Abstract
Culturing leaf protoplast-derived cells of the embryogenic alfalfa (Medicago sativa subsp. varia A2) genotype in the presence of low (1 μm) or high (10 μm) 2, 4-dichlorophenoxyacetic acid (2,4-D) concentrations results in different cell types. Cells exposed to high 2,4-D concentration remain small with dense cytoplasm and can develop into proembryogenic cell clusters, whereas protoplasts cultured at low auxin concentration elongate and subsequently die or form undifferentiated cell colonies. Fe stress applied at nonlethal concentrations (1 mm) in the presence of 1 μm 2,4-D also resulted in the development of the embryogenic cell type. Although cytoplasmic alkalinization was detected during cell activation of both types, embryogenic cells could be characterized by earlier cell division, a more alkalic vacuolar pH, and nonfunctional chloroplasts as compared with the elongated, nonembryogenic cells. Buffering of the 10 μm 2,4-D-containing culture medium by 10 mm 2-(N-morpholino)ethanesulfonic acid delayed cell division and resulted in nonembryogenic cell-type formation. The level of endogenous indoleacetic acid (IAA) increased transiently in all protoplast cultures during the first 4 to 5 d, but an earlier peak of IAA accumulation correlated with the earlier activation of the division cycle in embryogenic-type cells. However, this IAA peak could also be delayed by buffering of the medium pH by 2-(N-morpholino)ethanesulfonic acid. Based on the above data, we propose the involvement of stress responses, endogenous auxin synthesis, and the establishment of cellular pH gradients in the formation of the embryogenic cell type.
One of the characteristics of plant development is that somatic cell differentiation is reversible. This can be best demonstrated in in vitro systems where somatic plant cells can regain their totipotency and form embryos through the developmental pathway of somatic embryogenesis. Somatic embryo formation resembles zygotic embryogenesis in many aspects (for review, see Dodeman et al., 1997). However, beside the similarities, there are obvious differences: For example, whereas zygotes formed by the fusion of the egg and sperm cells are clearly determined to follow embryogenic development, somatic cells have to acquire competence to be able to respond to embryogenic signals and initiate embryogenesis. In carrot (Daucus carota), embryogenic cells of the proembryogenic cell mass are small, densely cytoplasmed, and full of starch grains, whereas nonembryogenic callus cells are large and highly vacuolated. This can be generalized for most embryogenic systems, including alfalfa (Medicago sativa), where protoplast-derived cells cultured at different 2, 4-dichlorophenoxyacetic acid (2,4-D) concentrations can develop into embryogenic or nonembryogenic cell types with the above characteristic morphologies (Bögre et al., 1990; Dudits et al., 1991). Nomura and Komamine (1985, 1995) showed that isolated, small, cytoplasm-rich carrot cells have the ability to develop to somatic embryos and go through an unequal first division parallel with the polarized synthesis of macromolecules. Even though some morphological (for review, see Yeung, 1995) and molecular (Kiyosue et al., 1992; Pennell et al., 1992, 1993; Schmidt et al., 1997; Perry et al., 1999) markers have already been associated with embryogenic competence, we still do not know how and why somatic plant cells can acquire an embryogenic fate.
Acquisition of embryogenic competence largely relies on dedifferentiation because the existing developmental information must be erased or altered to make the cells responsive for new signals (Dudits et al., 1991, 1995). The developmental switch from a differentiated and resting cell state to a dedifferentiated, dividing, embryogenic state likely involves the general reorganization of chromatin structure, overall reprogramming of gene expression, as well as cellular metabolism (Dudits et al., 1991, 1995). However, it is very difficult to dissect the specific cellular events related to the overlapping phases of dedifferentiation, cell cycle reactivation, and the acquisition of embryogenic competence.
One possible marker of dedifferentiation and cell activation is the cellular pH. Reactivation of quiescent cells was linked with characteristic changes in cellular pH gradients in animal and in yeast (Saccharomyces cerevisiae) cells. The modification of cytoplasmic pH (pHc) was found to be required for the control of the cell cycle, cell division, and growth (for review, see Frelin et al., 1988; Anand and Prasad, 1989; Swann and Whitaker, 1990; Whitaker, 1990). Tumorigenic cells of Chinese hamsters (Cricetulus griseus) have 0.2 pH units higher pHc than that of normal cells (Ober and Pardee, 1987). Prevention of cellular pH rise in sea urchin (Lytechinus pictus) eggs by different methods blocked pronuclear movements, diminished protein synthesis, and prevented cleavage (Swann and Whitaker, 1990). Contrary to this, the alkalinization of the cytoplasm with ammonia could reactivate the egg, and induce cyclin synthesis, p34cdc2 phosphorylation, and DNA replication (Whitaker, 1990). In slime mold (Dictyostelium discoideum), pHc determines different cell differentiation pathways (Gross et al., 1983). The pH of the medium was also found to influence somatic embryo induction and development in plants (Smith and Krikorian, 1990a, 1990b).
Auxin is considered to be the most important plant growth regulator in relation to cell division and differentiation, as well as in the induction of somatic embryogenesis. 2,4-D, an auxin analog herbicide, is especially effective in this latter process. Many in vitro somatic embryogenesis systems rely on the use of exogenous 2,4-D as an inducer. In carrot, one of the most extensively studied systems, it was proven that single cultured cells require 2,4-D to initiate embryo development (Nomura and Komamine, 1995). However, the continuous presence of this artificial auxin blocks further development and results in the accumulation of the already determined proembryogenic cell mass in the cultures (de Vries et al., 1988). In alfalfa, cultures of dedifferentiated cells (named as “microcallus suspension”) were initiated in the presence of 1-naphthaleneacetic acid and a short 2,4-D shock (as short as a few minutes) was sufficient to induce embryo development under the subsequent hormone-free conditions (Dudits et al., 1991). This suggests that 2,4-D has a specific signaling role in the initiation of somatic embryogenesis. A high concentration of exogenous auxin is needed to maintain the embryogenic nature of most embryogenic monocot cultures (KrishnaRaj and Vasil, 1995). Moreover, 2,4-D treatment could induce zygote development in unfertilized maize (Zea mays) egg cells, emphasizing its role as a general inducer of the embryogenic response (Kranz et al., 1995). However, it is not known how and why 2,4-D is so effective in the induction of embryogenic competence. 2,4-D was shown to influence the endogenous auxin (indoleacetic acid, IAA) metabolism in carrot cells, which was suggested to play significant roles in somatic embryo formation (Michalczuk et al., 1992b). However, 2,4-D may act not only as auxin (directly or indirectly), but also as a herbicide, and it may induce stress responses in plant cells (Grossmann, 2000). Early phases of somatic embryogenesis are characterized by the induction of many stress-related genes, which leads to the hypothesis that somatic embryogenesis is an extreme stress response of cultured plant cells (for review, see Dudits et al., 1991, 1995).
Here, we report on the use of the homogenous population of alfalfa leaf protoplast-derived cells to reveal the role of exogenous 2,4-D concentration and stress in the development of the embryogenic competent cell type characterized by parameters of cellular and external pH, cell morphology, cell division, and endogenous IAA levels.
RESULTS
Different Levels of Exogenously Applied 2,4-D and/or Fe Stress Influence the Development of Cultured Alfalfa Cells Derived from Leaf Protoplasts
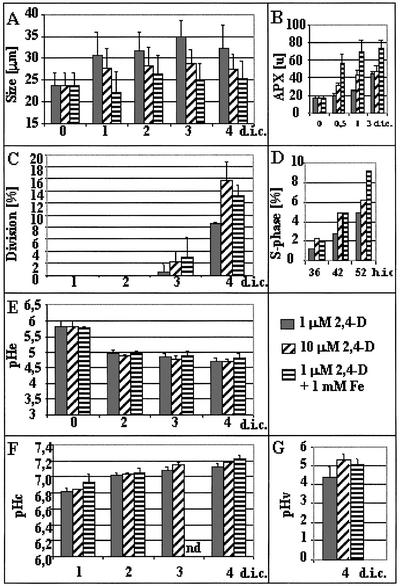
Leaf protoplasts of the embryogenic alfalfa genotype subsp. varia A2 developed differently if they were cultured at “low” (1 μm) or “high” (10 μm) 2,4-D concentrations. In the medium containing 1 μm 2,4-D, cells elongated during the first 4 to 5 d of culture (before their first cell division) and showed a significant increase in the volume of their central vacuole (Figs. 1A and 2A). Their cytoplasm and vacuoles were transparent and could not be strongly stained by toluidine blue, indicating a relatively low amount of proteins (Fig. 1B). Protoplasts subjected to higher (10 μm) 2,4-D concentration became densely cytoplasmed with several small vacuoles and had only a limited increase in their size followed by division with morphological asymmetry (Figs. 1C and 2A). The vacuoles in these cells were also dense and rich in proteins, as indicated by toluidine blue staining (Fig. 1D). Similar cell morphology has been observed upon the application of excess (1 mm) Fe to the medium containing only 1 μm 2,4-D (Fig. 1, E and F). This treatment significantly increased ascorbate peroxidase activity in the cells during the first 3 d of culture (Fig. 2B), indicating that this culture condition caused oxidative stress and the activation of the cellular defense system. The small, densely cytoplasmed cells developed under high 2,4-D or Fe stress conditions entered the division cycle approximately one-half of a day earlier than those grown in the presence of the lower 2,4-D concentration (Fig. 2. C and D). Although the timing of cell activation fluctuated from experiment to experiment (first divisions could be observed at the 3rd or 4th d), which could cause a significant variation in the cellular parameters determined at a given time point (e.g. compare Figs. 2 and 5), the trends of changes were the same in all experiments.
