Abstract
The classical dopamine hypothesis of schizophrenia postulates a hyperactivity of dopaminergic transmission at the D2 receptor. We measured in vivo occupancy of striatal D2 receptors by dopamine in 18 untreated patients with schizophrenia and 18 matched controls, by comparing D2 receptor availability before and during pharmacologically induced acute dopamine depletion. Acute depletion of intrasynaptic dopamine resulted in a larger increase in D2 receptor availability in patients with schizophrenia (19% ± 11%) compared with control subjects (9% ± 7%, P = 0.003). The increased occupancy of D2 receptors by dopamine occurred both in first-episode neuroleptic-naive patients and in previously treated chronic patients experiencing an episode of illness exacerbation. In addition, elevated synaptic dopamine was predictive of good treatment response of positive symptoms to antipsychotic drugs. This finding provides direct evidence of increased stimulation of D2 receptors by dopamine in schizophrenia, consistent with increased phasic activity of dopaminergic neurons.
Schizophrenia is a chronic mental illness characterized by disturbances of thoughts, perceptions, volition, and cognition. Schizophrenia typically emerges during late adolescence, and the course of the illness is usually characterized by alternating episodes of illness exacerbation and partial remission (1). Manifestations of the illness are commonly divided into positive (delusions, hallucinations, thought disorganization, paranoia), negative (lack of drive and motivation, alogia, social withdrawal), and cognitive symptoms (poor performance on cognitive tasks involving attention and working memory) (2, 3).
The prognosis of schizophrenia has been dramatically improved with the introduction of antipsychotic medications in the late fifties (4). Because all antipsychotic drugs are antagonists at the D2 receptor (5, 6), an alteration of dopamine transmission at this receptor has long been suspected to play a role in the pathophysiology of schizophrenia. Specifically, excess D2 transmission has been proposed to underlie positive symptomatology because these symptoms respond better to D2 receptor blockade compared with negative or cognitive symptoms (for references and review see ref. 7).
With the advance of brain imaging techniques, direct evidence suggestive of dysregulation of dopaminergic transmission in schizophrenia has emerged. First, four of five imaging studies have documented an increase in the striatal accumulation of [18F]fluorodopa or [11C]dopa in patients with schizophrenia (8–12). This increased uptake is consistent with increased activity of dopa decarboxylase, an enzyme involved in dopamine synthesis. However, the relationship between the increase in dopa decarboxylase activity revealed by these studies and the synaptic output of dopamine is unclear, since the reaction catalyzed by dopa decarboxylase is not the rate-limiting step of dopamine synthesis. Second, three of three studies have reported an increase in amphetamine-induced dopamine release in patients with schizophrenia (13–15). In these studies, changes in dopamine synaptic concentration were measured indirectly by the decrease in binding potential (BP) of the radiolabeled D2 receptor antagonists [11C]raclopride or (S)-(−)-3-[123I]iodo-2-hydroxy-6-methoxy-N-[(1-ethyl-2-pyrrolidinyl)methyl]benzamide ([123I]IBZM) after an amphetamine challenge. This exaggerated dopaminergic response to amphetamine associated with schizophrenia was detected at onset of illness and during periods of illness exacerbation, but not during remissions (16). A major limitation of these studies is that they measured changes in synaptic dopamine transmission after a nonphysiological challenge (i.e., amphetamine) and did not provide any information about synaptic dopamine levels at baseline—i.e., in the unchallenged state.
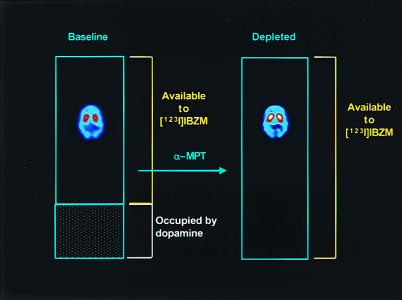
In this paper, we studied baseline occupancy of D2 receptors by dopamine in patients with schizophrenia and in healthy control subjects. D2 receptor availability was measured at baseline (i.e., in the absence of any pharmacological intervention) and during acute dopamine depletion. D2 receptor availability was measured with single-photon computerized emission tomography (SPECT) during constant infusion of the radiolabeled D2 receptor antagonist [123I]IBZM. Acute dopamine depletion was achieved by administration of high doses of the tyrosine hydroxylase inhibitor α-methyl-para-tyrosine (α-MPT) for 2 days (17, 18). Since this duration of treatment is too short to induce detectable D2 receptor up-regulation, the main difference between D2 receptor availability measured at baseline and in the depleted state is because of the unmasking of D2 receptors previously occupied by dopamine (19). Therefore, comparing D2 receptor availability at baseline and in the depleted state provided an indirect measure of the proportion of D2 receptors occupied by dopamine in the baseline state (Fig. 1).
