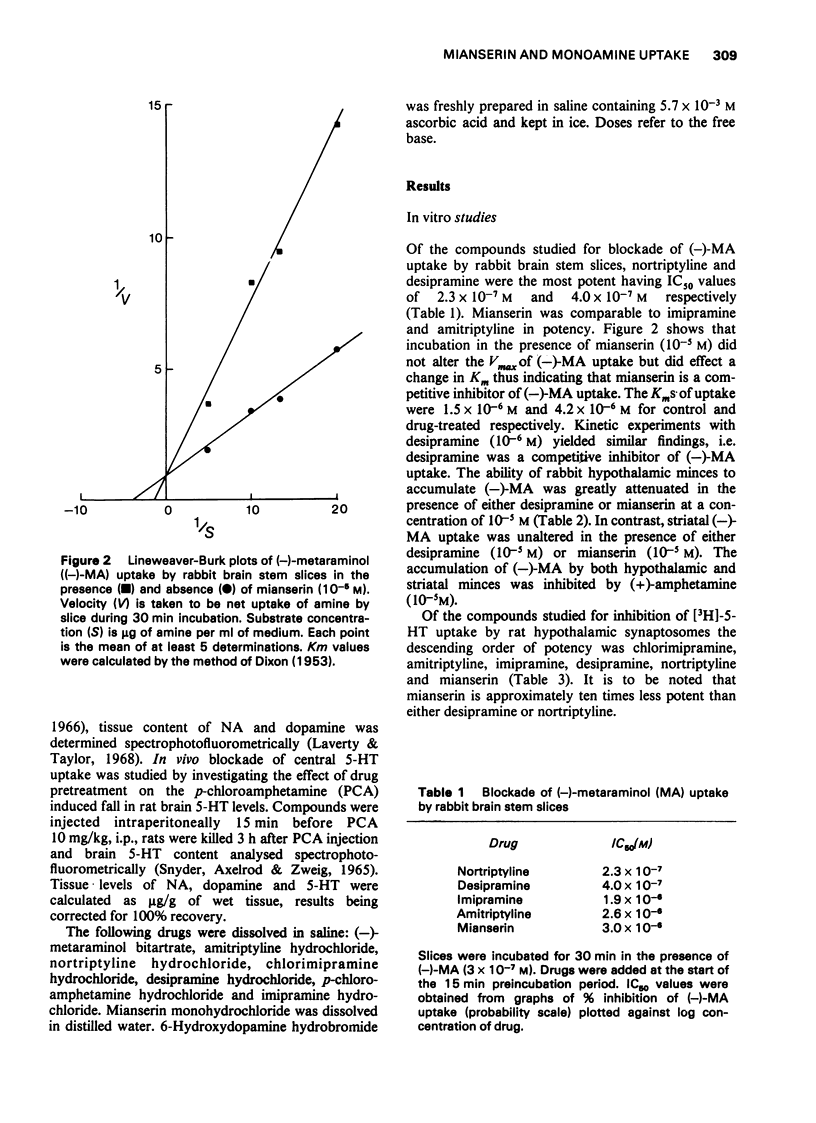
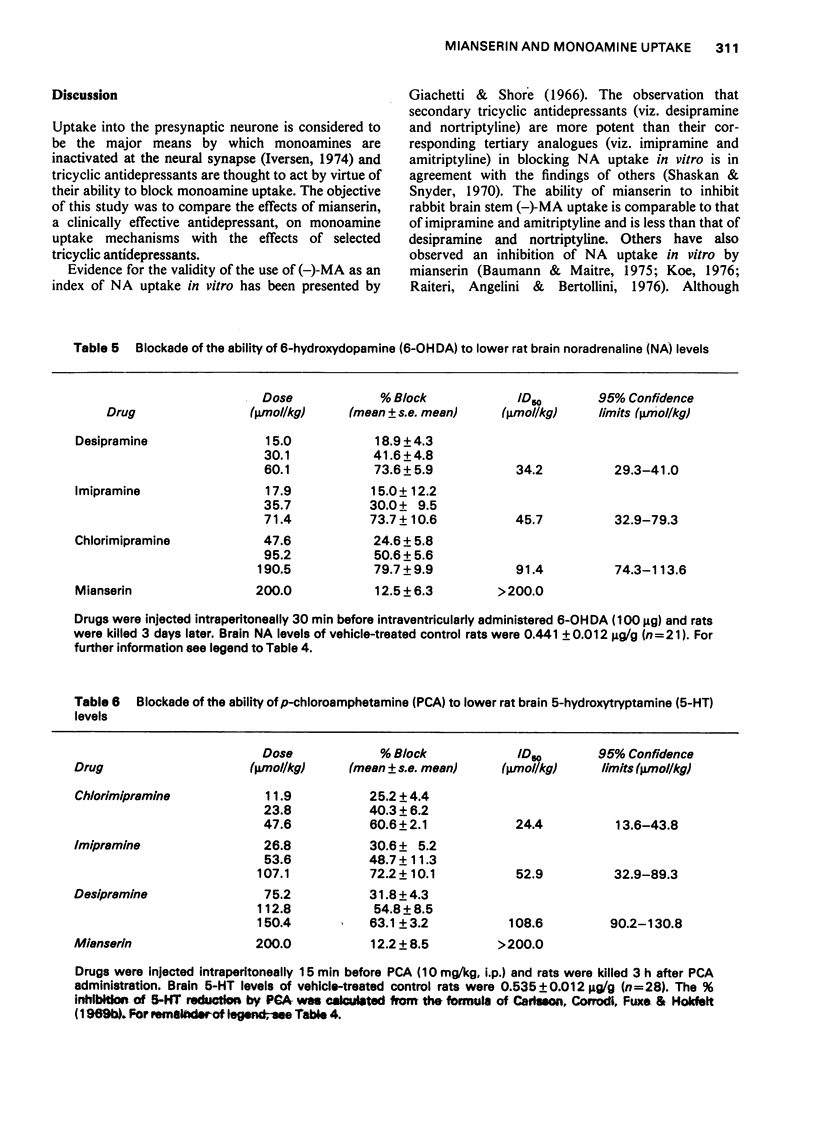
1 The effects of mianserin and of selected tricyclic antidepressants were compared in a number of monoamine uptake models. 2 The ability of mianserin to block the noradrenergic neurone membrane amine pump of rabbit brain stem slices was comparable to that of imipramine and amitriptyline and less than that of desipramine and nortriptyline. Both mianserin and desipramine were competitive inhibitors of noradrenaline uptake in vitro. The effect of mianserin on noradrenaline uptake in vivo was studied both peripherally and centrally. The ability of 6-hydroxydopamine to lower rat heart noradrenaline levels was found to be very sensitive to inhibition by tricyclic antidepressants. Mianserin was active in this model. However, its ability to block the 6-hydroxydopamine-induced fall in rat heart noradrenaline concentration was appreciably less than that of the tricyclics studied. 3 Mianserin, like tricyclic antidepressants, was essentially devoid of effect on dopamine uptake both in vitro and in vivo. 4 The ability of mianserin to inhibit [3H]-5-hydroxytryptamine uptake by rat hypothalamic synaptosomes was appreciably less than that of the tricyclic antidepressants studied. Mianserin was essentially devoid of effect on rat brain 5-hydroxytryptamine uptake in vivo. 5 It is concluded that in certain situations large doses of mianserin may block noradrenaline uptake in vivo. However, in no way does mianserin rival tricyclic antidepressants in blocking monoamine uptake in vivo. The clinical efficacy of mianserin cannot be attributed to inhibition of monoamine uptake.