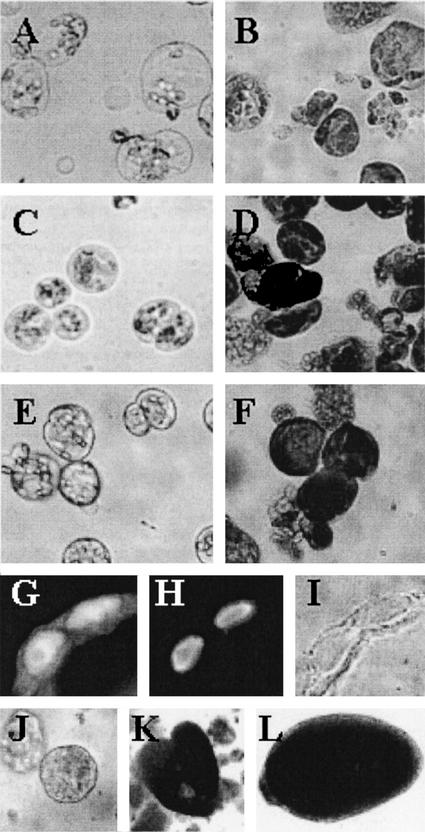
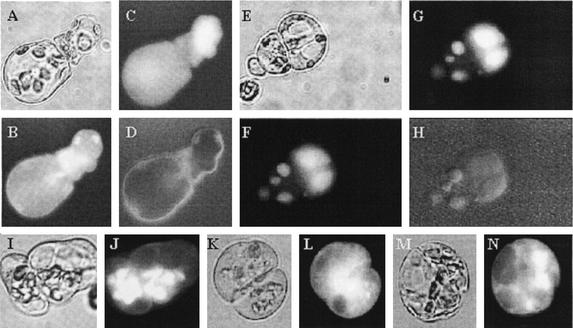
Figure 1.
Development of alfalfa subsp. varia A2 leaf protoplast-derived cells cultured at different 2,4-D and Fe (Fe-EDTA) concentrations. Leaf protoplast-derived cells of the embryogenic A2 alfalfa genotype cultured in a normal medium containing 100 μm Fe-EDTA at 1 μm 2,4-D (A and B) or 10 μm 2,4-D (C and D) concentration and in a medium with excess (1 mm) Fe-EDTA in the presence of 1 μm 2,4-D (E and F) at 5 d in culture (A, C, and E) and cells from the same cultures after staining by toluidine blue (B, D, and F). Cells of proembryogenic cell colonies express the AGL-15 epitope (Perry et al., 1999) in their nuclei as a characteristic of embryogenic cells (G, immunolabeling; H, nuclear staining by 4,6-diamidino-2-phenylindole; and I, transmitted light). Stages of somatic embryogenesis initiated from the proembryogenic colonies after decreasing the embryogenic (10 μm) 2,4-D concentration to 1 μm in the medium (J, globular; K, heart; and L, torpedo).
Figure 2.
Characterization of embryogenic and nonembryogenic protoplast-derived alfalfa cells formed under different conditions. A, Cell size expressed as the average of the length and width of the cells. Thirty cells were measured per treatment. B, Increase of ascorbate peroxidase activity indicating oxidative stress response of the cells due to excess Fe or 2,4-D in the medium. C, Cell division rate determined under a light microscope as the percentage of cells already divided at least once (at least 500 cells were counted). D, S phase progression followed by 5-bromo-2′-deoxyuridine (BrdU) incorporation into the nuclei of the cells during 36, 42, and 52 h of culture, respectively. E, The pH of the medium (pHe) at 0, 2, 3, and 4 d of culture. F, pHc of protoplast-derived cells grown under different conditions. Average pHc of 15 randomly selected cells. The pH values were determined using fluorescence microscopy following FDA staining and an in vitro calibration curve (see “Materials and Methods”). G, Vacuolar pH of protoplast-derived cells at 4 d of culture grown under different conditions. Average vacuolar pH of 15 randomly selected cells. The pH values were determined using fluorescence microscopy following BCECF-AM staining and an in vitro calibration curve (see “Materials and Methods”). Data are from at least three independent experiments. The se of the measurements is indicated on the bars. d.i.c., Days in culture; h.i.c., hours in culture; nd, no data.
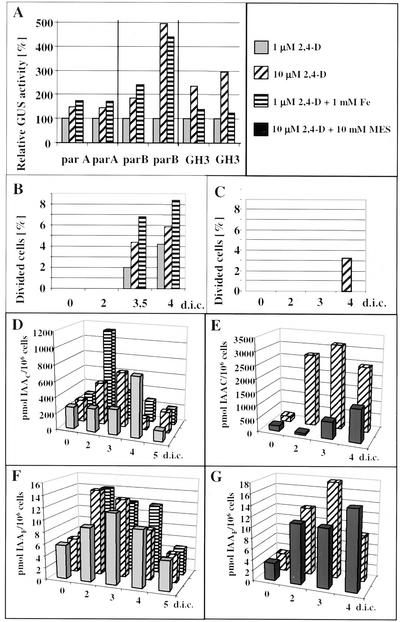
Figure 5.
Changes in endogenous IAA levels in leaf protoplast-derived cells. A, Transient expression of auxin responsive promoters in alfalfa leaf protoplasts cultured under embryogenic/nonembryogenic conditions. Leaf protoplasts were transfected with plasmid DNAs carrying chimeric GUS genes under the control of parA, parB, and GH3 promoters known to be auxin dependent. The protoplast-derived cells were cultured for 3 d in the presence of 1 μm 2,4-D, 10 μm 2,4-D, or 1 μm 2,4-D + 1 mm Fe-EDTA, collected by centrifugation, extracted, and the activity of the GUS enzyme has been determined fluorometrically as nanomoles of methylumbelliferon produced per milligram of protein per hour. The data of experiments in duplicate are presented as relative GUS activity considering the activity of 1 μm 2,4-D-cultured protoplasts as 100%. B through G, Dynamics of endogenous IAA accumulation in the protoplast-derived alfalfa cells as measured by microliquid chromatography with column switch coupled to electrospray tandem mass spectrometry after solid phase extraction. Frequency of cell division (B and C) and the changes in the cellular levels of endogenous conjugated (D and E) and free (F and G) forms of IAA have been determined during the first 4 to 5 d of protoplast culture as indicated. Cells were cultured under different conditions: in the presence of 1 and 10 μm 2,4-D as well as 1 μm 2,4-D with Fe stress (B–F) and in the presence of 10 μm 2,4-D with or without buffering of the medium with 10 mm MES (C–G), respectively.
The observed characteristic cell morphologies could be linked with the capability of somatic embryo formation under appropriate culture conditions. When the cells were cultivated in a medium with 1 μm 2,4-D and were then subcultured in fresh medium and embedded into alginate beads during the period of 3 to 5 d after protoplast isolation, most of them died and only a few cells could develop into undifferentiated cell colonies (callus). However, if the cells were grown for a period of 3 to 5 d in the presence of 10 or 1 μm 2,4-D + 1 mm Fe and were subsequently transferred to a medium containing only 1 μm 2,4-D, they formed globular, proembryo-like structures with high (above 80%) efficiency. The nuclei of the cells of these colonies could be stained by the antibody raised against the “agamous-like” protein AGL-15 of pea (Pisum sativum; Fig. 1, G–I). The translocation of the pea AGL-15 protein into the nucleus has been reported to be characteristic for embryogenic cells of different origin, including alfalfa somatic embryos (Perry et al., 1999). The proembryo-like colonies could develop into globular, polarized heart and torpedo-shaped somatic embryos (Fig. 1, J–L). Alginate or agarose embedding at the time of medium change (removal of high 2,4-D concentration) after 3 to 5 d of culture promoted this conversion (data not shown). Protoplast-derived cells cultured in the presence of 10 or 1 μm 2,4-D + 1 mm Fe remained viable with unchanged cell morphology for a long time (more than 1 month) in the same medium, but their sustained divisions were inhibited if they were not subcultured into fresh medium containing reduced levels of 2,4-D (data not shown).