Figure 1.
Schematic presentation of the experiment. The blue rectangles represent the total population of D2 receptors. At baseline, an unknown proportion of these receptors is occupied by dopamine (shaded area), and only a fraction of receptors are unoccupied and available to [123I]IBZM binding. After α-MPT-induced depletion of endogenous dopamine, all receptors are available to [123I]IBZM binding. Thus, comparing the [123I]IBZM binding potential at baseline and after dopamine depletion allows derivation of the proportion of D2 receptors that were masked by dopamine at baseline. The SPECT images display distribution of [123I]IBZM in one subject at baseline and after dopamine depletion (note the increase in specific binding in the striatum).
Materials and Methods
Subjects.
The protocol was approved by the Institutional Review Boards of the New York State Psychiatric Institute and Columbia Presbyterian Medical Center. Patients were recruited after voluntary admission to a research ward (Schizophrenia Research Unit, New York State Psychiatric Institute) and were inpatients throughout the study. Capacity to provide informed consent was evaluated by a psychiatrist not associated with the study. According to the recommendations of the National Alliance for the Mentally Ill (Arlington, VA), assent from involved family members was also obtained.
Inclusion criteria for patients were as follows: (i) diagnosis of schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; ref. 20); (ii) no other DSM-IV axis I diagnosis; (iii) no lifetime history of alcohol or substance abuse or dependence; (iv) absence of any psychotropic medication for at least 21 days before the study (with the exception of lorazepam, which was allowed at a maximal dose of 3 mg per day up to 24 h before the study); (v) no concomitant or past severe medical conditions; (vi) no pregnancy; (vii) no current suicidal or homicidal ideation; and (ix) ability to provide informed consent. Inclusion criteria for the control group were (i) absence of past or present neurological or psychiatric illnesses, including substance abuse; (ii) no concomitant or past severe medical conditions; (iii) no pregnancy; and (iv) informed consent. Groups were matched for age, gender, race, parental socioeconomic level [Hollingshead, A. B. (1975) Four Factor Index of Social Status (working paper published by the author, New Haven, CT)], cigarette smoking, and weight.
SPECT Protocol.
Subjects underwent two measurements of D2 receptor BP with SPECT, [123I]IBZM, and the sustained equilibrium/constant infusion technique (21). The first scan was obtained in control conditions (baseline scan), on the first day of the study (day 1). The second scan was obtained on day 3, after dopamine depletion induced by oral administration of α-MPT, given at a dose of 1 g every 6 h for 2 days (for a total dose of 8 g). Thus, two measures of BP were obtained in each subject: at baseline (BPBASE) and in the depleted state (BPDEPL).
SPECT experiments were carried out as previously described (21). Briefly, [123I]IBZM with specific activity >5,000 Ci/mmol (1 Ci = 37 GBq) and radiochemical purity >95% was prepared by direct electrophilic radioiodination of the desiodo precursor BZM (22). A total [123I]IBZM dose of 7.6 ± 1.7 mCi (mean ± SD) was given as a bolus, followed by a continuous infusion for the duration of the experiment (240 min). The bolus to hourly infusion ratio was 3.92. This protocol of administration induces a state of sustained binding equilibrium after 150 min (21). On both baseline and depleted days, SPECT data were acquired for 60 min, from 180 to 240 min after the initiation of [123I]IBZM administration. SPECT data were acquired on the PRISM 3000 (Picker, Cleveland, Ohio) with high-resolution fan beam collimators (resolution at full width half-maximum, 11 mm). Scanning was performed with the following acquisition parameters: continuous acquisition mode; matrix, 64 × 64 × 32; angular range, 120°; angular steps, 3; seconds per step, 18; frame duration, 12 min; number of frames, 5; radius of rotation, 13.5 cm.
α-MPT Administration.