Full text
PDF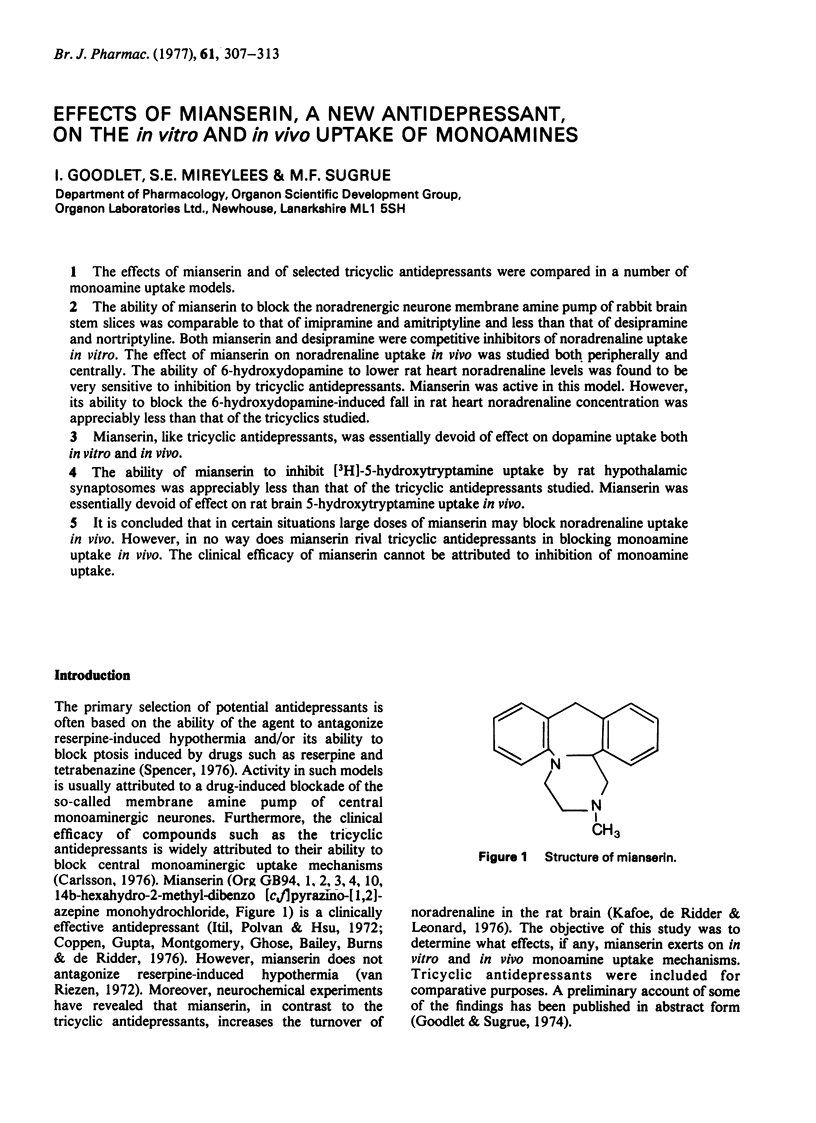




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berti F., Shore P. A. A kinetic analysis of drugs that inhibit the adrenergic neuronal membrane amine pump. Biochem Pharmacol. 1967 Nov;16(11):2091–2094. doi: 10.1016/0006-2952(67)90005-6. [DOI] [PubMed] [Google Scholar]
- Breese G. R., Traylor T. D. Developmental characteristics of brain catecholamines and tyrosine hydroxylase in the rat: effects of 6-hydroxydopamine. Br J Pharmacol. 1972 Feb;44(2):210–222. doi: 10.1111/j.1476-5381.1972.tb07257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A., Corrodi H., Fuxe K., Hökfelt T. Effect of antidepressant drugs on the depletion of intraneuronal brain 5-hydroxytryptamine stores caused by 4-methyl-alpha-ethyl-meta-tyramine. Eur J Pharmacol. 1969 Mar;5(4):357–366. doi: 10.1016/0014-2999(69)90113-7. [DOI] [PubMed] [Google Scholar]
- Carlsson A., Corrodi H., Fuxe K., Hökfelt T. Effects of some antidepressant drugs on the depletion of intraneuronal brain catecholamine stores caused by 4,alpha-dimethyl-meta-tyramine. Eur J Pharmacol. 1969 Mar;5(4):367–373. doi: 10.1016/0014-2999(69)90114-9. [DOI] [PubMed] [Google Scholar]
- Carlsson A., Fuxe K., Hamberger B., Lindqvist M. Biochemical and histochemical studies on the effects of imipramine-like drugs and (+)-amphetamine on central and peripheral catecholamine neurons. Acta Physiol Scand. 1966 Jul-Aug;67(3):481–497. doi: 10.1111/j.1748-1716.1966.tb03334.x. [DOI] [PubMed] [Google Scholar]
- Coppen A., Gupta R., Montgomery S., Ghose K., Bailey J., Burns B., de Ridder J. J. Mianserin hydrochloride: a novel antidepressant. Br J Psychiatry. 1976 Oct;129:342–345. doi: 10.1192/bjp.129.4.342. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Snyder S. H. Catecholamine uptake by synaptosomes in homogenates of rat brain: stereospecificity in different areas. J Pharmacol Exp Ther. 1969 Dec;170(2):221–231. [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris R. L., Shore P. A. Amine uptake and storage mechanisms in the corpus striatum of rat and rabbit. J Pharmacol Exp Ther. 1971 Oct;179(1):15–19. [PubMed] [Google Scholar]
- Evetts K. D., Iversen L. L. Effects of protriptyline on the depletion of catecholamines induced by 6-hydroxydopamine in the brain of the rat. J Pharm Pharmacol. 1970 Jul;22(7):540–543. doi: 10.1111/j.2042-7158.1970.tb10564.x. [DOI] [PubMed] [Google Scholar]