Manipulation of the External pH Influences Morphology and Division of Leaf Protoplast-Derived Alfalfa Cells
The pH of the medium containing leaf protoplast-derived cells dropped from the initial value of pH 5.8 to approximately pH 4.8 during the first 2 d of protoplast culture (Fig. 2E). At this time, no dividing cells could be observed and only a small percentage of the cells entered the S phase of the cell cycle (Fig. 2, C and D). Although the small, embryogenic cells tended to divide at a higher rate during the first 4 d of culture (Fig. 2, C and D) and probably entered the cell division cycle somewhat earlier than the elongated cells, the differences could not be correlated with the pH of the medium.
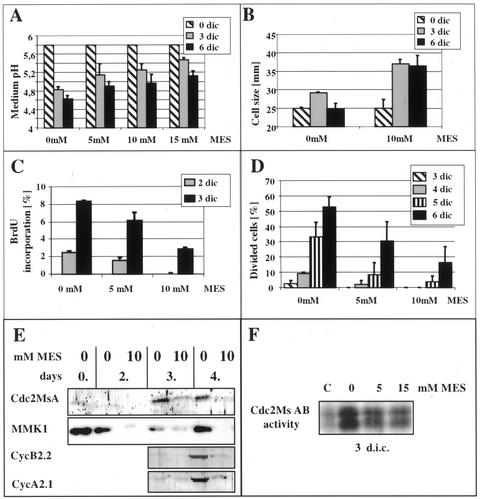
Considering the observed decrease in the external pH values during cellular reactivation, the effect of disturbed plasma membrane pH gradient on protoplast-derived cells was investigated. The medium containing 10 μm 2,4-D was buffered with MES and used for protoplast culture. By application of different MES concentrations (5, 10, and 15 mm), the acidification of the culture medium resulting from cellular activities could be differentially slowed down (Fig. 3A). The buffering effect was transient and ceased when the medium pH dropped below the efficient buffering range (pH 5.4–6.6) of MES. The slower rate of medium acidification resulted in increased cell elongation (Fig. 3B) and delayed cell division as determined by BrdU incorporation and cell division frequency (Fig. 3, C and D). The analysis of the accumulation of different cell division-related proteins like the mitogen-activated protein kinase MMK1, the alfalfa p34cdc2 homolog cdc2MsA/B kinase, and the A and B type mitotic cyclins Medsa:CycA;2.1 and Medsa:CycB;2.2, respectively, also confirmed the delay in the reactivation of cell division (Fig. 3E). The histone H1 phosphorylating activity of the immunoprecipitated cdc2MsA/B-related kinase complex also decreased due to medium buffering (Fig. 3F).
Figure 3.
Effect of buffering of the medium pH by MES on the development of leaf protoplast-derived cells cultured in the presence of 10 μm 2,4-D. A, Concentration-dependent effect of MES on delaying the acidification of the culture medium caused by the cells. B, Inhibition of medium acidification allows the cells to elongate in the presence of the otherwise inhibitory 10 μm 2,4-D concentration as shown by cell size. C and D, Cell division is delayed parallel with the delay in medium acidification as determined by BrdU incorporation (C) and cell division index (D). E, The appearance of cell activation/division-related proteins like a mitogen-activated protein kinase (MMK1), cyclin-dependent kinase (Cdc2MsA), and A and B type mitotic cyclins (Medsa;CycA2.1 and Medsa;CycB2.2) is also delayed due to medium buffering. F, Cdc2Ms AB-related histone phosphorylation activity is decreased in the cells cultured at buffered medium pH by MES. C, Freshly isolated protoplasts as control.
Cellular pH Increase during the Activation of Cell Division in Protoplast-Derived Cells
To reveal intracellular pH changes associated with cell activation, cell type, and division, cells were loaded with the pH-sensitive, cell-permeant fluorescein derivatives fluorescein diacetate (FDA) and Bis-(2-carboxyethyl)-5,6-carboxyfluorescein acetoxymethyl ester (BCECF-AM). The two dyes exhibited different cellular distribution (Fig. 4). FDA entered the cells instantaneously and formed free fluorescein showing mainly cytoplasmic labeling (Fig. 4, A–D). However, in elongating, vacuolized cells (1 μm 2,4-D), it started to accumulate in the chloroplasts after 5 to 10 min (Fig. 4, I and J). This chloroplast labeling was never observed in the small, compact embryogenic cells developed under 10 μm 2,4-D or Fe-stress conditions (Fig. 4, K–N). Application of carbonyl cyanide-m-chlorophenol hydrazone, a compound known to remove membrane pH gradients (Homann, 1971), prevented the accumulation of fluorescein in the chloroplasts, which demonstrates that this process is pH dependent (data not shown).
Figure 4.
Cellular distribution of fluorescent dyes used to measure intracellular pH. A through D, Example of FDA staining used for the determination of pHc. The cells shown were grown at 1 μm 2,4-D for 4 d (A, transmitted light; B, pH-dependent and -independent FDA fluorescence together at 490 nm; C, fluorescence dependent only on dye distribution at 440 nm; and D, pH-dependent fluorescence ratio image). The images were taken 5 min after FDA loading when the dye is predominantly in the cytoplasm. E through H, Example of BCECF staining of cells used to determine vacuolar pH. The cells shown were grown at 10 μm 2,4-D for 4 d (E, transmitted light; F, pH-dependent and -independent BCECF fluorescence together at 490 nm; G, fluorescence dependent only on dye distribution at 440 nm; and H, pH-dependent fluorescence ratio image). The images were taken 30 min after BCECF-AM loading. I through N, Differential FDA accumulation in the chloroplasts of protoplast-derived alfalfa cells under different culture conditions. Transmitted light (I, K, and M) and fluorescent (J, L, and N) microscopic images of cells grown at 1 μm 2,4-D (I and J), 10 μm 2,4-D (K and L), and 1 μm 2,4-D with 1 mm Fe (M and N), respectively. The images were taken 30 min after FDA loading when the dye accumulated in the chloroplasts in the elongated cells grown in the presence of 1 μm 2,4-D.
BCECF-AM, the free acid of what is known to be better retained within the cells as compared with fluorescein, required longer incubation time to enter the cells, gave much lower level of fluorescence intensity, and quickly accumulated in the vacuole independently from the growth conditions (Fig. 4, E–H).
Due to these differences in the cellular distribution and in fluorescence intensity, FDA has been used to determine pHc changes (within 5 min after loading), and BCECF-AM has been applied to detect changes in vacuolar pH (30 min after loading). The ratio between pH-dependent (excited at 490 nm) and pH-independent (excited at 440 nm) fluorescence of fluorescein and BCECF acid, respectively, was determined in 15 to 20 individual cells in three independent protoplast cultures (Fig. 2, F and G). In both culture types (embryogenic versus nonembryogenic), the pHc (Fig. 2F) was increased during cell reactivation followed by cell division activity (Fig. 2, C and D). However, no significant differences could be determined by comparison of the pHc values in cells grown under different conditions, although a slightly higher increase in the values was consistently found in all three experiments at 4 d of culture in the 10 μm 2,4-D or Fe stress-cultured cells (Fig. 2F). The vacuolar pH was also increased parallel with cell activation and division in all the tested cultures from pH 3.5 to 4 (measured at the 1st d of culture) to pH 4.5 to 5.2 (measured 3 d later). In contrast to pHc, a characteristic difference in vacuolar pH was observed between cells cultured with 1, 10, or 1 μm 2,4-D and Fe stress at the 4th d of culture. The difference in vacuolar alkalinization caused by the increased 2,4-D or Fe concentration reached nearly one pH unit by this time (Fig. 2G).
Transient Increase in the Endogenous IAA Level Is Associated with the Activation of Cell Division and the Formed Cell Type
The key role of IAA in zygotic embryo development and somatic embryogenesis has been suggested by several experimental findings (e.g. Michalczuk et al., 1992a, 1992b; Ribnicky et al., 2001). Our present observation that Fe stress could mimic the effect of high concentrations of 2,4-D in cellular responses encouraged the analysis of endogenous IAA levels in the protoplast-derived cells as a potential factor determining embryogenic development. In the first attempt, transient expression of chimeric β-glucuronidase (GUS) genes driven by different auxin-responsive promoters was measured in transfected protoplasts cultured under 1, 10, and 1 μm 2,4-D + Fe stress conditions. In all experiments (three promoters in two replications each), increased auxin responses were found under higher 2,4-D or Fe-stress conditions as indicated by the promoter activities (Fig. 5A). To verify that these increased promoter activities reflected higher endogenous IAA levels, the amounts of this hormone were directly measured from 250 to 500 thousand protoplast-derived cells, respectively, at different time points during culture, in two repetitions. These studies revealed a transient increase of cellular IAA concentrations under all three culturing conditions during the investigated 4- to 5-d period preceding cell division (Fig. 5, B, D, and F). These data suggest de novo auxin synthesis in the protoplast-derived cells. The increase was more pronounced in the level of IAA conjugates, but free IAA levels were also elevated. The peak of this transient IAA increase occurred approximately 1 d earlier in small, dense embryogenic-type cells grown in the presence of 10 μm 2,4-D or Fe stress as compared with the levels observed in elongated, vacuolized cells grown in the presence of 1 μm 2,4-D only.