The dose and frequency of α-MPT administration (1 g four times a day for 2 days) were selected to provide and maintain significant inhibition of tyrosine hydroxylase activity (23). α-MPT was given for 2 days, based on the expectation that this duration of treatment would be adequate to produce marked dopamine depletion but too short to induce significant D2 receptor up-regulation (see discussion in ref. 19). The first α-MPT dose was given on the evening of day 1 (8 p.m.). On day 2, 1 g of α-MPT was administered at 7 a.m., noon, 6 p.m., and 11 p.m. On day 3, 1 g of α-MPT was given at 7 a.m., noon, and 1 h before the beginning of the second scanning session (5 p.m.). To prevent the formation of α-MPT crystals in the urine, subjects were instructed to drink at least 4 liters of fluids per day, starting the day before the study, and fluid intake was carefully monitored (23, 24). Urinalysis was performed daily. Clinical evaluation, including orthostatic blood pressure monitoring, was obtained four times a day. Trough plasma α-MPT levels were measured 4 h after the seventh dose by using gas chromatography/mass spectrometry.
Image Analysis.
Frames were reconstructed by using a Butterworth filter (cutoff = 1 cm, power factor = 10) and transferred into the MEDx software system (Sensor Systems, Sterling, VA). Frames were realigned to each other, using a least-squares algorithm for within-modalities coregistration (automated image registration, AIR) (25). The four slices with highest striatal uptake were summed, and attenuation was corrected assuming uniform attenuation within an ellipse drawn around the subject's head (attenuation coefficient μ = 0.10 cm2/g). For each subject, the same attenuation ellipse was used for scans 1 and 2. Standard regions of interest of constant size (striatum 15,820 mm3, frontal 57,656 mm3, occipital 58,497 mm3) were positioned on the summed images. Right and left striatal regions were averaged. The average of frontal and occipital regions was used to define the nonspecific uptake. The image analysis protocol used here was different from the image analysis protocol used in our previously published feasibility study (19). Analysis parameters were selected here to provide maximal protection against noise. For example, the size of the striatal region of interest (ROI) was larger than in the previous study. A larger ROI size protects against noise but increases the partial voluming effect. Similarly, we selected here a Butterworth filter rather than a Wiener filter, as in the previous study. The Butterworth filter is more robust with respect to noise than the Wiener filter, but, in phantom experiments, the slope of the relationship between true and measured target to background ratios was 0.69 for Butterworth versus 0.99 for Wiener (unpublished data).
Specific binding was defined as the difference between striatal activity and nonspecific activity. BP was calculated as the ratio of specific to nonspecific activity. The α-MPT-induced increase in BP was calculated as the difference between the BPBASE and BPDEPL, and this difference was expressed in percentage of BPBASE. BP is related to the receptor number (Bmax) and affinity for [123I]IBZM (Kd) such that BP = Bmax/Kd(1 + FDA/Ki)V2, where FDA is the temporal and spatial average concentration of free synaptic dopamine, Ki is the inhibition constant of dopamine for [123I]IBZM, and V2 is the distribution volume of the nonspecific compartment. Bmax, Kd, Ki, and V2 are unaffected by this α-MPT regimen. It follows that, because FDA during depleted conditions is negligible compared with Ki, the increase in BP induced by α-MPT is linearly related to baseline FDA by FDA = Ki(a − 1), where a is the ratio of BPDEPL to BPBASE (see derivation in ref. 19).
Clinical Assessment.
Severity of clinical symptoms were measured with the Positive and Negative Symptom Scale (PANSS) (26). The PANSS includes a positive symptom subscale, a negative symptom subscale, and a general psychopathology subscale.
Baseline ratings were obtained 60 min before the first scanning session. Ratings in the depleted state were obtained 60 min before the scanning session on the depleted day. After the completion of the scans, patients were started on antipsychotic medication and remained on the research ward for the initial 6 weeks of treatment. The choice of the antipsychotic agent, as well as the dose, was determined by the treating psychiatrist on the basis of clinical indication. Clinical ratings were also obtained after 1 week and 6 weeks of antipsychotic treatment.
Statistical Analysis.
Between-group comparisons were performed by using factorial ANOVA or repeated-measure ANOVA with group × effect interaction, as appropriate. Relationships between continuous variables were analyzed with the Pearson product moment correlation coefficient. A probability value of 0.05 was selected as significance level.
Results
Group Composition.