- Ghose K., Coppen A., Turner P. Autonomic actions and interactions of mianserin hydrochloride (Org. GB 94) and amitriptyline in patients with depressive illness. Psychopharmacology (Berl) 1976 Sep 17;49(2):201–204. doi: 10.1007/BF00427291. [DOI] [PubMed] [Google Scholar]
- Giachetti A., Shore P. A. Studies in vitro of amine uptake mechanisms in heart. Biochem Pharmacol. 1966 May;15(5):607–614. doi: 10.1016/0006-2952(66)90028-1. [DOI] [PubMed] [Google Scholar]
- Glowinski J., Iversen L. L. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966 Aug;13(8):655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- Itil T. M., Polvan N., Hsu W. Clinical and EEG effects of GB-94, a "tetracyclic" antidepressant (EEG model in discovery of a new psychotropic drug). Curr Ther Res Clin Exp. 1972 Jul;14(7):395–413. [PubMed] [Google Scholar]
- Iversen L. L. Uptake mechanisms for neurotransmitter amines. Biochem Pharmacol. 1974 Jul 15;23(14):1927–1935. doi: 10.1016/0006-2952(74)90250-0. [DOI] [PubMed] [Google Scholar]
- Kafoe W. F., De Ridder J. J., Leonard B. E. The effect of a tetracyclic antidepressant compound, Org GB94, on the turnover of biogenic amines in rat brain. Biochem Pharmacol. 1976 Nov 15;25(22):2455–2460. doi: 10.1016/0006-2952(76)90447-0. [DOI] [PubMed] [Google Scholar]
- Koe B. K. Molecular geometry of inhibitors of the uptake of catecholamines and serotonin in synaptosomal preparations of rat brain. J Pharmacol Exp Ther. 1976 Dec;199(3):649–661. [PubMed] [Google Scholar]
- Laverty R., Taylor K. M. The fluorometric assay of catecholamines and related compounds: improvements and extensions to the hydroxyindole technique. Anal Biochem. 1968 Feb;22(2):269–279. doi: 10.1016/0003-2697(68)90316-3. [DOI] [PubMed] [Google Scholar]
- Leonard B. E. Some effects of a new tetracyclic anti-depressant compound, Org GB 94, on the metabolism of monoamines in the rat brain. Psychopharmacologia. 1974 Apr 23;36(3):221–236. doi: 10.1007/BF00421804. [DOI] [PubMed] [Google Scholar]
- Malmfors T., Sachs C. Degeneration of adrenergic nerves produced by 6-hydroxydopamine. Eur J Pharmacol. 1968 Apr;3(1):89–92. doi: 10.1016/0014-2999(68)90056-3. [DOI] [PubMed] [Google Scholar]
- Meek J. L., Fuxe K., Carlsson A. Blockade of p-chloromethamphetamine induced 5-hydroxytryptamine depletion by chlorimipramine, chlorpheniramine and meperidine. Biochem Pharmacol. 1971 Mar;20(3):707–709. doi: 10.1016/0006-2952(71)90156-0. [DOI] [PubMed] [Google Scholar]