To further confirm a correlation between the timing of cell division reactivation and endogenous IAA levels, the concentration of endogenous IAA has been measured in cells grown in MES-buffered medium in the presence of 10 μm 2,4-D (Fig. 5, C, E, and G). These results showed a delay in endogenous IAA synthesis due to the buffering of the medium pH. Moreover, the free IAA levels were much less affected than the conjugated IAA forms, although a delay in both peaks was obvious in the presence of 10 mm MES (Fig. 5, E and G).
DISCUSSION
In plants, embryos can develop not only from fertilized but also from unfertilized egg cells as well as from somatic cells. Up to now, no universal signal has been known that renders the cells to be embryogenic. The zygote, formed as a consequence of egg cell fertilization, is clearly determined to follow the embryogenic cell fate. In all other ways leading to plant embryogenesis, including apomixis and somatic embryogenesis, the transition phase toward competent and embryogenic cell types is much less defined. Synchronous, direct embryogenesis from single somatic plant cells in vitro is the most amenable experimental system for studying such a transition, as investigations can be made at the single cell as well as at the cell population level, allowing the use of different experimental approaches.
Stress, Cell Morphology, Division Rate, and Formation of the Embryogenic Cell Type
In alfalfa, leaf protoplasts can be used as a single cell system to induce the embryogenic response (Bögre et al., 1990; Song et al., 1990; Dudits et al., 1991). In the presence of an embryogenic concentration of 2,4-D, the vacuolated leaf mesophyll protoplasts form small, spherical cells with a dense cytoplasm (Bögre et al., 1990; Dudits et al., 1991). These protoplasts divide at a higher rate than leaf protoplasts isolated from a nonembryogenic genotype (Bögre et al., 1990). In the present study, we have shown that cells in the proembryogenic clusters developed from these type of small, densely cytoplasmed cells accumulate the embryogenic AGL-15 protein (Perry et al., 1999) in their nuclei (Fig. 1, G–I). In other experimental systems, direct somatic embryos were also formed by cells that are small in size, have a dense cytoplasm, and are metabolically very active (Nomura and Komamine, 1985; Song et al., 1990; Blervacq et al., 1995; Yeung, 1995). It can be hypothesized that the small, protoplast-derived, cytoplasmically rich alfalfa cells represent a dedifferentiated cell state with the potency to initiate a new developmental program. As such, they are the analogs of the “state 0” embryogenic competent cells defined by Nomura and Komamine in carrot cultures (Nomura and Komamine, 1995). Any information about factors responsible for the formation of this specific cell type in vitro can provide clues to reveal the physiological and molecular backgrounds of the flexible differentiation/dedifferentiation of somatic plant cells.
Induction of embryo development from somatic plant cells is often accompanied with cellular stress (for review, see Dudits et al., 1991, 1995). Moreover, 2,4-D, the most frequently used compound to initiate somatic embryo development, is known to induce many stress-related genes (e.g. Györgyey et al., 1991; Davletova et al., 2001). To provide further pieces of evidence that stress reactions are involved in the process of somatic embryogenesis, leaf protoplast-derived cells of alfalfa were cultured under nonembryogenic (1 μm) 2,4-D concentrations in the presence of 1 mm Fe as a stress treatment. Like other transition metals, Fe can cause oxidative stress in plant cells (e.g. Sinha et al., 1997). Caro and Puntarulo (1996) observed that the addition of Fe-EDTA in vivo up to an exogenous concentration of 5 × 10−4 m gives rise to an increase in the Fe content of the tissues accompanied by oxidative stress in the roots of soybean (Glycine max). Here, we applied 1 × 10−3 m Fe-EDTA to the protoplasts and found that this treatment increases the activity of ascorbate peroxidase, a scavenger for hydrogen peroxide, within 1 d. Activation of this enzyme indicates an oxidative stress response in the cells. Vansuyt et al. (1997) also demonstrated that Fe overfertilization in oilseed rape (Brassica napus) seedlings caused rapid accumulation of ascorbate peroxidase mRNA, a marker of oxidative stress (e.g. Mittler et al., 1999; Yoshimura et al., 2000).
As shown in Figure 2, increased Fe concentration resulted in the development of the same cell morphology as in the case of the “high,” or embryogenic, 2,4-D concentration (10 μm): reduced cell size and earlier entry into the cell division cycle. Similar results could be obtained by other oxidative stress-inducing agents like copper, menadion, paraquat, or alloxan applied at nonlethal concentrations (T. Pasternak, G. Potters, P. Miskolczi, H. Asard, D. Dudits, and A. Fehér, unpublished data). These cells could also form proembryogenic cell clusters upon subculture into fresh medium without the stress-inducing agent. The effect was independent of the specific presence of 2,4-D, but was dependent on exogenous auxin in the medium: Similar effects could be observed in the presence of 10 μm 1-naphthaleneacetic acid, whereas in the absence of any auxin, cells died under all stress conditions (T. Pasternak and A. Fehér, unpublished data).
Parallel activation of auxin and stress signaling may be a key event in cellular adaptation reprogramming the gene expression pattern, cellular metabolism, and physiology resulting in totipotency and embryogenic competence of somatic plant cells. However, further experiments are needed to validate this hypothesis.
Cell Activation and External/Cellular pH
The two different cell types formed due to different auxin/Fe concentrations from alfalfa leaf protoplasts could be characterized not only by morphological parameters like cell size, elongation, vacuolization, and the density of the cytoplasm as well as earlier cell divisions, but also by well-defined changes in the pH of their cellular compartments. Although cytoplasmic alkalinization occurred during cell reactivation and division under embryogenic and nonembryogenic conditions, the pH values in the vacuoles as well as in the chloroplasts may serve as indicators of the cell type formed.
In plants, there are only a limited number of examples for the physiological role of long-term changes in cellular pH (for review, see Kurkdjian and Guern, 1989; Pichon and Desbiez, 1994). In Bidens pilosa, Pichon and Desbiez (1994) found that pHc correlated with cell division. An alkalinization was promoting the cell cycle in the meristematic region of the hypocotyl, whereas acidification inhibited cell cycle activity. Initiation of root hair cells of Arabidopsis could be characterized similarly by the acidification of the apoplast and alkalinization of the cytoplasm (Bibikova et al., 1998). In good agreement with data obtained by studies on other eukaryotic cell types (for review, see Frelin et al., 1988), our observations showed the increase of pHc of alfalfa leaf protoplast-derived cells in parallel with cell activation and division. We cannot know whether cytoplasmic alkalinization is itself a mitotic signal, a prerequisite, or only a consequence of cell activation. In animal cells, it has been proposed that alkaline cellular pH has a permissive effect for the pathway leading to DNA synthesis rather than being a mitotic signal per se (Pouyssegur et al., 1984, 1985).
The fact that buffering of the medium pH by MES in the case of alfalfa leaf protoplasts could change the morphology and division of the alfalfa leaf-derived cells with a simultaneous delay in the accumulation of cell cycle-related proteins suggests that not only the value of intracellular pH but the establishment of the plasma membrane pH/proton gradient is of primary importance for reactivation (dedifferentiation) and division of these cells. The intracellular distribution and effect of MES is not known. Because the effective buffering range of MES (pH 5.4–6.6) differs from the intracellular values (above 6.6 for cytoplasmic and below 5.4 for vacuolar pH), it is likely to exert its effect primarily through buffering the external pH. It can be suggested that buffering of the medium forces the cells to excrete more protons to maintain the required gradients. It is also supported by the observations that this buffering effect is not preventing, but is only decreasing the rate of medium acidification. As soon as the medium pH decreased below 5.4 due to cellular activities, the cells were released from the blockage in reactivation and division.
Buffering of the apoplast during the initiation of root hair cells of Arabidopsis resulted in the inhibition of elongation, whereas preventing the local increase of pHc by butyrate did not influence the cell wall acidification or root hair initiation (Bibikova et al., 1998). These observations also highlight the importance of apoplastic pH in the control of cell architecture and morphogenesis in vivo.
Although we could not establish a clear relationship between embryogenic competence and the degree of cytoplasmic alkalinization, embryogenic-type cells had a tendency to exhibit slightly higher cytoplasmic and lower medium pH values compared with the vacuolated cells (Fig. 2). This may be related to the earlier activation of division events in these cells.
Intracellular pH of the Embryogenic Cell Type
A relation between medium (and cellular) pH and developmental state has been suggested by experiments where wounded carrot zygotic embryos were cultured in the presence of 1 μm NH4Cl (Smith and Krikorian, 1990a, 1990b). A long-term culture of preglobular stage proembryos could be established through NH4Cl-induced cellular alkalinization with a parallel decrease of medium pH (down to pH 4), which likely correlated with increased pHc. The development of embryos could only be progressed if the medium pH was raised to approximately pH 5.7. It has already been shown that cells of an alfalfa embryogenic type of callus have higher average intracellular pH values in comparison with cells from a nonembryogenic type (Schaefer, 1985). Our experiments with leaf protoplast-derived cells of alfalfa also demonstrated that the developmental pathway of the cells could be altered due to the delay of cell reactivation caused by medium buffering: In the presence of embryogenic 2,4-D concentrations, the cells became elongated and highly vacuolized if the medium was supplemented with 10 mm MES.