Twenty-four patients and 22 controls were recruited for this study. Eighteen patients and 18 controls completed the protocol. Reasons for noncompletion (n = 10) included radiolabeling failure on the day of the second scan (n = 1), technical problems with [123I]IBZM infusion during the second scan (n = 1), and intolerance to the side effects of α-MPT (n = 8), resulting in withdrawal from the study. Common side effects included sedation, orthostatism, irritability, and bradykinesia. These side effects spontaneously resolved within 12 h of the last α-MPT administration. Uncommon side effects included acute dystonia (observed in 4 subjects), which resolved within minutes after diphenhydramine administration, and crystalluria (n = 3), which resolved within 4 h upon i.v. fluid administration.
Completers were matched for age, gender, parental socioeconomic status, cigarette smoking, and weight (Table 1). Among the 18 patients, 8 were antipsychotic naive and experiencing a first episode of illness. Ten patients were chronic and had been previously treated with antipsychotic drugs. In the previously treated group, the interval between the last antipsychotic administration and the first scan was 139 ± 146 days. These previously treated patients were experiencing an episode of illness exacerbation at the time of recruitment and were admitted to the hospital for clinical reasons. Total PANNS scores were 71 ± 12 in the first-episode patients and 63 ± 11 in the chronic patients.
Table 1.
Demographic and clinical variables
| Variable | Value
|
|
|---|---|---|
| Controls | Patients with schizophrenia | |
| n | 18 | 18 |
| Age, years | 31 ± 8 | 31 ± 8 |
| Gender (F/M) | 7/11 | 7/11 |
| Race (AA/H/C/AS) | 5/5/7/1 | 7/5/5/1 |
| Parental SES | 40 ± 17 | 43 ± 16 |
| Subject SES | 45 ± 13 | 26 ± 10* |
| Smokers (Yes/No) | 2/16 | 2/16 |
| Weight, kg | 67 ± 22 | 78 ± 14 |
| Neuroleptic-free/neuroleptic-naive | — | 10/8 |
| Neuroleptic-free interval, days | — | 139 ± 146 |
AA, Afro-Americans; H, Hispanics; C, Caucasians; AS, Asians; SES, socioeconomic status. *, Significantly different from controls, unpaired t test, P < 0.001.
Dopamine Levels: Patients Versus Controls.
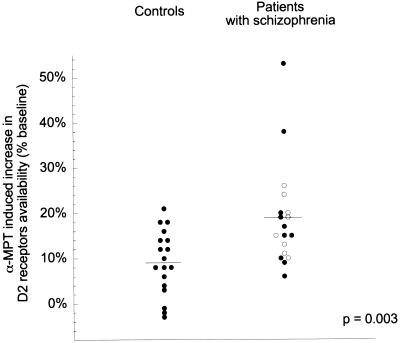
D2 receptor availability was significantly higher after α-MPT exposure compared with baseline, and this effect was larger in patients compared with controls (repeated-measure ANOVA, patients versus controls: α-MPT effect, P < 0.001; α-MPT × group interaction: P = 0.0034, Table 2). Removal of endogenous dopamine by α-MPT treatment increased D2 receptor availability by 9% ± 7% in controls and by 19% ± 11% in patients with schizophrenia (Fig. 2). The variance of the α-MPT effect on D2 receptor availability tended to be larger in schizophrenic patients compared with controls (variance ratio 0.40, P = 0.072). Nonparametric tests (Mann–Whitney U) were also used to compare α-MPT-induced increase in D2 receptor availability between groups, and this analysis revealed similar results (P = 0.0065). Two patients exhibited α-MPT-induced increase in D2 receptor availability of 53% and 38%, respectively, values corresponding to 3.0 and 1.7 SD above the mean of the schizophrenic group. After removal of these two patients, α-MPT-induced increase in D2 receptor availability was 15% ± 6% in patients with schizophrenia (n = 16) versus 9% ± 7% in controls (n = 18). This difference was as significant (P = 0.0033) as the difference observed in the complete data set (P = 0.0034). No experimental factors were identified that would justify excluding these two observations.
Table 2.