- Neff N. H., Costa E. The influence of monoamine oxidase inhibition on catecholamine synthesis. Life Sci. 1966 May;5(10):951–959. doi: 10.1016/0024-3205(66)90204-9. [DOI] [PubMed] [Google Scholar]
- Noble E. P., Wurtman R. J., Axelrod J. A simple and rapid method for injecting H3-norepinephrine into the lateral ventricle of the rat brain. Life Sci. 1967 Feb 1;6(3):281–291. doi: 10.1016/0024-3205(67)90157-9. [DOI] [PubMed] [Google Scholar]
- Raiteri M., Angelini F., Bertollini A. Comparative study of the effects of mianserin, a tetracyclic antidepressant, and of imipramine on uptake and release of neurotransmitters in synaptosomes. J Pharm Pharmacol. 1976 Jun;28(6):483–488. doi: 10.1111/j.2042-7158.1976.tb02770.x. [DOI] [PubMed] [Google Scholar]
- SHORE P. A., ALPERS H. S. FLUOROMETRIC ESTIMATION OF METARAMINOL AND RELATED COMPOUNDS. Life Sci. 1964 Jun;3:551–554. doi: 10.1016/0024-3205(64)90165-1. [DOI] [PubMed] [Google Scholar]
- STONE C. A., PORTER C. C., STAVORSKI J. M., LUDDEN C. T., TOTARO J. A. ANTAGONISM OF CERTAIN EFFECTS OF CATECHOLAMINE-DEPLETING AGENTS BY ANTIDEPRESSANT AND RELATED DRUGS. J Pharmacol Exp Ther. 1964 May;144:196–204. [PubMed] [Google Scholar]
- Shaskan E. G., Snyder S. H. Kinetics of serotonin accumulation into slices from rat brain: relationship to catecholamine uptake. J Pharmacol Exp Ther. 1970 Nov;175(2):404–418. [PubMed] [Google Scholar]
- Snyder S. H., Axelrod J., Zweig M. A sensitive and specific fluorescence assay for tissue serotonin. Biochem Pharmacol. 1965 May;14(5):831–835. doi: 10.1016/0006-2952(65)90102-4. [DOI] [PubMed] [Google Scholar]
- Spencer P. S. Animal models for screening new agents. Br J Clin Pharmacol. 1976 Feb;3(1 Suppl 1):5–12. doi: 10.1111/j.1365-2125.1976.tb03706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue M. F., Shore P. A. The mode of sodium dependency of the adrenergic neuron amide carrier. Evidence for a second, sodium-dependent, optically specific and reserpine-sensitive system. J Pharmacol Exp Ther. 1969 Dec;170(2):239–245. [PubMed] [Google Scholar]
- VonVoigtlander P. F., Losey E. G. On the use of selective neurotoxic amine analogs to measure the blockade of norepinephrine and 5-hydroxytryptamine uptake systems by antidepressants. Res Commun Chem Pathol Pharmacol. 1976 Mar;13(3):389–400. [PubMed] [Google Scholar]
- van Riezen H. Different central effects of the 5-HT antagonists mianserine and cyproheptadine. Arch Int Pharmacodyn Ther. 1972 Aug;198(2):256–269. [PubMed] [Google Scholar]