As shown in the present work, the small, cytoplasm-rich embryogenic-type cells have a vacuolar pH approximately one unit higher than the elongated, differentiating cells at the 4th d of culture. Plant cells have different types of vacuoles with different functions, including the large lytic-type of vacuoles characteristic of differentiated cells and small storage-type vacuoles of meristematic cells (for review, see Wink, 1993; Marty, 1999; Ratajczak, 2000). We suppose that the large difference observed in our experimental system in the vacuolar pH of the two different cell types is related to the differences in the vacuolar functions linked to the fate of these cells: Elongated, differentiated cells have large, central, probably lytic-type of vacuoles with more acidic pH, whereas the small dedifferentiated cells have several small, likely storage-type vacuoles characterized by low transparency under light microscopy and strong staining with toluidine blue indicating high protein content. There are several in vivo pieces of evidence suggesting a direct link between vacuolar H+ transport, cell morphology, and development. The vacuolar H+-ATPase-driven osmotic uptake of water into the central vacuole plays an important role in cell expansion (Wink, 1993). Moreover, in carrot, antisense mRNA-mediated inhibition of the tonoplast ATPase resulted in reduced cell expansion (Gogarten et al., 1992). The Arabidopsis det3 mutant has organ-specific defects in cell elongation and a failure arresting the apical meristem (Schumacher et al., 1999). The det3 gene has been identified as encoding the C-subunit of the vacuolar H+-ATPase (Schumacher et al., 1999).
Another very interesting characteristic of the dedifferentiated, embryogenic cells is the distribution of FDA, a pH indicator fluorescent dye. In this cell type, fluorescein was hardly detectable in the chloroplasts; the dye was localized only in the cytoplasm. In contrast, in the highly vacuolated cells, fluorescein accumulated in the chloroplast very quickly (within 10 min) in a pH-dependent manner. Although FDA can easily pass through cell membranes, the negatively charged fluorescein ions can be retained in acidic compartments. Photosynthetic electron transport results in the establishment of a ΔpH across the thylakoid membrane of chloroplasts significantly acidifying the thylakoid lumen (pH approximately 5.0) versus the stroma (pH approximately 8.0). We can assume that FDA accumulation in the chloroplasts (thylakoids) is related to functional electron transport of protoplast-derived cells under light excitation during microscopic investigation. The establishment of this trans-thylakoid pH gradient is missing in the embryogenic-type cells, which may indicate the fast dedifferentiation of chloroplasts and the loss of their functionality in these cells.
Endogenous IAA Synthesis and the Development of the Embryogenic Cell Type
Despite the absolute requirement of in vitro-cultured plant cells for exogenous auxins to sustain growth, cultured plant cells produce substantial amounts of the native auxin, IAA. Higher endogenous IAA concentrations have been shown in different species/explants as being associated with an increased embryogenic response (Rajasekaran et al., 1987; Ivanova et al., 1994; Michalczuk and Druart, 1999; Jimenez and Bangerth, 2001). In carrot cells, exogenous 2,4-D stimulates the accumulation of large amounts of endogenous IAA (Michalczuk et al., 1992a, 1992b). Michalczuk et al. (1992a, 1992b) hypothesized that the embryogenic competence of carrot cells is closely associated with a severalfold increase in endogenous IAA levels. It was also suggested that 2,4-D acts on the cells not directly as a strong auxin, but through affecting endogenous auxin metabolism (Michalczuk et al., 1992b). Ribnicky et al. (2001) also recently reported that an increase in endogenous IAA synthesis is associated with fertilization and early (up to the globular stage) zygotic embryogenesis in carrot.
In the present study, transient expression assays using auxin-inducible promoters linked to the reporter gene coding for GUS indicated an increased auxin response of embryogenic cells. The fact that Fe stress also enhanced the activity of the promoter indicated that this increase is not only due to the exogenous 2,4-D concentration. As the inducibility of these promoters by stress is unclear (Kusaba et al., 1996; Kitamiya et al., 2000), the endogenous IAA concentrations were determined in the protoplast-derived cells grown under embryogenic and nonembryogenic conditions. De novo synthesis of IAA in the protoplast-derived cells was indicated under all the examined conditions. The level of conjugated IAA forms changed considerably during the first 4 to 5 d of culture, whereas the free IAA content fluctuated to a lower extent.
In hormone-autotrophic embryogenic and a hormone-dependent nonembryogenic Prunus incisa × serrula calli, the levels of free and esterified IAA were comparable, whereas the amide-conjugated forms were significantly higher in the embryogenic calli (Michalczuk and Druart, 1999). There is substantial evidence to support the hypothesis that not only free but also conjugated IAA might have biological activity (e.g. Spena et al., 1991). It is also obvious that the significant increase in the cellular level of conjugated IAA forms has to be preceded by the synthesis of free IAA. More detailed analyses during the first 2 d of culture would be required to clarify the differences in free IAA levels due to the different culture conditions. Moreover, it also has to be taken into account that IAA as a weak acid easily penetrates through cellular membranes, therefore, cellular pH gradients can be important factors determining intracellular distribution. We can hypothesize that in the elongated cell type due to the more acidic vacuolar and chloroplast/thylakoid pH, more IAA ions can be trapped in these cell compartments than in the embryogenic competent cells. It is also to be emphasized that we calculated the endogenous IAA concentration on the basis of the number of cells and not of the fresh weight. Considering the differences in the size and morphology (vacuolarization) of the cells, the differences would be much higher with data based on fresh weight.
Although a transient increase in cellular IAA concentration could be observed under embryogenic and nonembryogenic conditions, the timing of IAA accumulation was different. The appearance of a peak in the endogenous IAA levels correlated with the cell type developed from the protoplasts. In cultures where the leaf protoplasts directly developed into the small embryogenic cell type due to high 2,4-D concentrations or to the presence of oxidative stress-inducing excess Fe-EDTA, the IAA peak could be observed at the 2nd or 3rd d of culture. In cultures where the cell elongation preceded cell division due to lower exogenous 2,4-D concentrations or to a buffered medium pH, the cellular IAA concentration reached the highest level only at the 4th d of culture. A similar alteration in endogenous IAA levels was observed in immature zygotic embryos of sunflower (Helianthus annuus) induced to form somatic embryos (Charriére et al., 1999). In the sunflower culture system, cells could be induced to form adventitious shoots or somatic embryos by simply modifying the Suc content in the culture medium. The tissues grown under embryogenic conditions showed a four times increase in their IAA content as compared with those tissues following the caulogenic pathway. The timing of the peak (at approximately 24 h of culture) correlated well with the time of the irreversible determination of the morphogenetic response (Charriére et al., 1999). These data are in good agreement with our results, strengthening the hypothesis that a transient increase in the endogenous IAA concentration can be involved in determining the developmental pathway of cultured plant cells. In both systems, the IAA peak correlated more or less with the reactivation of cell division.
A Model to Summarize the Observed Cellular Changes Caused by Auxin and Stress Responses during the Formation of the Embryogenic Cell Type
The developmental pathway followed by alfalfa leaf protoplast-derived cells could be easily manipulated by changing the culture conditions. The observation that the same cell type was obtained under different treatments facilitated the establishment of correlations among the cellular parameters determined. A faster reactivation (entering cell division) of the cells due to high or low 2,4-D concentration with Fe (oxidative) stress, respectively, was linked with smaller size and smaller vacuoles, higher vacuolar pH, more dense cytoplasm and vacuoles, and earlier chloroplast dedifferentiation as compared with cells grown in the presence of only 1 μm 2,4-D. Furthermore, the buffering of the medium not only caused a delay in cell activation, but completely prevented all the above mentioned cellular changes in the presence of the embryogenic (10 μm) 2,4-D concentrations. Considering the further development of leaf protoplast-derived alfalfa cells, these observations suggest that the timing of the reactivation of cell division is of primary importance. Although exogenous auxin was required for the reactivation and division of the leaf protoplast-derived cells, it was probably not the main determinant of the formed cell type as at the same 2,4-D concentration, cell development could be altered by changing culture conditions (stress, medium pH). However, the timing of endogenous IAA synthesis correlated with the timing of cell activation and the cell type developed under all culture conditions used in this study.
The above observations are summarized in Figure 6. Exogenous hormones, especially auxin and cytokinin (see Pasternak et al., 2000), are needed for the activation of somatic cells and for the entry to the division cycle. However, parallel application of mild stress may interfere with cellular activation, endogenous IAA metabolism, and the establishment of cellular pH gradients. Due to the induced cellular adaptation mechanisms, finally, the entire cellular metabolism is altered and results in the development of small, meristematic cells that may regain totipotency and can follow the embryogenic pathway if the stress factor is removed. This model can be further verified by detailed studies revealing the correlations between cell fate and oxidative stress. These investigations are currently in progress.
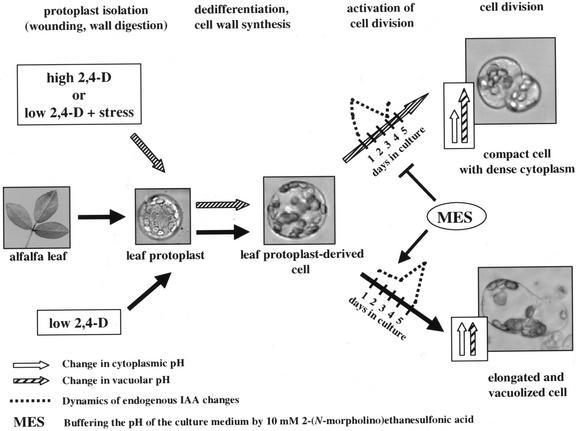
Figure 6.
A model summarizing the observed cellular changes related to the formation of the vacuolized and embryogenic cell types, respectively. Exogenous auxin is required for cell activation characterized by an increase in pHc, but the morphology and following development of the cells can be modulated by parallel mild stress treatments. The time required for endogenous auxin synthesis and the activation of cell division is shortened and the vacuolar pH is raised under high 2,4-D concentrations or mild stress conditions. Buffering of the medium pH by MES delays endogenous auxin synthesis and consequently delays cell activation and blocks the developmental pathway toward the formation of the small, cytoplasmically rich embryogenic cell type.
MATERIALS AND METHODS
Protoplast Culture and Transformation
All procedures of protoplast isolation and culture were performed essentially as described previously (Pasternak et al., 2000). Protoplasts were cultured in Kao and Michayluk medium (Kao and Michayluk, 1975) supplemented with the appropriate growth regulators, at a cell density of 105 protoplasts mL−1. Fe stress was applied by increasing the concentration of Fe-EDTA 10-fold in the culture medium. If MES was applied, it was dissolved directly in the medium at the given concentrations.
For transient expression assays, protoplasts were transformed according to the protocol described by Negrutiu et al. (1987). The plasmids carrying parA, parB, and GH3 promoters (Hagen et al., 1991; Takahashi and Nagata, 1992; Takahashi et al., 1994) fused to the GUS gene were kindly provided by Prof. Toshiyuki: Nagata (Tokyo) and Prof. Tom Guifoyle (Columbia), respectively. The activity of the GUS enzyme was determined as described elsewhere (Bilgin et al., 1999). The data were calculated as nanomoles methylumbelliferon produced per milligram of protein per hour.
Determination of Cell Size, S Phase, and Cell Division Frequency
Cell size was determined under a light microscope as the average of the length and width of the cells. At least 30 randomly chosen cells were measured per dish.
To determine the frequency of cells passing through the S phase of the cell cycle, the cells were cultured in the presence of 15 μm BrdU in the dark and samples were taken at the indicated time points. The percentage of cells incorporating BrdU into their DNA was determined after the isolation of nuclei as described by Pasternak et al. (2000) using a primary anti-BrdU antibody from Amersham (Buckinghamshire, UK) and fluorescein isothiocyanate-labeled anti-mouse immunoglobulin G (Sigma, St. Louis). Fluorescence microscopy was performed with an Axiovert 135 M fluorescence microscope (Zeiss, Oberkochen, Germany), and the excitation was at 490 nm for fluorescein isothiocyanate and 365 nm for 4,6-diamidino-2-phenylindole. The frequency of divided cells was determined under a light microscope by counting the cells that had already formed a division plate. A minimum of 500 randomly chosen cells were investigated for S phase/cell division at each time point, except at the early stages of protoplast culture when BrdU incorporation or cell division frequency was below 5% and 1,000 to 1,500 cells were observed.
Ascorbate Peroxidase Activity
Ascorbate peroxidase activity was determined spectrophotometrically based on the disappearance of ascorbate from the following reaction mixture at 290 nm: 50 mm potassium phosphate buffer, 5 mm ascorbate, 1 μm EDTA, 10 μg of protein, and 0.03% (v/v) hydrogen peroxide.
Immunological Techniques
For the immunoblots, cells were harvested by centrifugation and were frozen in liquid nitrogen after the indicated period of culture. Extraction and immunoblotting of proteins onto polyvinylidene difluoride (Millipore, Bedford, MA) membranes after SDS-PAGE were performed based on standard procedures as described by Pasternak et al. (2000). The primary antibodies against the proteins Cdc2Ms A/B and Medsa;CycB2.2 were described by Magyar et al. (1997), the Medsa;CycA2.1 by Roudier et al. (2000), and the MMK1 kinase by Bögre et al. (1999). Peroxidase-conjugated anti-rabbit polyvalent immunoglobulins (Sigma) were used as secondary antibody. The signal was developed by the super signal chemiluminescent substrate (Pierce, Rockford, IL) according to the manufacturer's instructions. Cdc2-related histone kinase activity was determined according to Magyar et al. (1997).
Cell fixation and indirect immunofluorescence using the anti-AGL-15 antibody (Perry et al., 1999) was carried out exactly as described earlier by Ayaydin et al. (2000).
Intracellular pH Determination
The pH-sensitive fluorescent dyes BCECF and its cell permeant acetoxymethyl ester derivate (BCECF-AM) were purchased from Molecular Probes (Eugene, OR). Fluorescein and its cell permeant derivate FDA were from Sigma. All of the dyes were dissolved in dimethyl sulfoxide at the concentrations of 1 mm for BCECF or BCECF-AM and 2 mm for fluorescein or FDA, respectively, and were stored at −20°C. For cell labeling, small aliquots of the cells (approximately 200 μL of culture) were harvested and transferred to 1.5-mL reaction tubes containing the appropriate amount of the dye solution. Both dyes were used in a final concentration of 2 μm. FDA fluorescence was measured at 5 min, and BCECF-AM fluorescence at 30 min after loading the cells with the dye, respectively. Fluorescent signals were detected by a laser scanning confocal microscope (LSM 410; Zeiss) and a fluorescence microscope (Axiovert 135 M; Zeiss). For ratiometric pH measurements, the excitation was at 440 ± 10 and 470 ± 20 nm, respectively. Fluorescence passing through a 515-nm dichroic mirror and a 535 ± 10- or 540 ± 25-nm bandpass filter, respectively, was recorded with a CCD camera. A 25% transmittance neutral density filter was used between the light source and the filter to decrease excitation energy and minimize photobleaching. Excitation time was kept to 2 s for BCECF-AM and 0.125 s for FDA also to minimize dye bleaching. In this case, photobleaching of the dyes represented less than 5% of fluorescence per observation/scan. Autofluorescence represented less than 1% of the total signal from dye-loaded protoplasts and did not change with time or experimental treatment. Background fluorescence intensity (together with dark camera level) was measured based on the average background signal from each individual image from an area next to the samples and was subtracted from the fluorescent intensity of the cells. Arithmetic operation was used to distinguish the pH-dependent fluorescence obtained at 470 nm excitation from the pH-independent fluorescence obtained at 440 nm excitation. An average ratio was calculated and converted to a pH value using in vitro calibration curves. In vitro calibration of pH-dependent fluorescence was made in a buffer containing 100 mm KCl, 30 mm NaCl, 500 mm mannitol, 25 mm MES, and 25 mm HEPES. The fluorescence ratio of the buffers was determined using the digital image analyzer as described above. The pH range of the calibration curve was from pH 6 to 8 in the buffer containing 2 μm free fluorescein and was from pH 4.5 to 6.5 in the buffer containing 2 μm BCECF.
Measurement of the Endogenous IAA Concentrations
Cellular concentrations of conjugated and free endogenous IAA in samples of 250 to 500 thousand cells have been determined by microliquid chromatography with column switch coupled to electrospray tandem mass spectrometry after solid phase extraction in two independent experiments following Prinsen et al. (1998, 2000). The data obtained are expressed in picomoles as a function of cell number (picomoles per million cells).
Supplementary Material
ACKNOWLEDGMENTS
We thank Era Kondorosi, László Bögre, Herbert Hirt, and Donna E. Fernandez for the cyclin, MMK1, and AGL-15 antibodies, respectively.
Footnotes
This work was supported by the Bilateral Flemish-Hungarian Collaboration (grant no. B–5/98), by the European Union International Cooperation Copernicus grant (no. IC15–CT96–0906), by Orszàgos Tudomànyos Kutatási Aloup T034818, and by Crop Design N.V. (Gent, Belgium). A.F. is the recipient of the János Bólyai research fellowship. G.P. is Aspirant at the Fund for Scientific Research-Flanders (FWO-Vlaanderen). This is a contribution of the University of Nebraska Agricultural Research Division (Lincoln; journal series no. 13,694).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.000810.
LITERATURE CITED
- Anand S, Prasad R. Rise in intracellular pH is concurrent with “start” progression of Saccharomyces cerevisiae. J Gen Microbiol. 1989;135:2173–2179. doi: 10.1099/00221287-135-8-2173. [DOI] [PubMed] [Google Scholar]
- Ayaydin F, Vissi E, Meszaros T, Miskolczi P, Kovacs I, Feher A, Dombradi V, Erdodi F, Gergely P, Dudits D. Inhibition of serine/threonine-specific protein phosphatases causes premature activation of cdc2MsF kinase at G2/M transition and early mitotic microtubule organisation in alfalfa. Plant J. 2000;23:85–96. doi: 10.1046/j.1365-313x.2000.00798.x. [DOI] [PubMed] [Google Scholar]
- Bibikova TN, Jacob T, Dahse I, Gilroy S. Localized changes in apoplastic and cytoplasmic pH are associated with root hair development in Arabidopsis thaliana. Development. 1998;125:2925–2934. doi: 10.1242/dev.125.15.2925. [DOI] [PubMed] [Google Scholar]
- Bilgin M, Dedeoglu D, Omirulleh S, Peres A, Engler G, Inzé D, Dudits D, Feher A. Meristem, cell division and S phase-dependent activity of wheat histone H4 promoter in transgenic maize plants. Plant Sci. 1999;143:35–44. [Google Scholar]
- Blervacq AS, Dubois T, Dubois J, Vasseur J. First divisions of somatic embryogenic cells in Cichorium hybrid “474.”. Protoplasma. 1995;186:163–168. [Google Scholar]
- Bögre L, Calderini O, Binarova P, Mattauch M, Till S, Kiegerl S, Jonak C, Pollaschek C, Barker P, Huskisson NS et al. A MAP kinase is activated late in plant mitosis and becomes localized to the plane of cell division. Plant Cell. 1999;11:101–113. [PMC free article] [PubMed] [Google Scholar]
- Bögre L, Stefanov I, Ábrahám M, Somogyi I, Dudits D. Differences in the responses to 2, 4-dichlorophenoxyacetic acid (2, 4-D) treatment between embryogenic and non-embryogenic lines of alfalfa. In: Nijkamp HJJ, van der Plaas LHW, Van Aartrijk J, editors. Progress in Plant Cellular and Molecular Biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1990. pp. 427–436. [Google Scholar]
- Caro A, Puntarulo S. Effect of in vivo iron supplementation on oxygen radical production by soybean roots. Biochim Biophys Acta. 1996;1291:245–251. doi: 10.1016/s0304-4165(96)00071-2. [DOI] [PubMed] [Google Scholar]
- Charriére F, Sotta B, Miginiac É, Hahne G. Induction of adventitious or somatic embryos on in vitro cultured zygotic embryos of Helianthus annuus: variation of endogenous hormone levels. Plant Physiol Biochem. 1999;37:751–757. [Google Scholar]
- Davletova S, Meszaros T, Miskolczi P, Oberschall A, Torok K, Magyar Z, Dudits D, Deak M. Auxin and heat shock activation of a novel member of the calmodulin like domain protein kinase gene family in cultured alfalfa cells. J Exp Bot. 2001;52:215–221. [PubMed] [Google Scholar]
- de Vries SC, Booij H, Meyerink P, Huisman G, Wilde HD, Thomas TL, van Kammen A. Acquisition of embryogenic potential in carrot cell-suspension cultures. Planta. 1988;176:196–204. doi: 10.1007/BF00392445. [DOI] [PubMed] [Google Scholar]
- Dodeman V, Ducreux G, Kreis M. Zygotic embryogenesis versus somatic embryogenesis. J Exp Bot. 1997;48:1493–1509. [Google Scholar]
- Dudits D, Bögre L, Györgyey J. Molecular and cellular approaches to the analysis of plant embryo development from somatic cells in vitro. J Cell Sci. 1991;99:475–484. [Google Scholar]
- Dudits D, Györgyey J, Bögre L, Bakó L. Molecular biology of somatic embryogenesis. In: Thorpe TA, editor. In Vitro Embryogenesis in Plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 267–308. [Google Scholar]
- Frelin C, Vigne P, Ladoux A, Lazdunski M. The regulation of the intracellular pH in cells from vertebrates. Eur J Biochem. 1988;174:3–14. doi: 10.1111/j.1432-1033.1988.tb14055.x. [DOI] [PubMed] [Google Scholar]
- Gogarten JP, Fichmann J, Braun Y, Morgan L, Styles P, Taiz SL, DeLapp K, Taiz L. The use of antisense mRNA to inhibit the tonoplast H+ ATPase in carrot. Plant Cell. 1992;4:851–864. doi: 10.1105/tpc.4.7.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JD, Bradbury J, Kay RR, Peacey MJ. Intracellular pH and the control of cell differentiation in Dictyostelium discoideum. Nature. 1983;303:244–245. doi: 10.1038/303244a0. [DOI] [PubMed] [Google Scholar]
- Grossmann K. Mode of action of auxinic herbicides: a new ending to a long, drawn out story. Trends Plant Sci. 2000;5:506–508. doi: 10.1016/s1360-1385(00)01791-x. [DOI] [PubMed] [Google Scholar]
- Gyorgyey J, Gartner A, Nemeth K, Magyar Z, Hirt H, Heberle-Bors E, Dudits D. Alfalfa heat shock genes are differentially expressed during somatic embryogenesis. Plant Mol Biol. 1991;16:999–1007. doi: 10.1007/BF00016072. [DOI] [PubMed] [Google Scholar]
- Hagen G, Martin G, Li Y, Guilfoyle TJ. Auxin-induced expression of the soybean GH3 promoter in transgenic tobacco plants. Plant Mol Biol. 1991;17:567–579. doi: 10.1007/BF00040658. [DOI] [PubMed] [Google Scholar]
- Homann PH. Electron transport and fluorescence of isolated chloroplasts. Biochim Biophys Acta. 1971;245:129–143. doi: 10.1016/0005-2728(71)90015-6. [DOI] [PubMed] [Google Scholar]
- Ivanova A, Velcheva M, Denchev P, Atanassov A, Van Onckelen H. Endogenous hormone levels during direct somatic embryogenesis in Medicago falcata. Physiol Plant. 1994;92:85–89. [Google Scholar]
- Jimenez VM, Bangerth F. Endogenous hormone levels in explants and in embryogenic and non-embryogenic cultures of carrot. Physiol Plant. 2001;111:389–395. doi: 10.1034/j.1399-3054.2001.1110317.x. [DOI] [PubMed] [Google Scholar]
- Kao KN, Michayluk M. Nutritional requirements for growth of Vicia hajstana cells and protoplasts at a very low population density in liquid media. Planta. 1975;126:105–110. doi: 10.1007/BF00380613. [DOI] [PubMed] [Google Scholar]
- Kitamiya E, Suzuki S, Sano T, Nagata T. Isolation of two genes that were induced upon the initiation of somatic embryogenesis on carrot hypocotyls by high concentrations of 2,4-D. Plant Cell Rep. 2000;19:551–557. doi: 10.1007/s002990050772. [DOI] [PubMed] [Google Scholar]
- Kiyosue T, Yamaguchi-Shinozaki K, Shinozaki K, Higashi K, Satoh S, Kamada H, Harada H. Isolation and characterization of a cDNA that encodes ECP31, an embryogenic-cell protein from carrot. Plant Mol Biol. 1992;19:239–249. doi: 10.1007/BF00027345. [DOI] [PubMed] [Google Scholar]
- Kiyosue T, Yamaguchi-Shinozaki K, Shinozaki K, Kamada H, Harada H. cDNA Cloning of ECP40, an embryogenic-cell protein in carrot, and its expression during somatic and zygotic embryogenesis. Plant Mol Biol. 1993;21:1053–1068. doi: 10.1007/BF00023602. [DOI] [PubMed] [Google Scholar]
- Kranz E, von Wiegen P, Lörz H. Early cytological events after induction of cell division in egg cells and zygote development following in vitro fertilization with angiosperm gametes. Plant J. 1995;8:9–23. [Google Scholar]
- KrishnaRaj S, Vasil IK. Somatic embryogenesis in herbaceous monocots. In: Thorpe TA, editor. In Vitro Embryogenesis in Plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 471–540. [Google Scholar]
- Kurkdjian A, Guern J. Intracellular pH: measurement and importance in cell activity. Annu Rev Plant Physiol. 1989;40:277–303. [Google Scholar]
- Kusaba M, Takahashi Y, Nagata T. A multiple-stimuli-responsive as-1-related element of parA gene confers responsiveness to cadmium but not to copper. Plant Physiol. 1996;111:1161–1167. doi: 10.1104/pp.111.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar Z, Meszaros T, Miskolczi P, Deak M, Feher A, Brown S, Kondorosi E, Athanasiadis A, Pongor S, Bilgin M et al. Cell cycle phase specificity of putative cyclin-dependent kinase variants in synchronized alfalfa cells. Plant Cell. 1997;9:223–235. doi: 10.1105/tpc.9.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty F. Plant vacuoles. Plant Cell. 1999;11:587–600. doi: 10.1105/tpc.11.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalczuk L, Cooke TJ, Cohen JD. Auxin levels at different stages of carrot somatic embryogenesis. Phytochemistry. 1992a;31:1097–1103. [Google Scholar]
- Michalczuk L, Druart P. Indole-3-acetic acid metabolism in hormone-autotrophic, embryogenic callus of Inmil cherry rootstock (Prunus incisa × serrula “GM 9”) and in hormone-dependent, non-embryogenic calli of Prunus incisa × serrula and Prunus domestica. Physiol Plant. 1999;107:426–432. [Google Scholar]
- Michalczuk L, Ribnicky DM, Cooke TJ, Cohen JD. Regulation of indole-3-acetic acid biosynthetic pathways in carrot cell cultures. Plant Physiol. 1992b;100:1346–1353. doi: 10.1104/pp.100.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Lam E, Shulaev V, Cohen M. Signals controlling the expression of cytosolic ascorbate peroxidase during pathogen-induced programmed cell death in tobacco. Plant Mol Biol. 1999;39:1025–1035. doi: 10.1023/a:1006110223774. [DOI] [PubMed] [Google Scholar]
- Negrutiu I, Shillito R, Potrykus I, Biasini G, Sala F. Hybrid genes in the analysis of transformation conditions: setting up a simple method for direct gene transfer to protoplasts. Plant Mol Biol. 1987;8:363–373. doi: 10.1007/BF00015814. [DOI] [PubMed] [Google Scholar]
- Nomura K, Komamine A. Identification and isolation of single cells that produce somatic embryos at a high frequency in a carrot cell suspension culture. Plant Physiol. 1985;79:988–991. doi: 10.1104/pp.79.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura K, Komamine A. Thorpe TA, ed, In Vitro Embryogenesis in Plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. Physiological and biological aspects of somatic embryogenesis; pp. 249–266. [Google Scholar]
- Ober SS, Pardee AB. Intracellular pH is increased after transformation of Chinese hamster embryo fibroblasts. Proc Natl Acad Sci USA. 1987;84:2766–2770. doi: 10.1073/pnas.84.9.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak T, Miskolczi P, Ayaydin F, Mészáros T, Dudits D, Fehér A. Exogenous auxin and cytokinin dependent activation of CDKs and cell division in leaf protoplast-derived cells of alfalfa. Plant Growth Regul. 2000;32:129–141. [Google Scholar]
- Pennell RI, Janniche L, Scofield GN, Booij H, de Vries SC, Roberts K. Identification of a transitional cell state in the developmental pathway to carrot somatic embryogenesis. J Cell Biol. 1992;119:1371–1380. doi: 10.1083/jcb.119.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SE, Lehti MD, Fernandez DE. The MADS-domain protein AGAMOUS-like 15 accumulates in embryonic tissues with diverse origins. Plant Physiol. 1999;120:121–130. doi: 10.1104/pp.120.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichon O, Desbiez M-O. Is cytoplasmic pH involved in the regulation of cell cycle in plants? Physiol Plant. 1994;92:261–265. [Google Scholar]
- Pouyssegur J, Franchi A, L'Allemain G, Paris S. Cytoplasmic pH, a key determinant of growth factor-induced DNA synthesis in quiescent fibroblasts. FEBS Lett. 1985;190:115–119. doi: 10.1016/0014-5793(85)80439-7. [DOI] [PubMed] [Google Scholar]
- Pouyssegur J, Sardet C, Franchi A, L'Allemain G, Paris S. A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH. Proc Natl Acad Sci USA. 1984;81:4833–4837. doi: 10.1073/pnas.81.15.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinsen E, Van Dongen W, Esmans EL, Van Onckelen H. Micro and capillary LC-MS/MS: a new dimension in phytohormone research. J Chromatogr A. 1998;826:25–37. [Google Scholar]
- Prinsen E, Van Laer S, Sevgi Ö, Van Onckelen H. Auxin analysis. In: Tucker GA, editor. Plant Hormone Protocols. Totowa, NJ: Humana Press; 2000. pp. 49–65. [Google Scholar]
- Rajasekaran K, Hein MB, Davis GC, Carnes MG, Vasil IK. Exogenous growth regulators in leaves and tissue cultures of Pennisetum purpureum Schum. J Plant Physiol. 1987;130:13–25. [Google Scholar]
- Ratajczak R. Structure, function and regulation of the plant vacuolar H+-translocating ATPase. Biochim Biophys Acta. 2000;1465:17–36. doi: 10.1016/s0005-2736(00)00129-2. [DOI] [PubMed] [Google Scholar]
- Ribnicky DM, Cohen JD, Hu WS, Cooke TJ. An auxin surge following fertilization in carrots: a mechanism for regulating plant totipotency. Planta. 2001;214:505–509. doi: 10.1007/s004250100639. [DOI] [PubMed] [Google Scholar]
- Roudier F, Fedorova E, Gyorgyey J, Feher A, Brown S, Kondorosi A, Kondorosi E. Cell cycle function of a Medicago sativa A2-type cyclin interacting with a PSTAIRE-type cyclin-dependent kinase and a retinoblastoma protein. Plant J. 2000;23:73–83. doi: 10.1046/j.1365-313x.2000.00794.x. [DOI] [PubMed] [Google Scholar]
- Schaefer J. Regeneration in alfalfa tissue culture. Plant Physiol. 1985;79:584–589. doi: 10.1104/pp.79.3.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt ED, Guzzo F, Toonen MA, de Vries SC. A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development. 1997;124:2049–2062. doi: 10.1242/dev.124.10.2049. [DOI] [PubMed] [Google Scholar]
- Schumacher K, Vafeados D, McCarthy M, Sze H, Wilkins T, Chory J. The Arabidopsis det3 mutant reveals a central role for the vacuolar H+-ATPase in plant growth and development. Genes Dev. 1999;13:3259–3270. doi: 10.1101/gad.13.24.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Gupta M, Chandra P. Oxidative stress induced by iron in Hydrilla verticillata (l.f.) Royle: response of antioxidants. Ecotoxicol Environ Saf. 1997;38:286–291. doi: 10.1006/eesa.1997.1598. [DOI] [PubMed] [Google Scholar]
- Smith DL, Krikorian AD. pH control of carrot somatic embryogenesis. In: Nijkamp HJJ, Van der Plas LHW, Van Aartrijk J, editors. Progress in Plant Cellular and Molecular Biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1990a. pp. 449–453. [Google Scholar]
- Smith DL, Krikorian AD. Somatic proembryo production from excised, wounded zygotic carrot embryos on hormone-free medium: evaluation of the effects of pH, ethylene and activated charcoal. Plant Cell Rep. 1990b;9:34. [PubMed] [Google Scholar]
- Song J, Sorensen EL, Liang GH. Direct embryogenesis from single mesophyll protoplasts in alfalfa (Medicago sativa L.) Plant Cell Rep. 1990;9:21–25. doi: 10.1007/BF00232128. [DOI] [PubMed] [Google Scholar]
- Spena A, Prinsen E, Fladung M, Schulze SC, Van Onckelen H. The indoleacetic acid-lysine synthetase gene of Pseudomonas syringae subsp. savastanoi induces developmental alterations in transgenic tobacco and potato plants. Mol Gen Genet. 1991;227:205–212. doi: 10.1007/BF00259672. [DOI] [PubMed] [Google Scholar]
- Swann K, Whitaker MJ. Second messengers at fertilization in sea-urchin eggs. J Reprod Fertil Suppl. 1990;42:141–153. [PubMed] [Google Scholar]
- Takahashi Y, Ishida S, Nagata T. Function and modulation of expression of auxin-regulated genes. Int Rev Cytol. 1994;152:109–144. doi: 10.1016/s0074-7696(08)62555-3. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Nagata T. parB: an auxin-regulated gene encoding glutathione S-transferase. Proc Natl Acad Sci USA. 1992;89:56–59. doi: 10.1073/pnas.89.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansuyt G, Lopez F, Inzé D, Briat JF, Fourcroy P. Iron triggers a rapid induction of ascorbate peroxidase gene expression in Brassica napus. FEBS Lett. 1997;410:195–200. doi: 10.1016/s0014-5793(97)00587-5. [DOI] [PubMed] [Google Scholar]
- Whitaker MJ. Cell cycle control proteins are second messenger targets at fertilization in sea-urchin eggs. J Reprod Fertil Suppl. 1990;42:199–204. [PubMed] [Google Scholar]
- Wink M. Τhe plant vacuole: a multifunctional compartment. J Exp Bot. 1993;44:231–246. [Google Scholar]
- Yeung EC. Structural and developmental patterns in somatic embryogenesis. In: Thorpe TA, editor. In Vitro Embryogenesis in Plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 205–248. [Google Scholar]
- Yoshimura K, Yabuta Y, Ishikawa T, Shigeoka S. Expression of spinach ascorbate peroxidase isoenzymes in response to oxidative stresses. Plant Physiol. 2000;123:223–234. doi: 10.1104/pp.123.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.