Effect of dopamine depletion of D2 receptor availability in controls and patients with schizophrenia
| Subjects | n | [123I]IBZM BP
|
α-MPT effect on [123I]IBZM BP, % | α-MPT in plasma, μg/ml | |
|---|---|---|---|---|---|
| Baseline | Post-α-MPT | ||||
| Controls | 18 | 0.72 ± 0.09 | 0.79 ± 0.10 | 9 ± 7 | 19 ± 6 |
| Patients with schizophrenia | 18 | 0.75 ± 0.10 | 0.89 ± 0.1* | 19 ± 11** | 21 ± 6 |
*, Significantly different from controls, unpaired t test, P = 0.009; **, significantly different compared with controls, repeated-measure ANOVA, group × effect interaction, P = 0.0034.
Figure 2.
Increase in [123I]IBZM BP to D2 receptors after acute dopamine depletion in controls and patients with schizophrenia (●, previously treated patients; ○, first-episode neuroleptic-naive patients).
No effect of age was observed on α-MPT-induced increase in D2 receptor availability, neither in the entire sample (r2 = 0.01, P = 0.47) nor in the groups considered separately (patients: r2 = 0.03, P = 0.51; controls: r2 = 0.01, P = 0.63). No effect of gender was observed on α-MPT-induced increase in D2 receptor availability, neither in the entire sample (P = 0.40) nor in the groups considered separately (patients: P = 0.58; controls: P = 0.46).
The differential effect of α-MPT between patients and controls was not due to differences in α-MPT bioavailability. α-MPT plasma levels were not different in controls (19 ± 6 μg/ml) and patients (21 ± 6 μg/ml, P = 0.522). α-MPT plasma levels were not correlated with the magnitude of the α-MPT effect on D2 receptor availability, neither in the controls (r2 = 0.002, P = 0.86) nor in the patients (r2 = 0.10, P = 0.22).
D2 Receptor Binding Potential: Patients Versus Controls.
At baseline, no difference was observed in D2 receptor availability between controls and patients with schizophrenia (P = 0.377). However, after removal of endogenous dopamine, D2 receptor availability was significantly higher in patients with schizophrenia compared with controls (P = 0.009; Table 2).
Effect of Previous Neuroleptic Treatment.
As summarized in Table 3, α-MPT effect on D2 receptor availability was not statistically different between drug-naive (n = 8, 17% ± 6%) and previously treated patients (n = 10, 20% ± 15%, repeated-measure ANOVA, drug-naive patients versus previously treated patients: α-MPT × group interaction: P = 0.46). Both groups were significantly different from controls (repeated-measure ANOVA, drug-naive patients versus controls: α-MPT × group interaction: P = 0.0088; previously treated patients versus controls: α-MPT × group interaction: P = 0.0082). In previously treated patients, no relationship was observed between duration of the drug-free interval and α-MPT effect on D2 receptor availability (r2 = 0.16, P = 0.27). Thus, the increased occupancy of D2 receptors by dopamine observed in patients with schizophrenia was present at onset of illness and was not a consequence of previous neuroleptic administration.
Table 3.
Effect of dopamine depletion of D2 receptor availability in controls and first-episode and chronic patients with schizophrenia
| Subjects | n | Age, years | Gender (M/F) | [123I]IBZM BP
|
α-MPT effect on [123I]IBZM BP, % | α-MPT in plasma, μg/ml | |
|---|---|---|---|---|---|---|---|
| Baseline | Post-α-MPT | ||||||
| Controls | 18 | 31 ± 8 | 7/11 | 0.72 ± 0.09 | 0.79 ± 0.10 | 9 ± 7 | 19 ± 6 |
| Patients with schizophrenia | |||||||
| Neuroleptic-naive first-episode | 8 | 30 ± 7 | 5/3 | 0.72 ± 0.07 | 0.84 ± 0.06 | 17 ± 6** | 21 ± 8 |
| Neuroleptic-free chronic | 10 | 32 ± 9 | 2/8 | 0.78 ± 0.12 | 0.93 ± 0.15* | 20 ± 15** | 21 ± 5 |
*, Significantly different from controls, unpaired t test, P < 0.05; **, significantly different compared with controls, repeated-measure ANOVA, group × effect interaction, P < 0.01.
In contrast, the higher D2 receptor BP measured after endogenous dopamine depletion reached significance only in the previously treated group (P = 0.038), not in the drug-naive group (P = 0.188). In previously treated patients, D2 receptor availability was related to the drug-free interval, i.e., larger in patients with shorter washout periods (r2 = 0.47, P = 0.03). Thus, there was indirect evidence that the higher D2 receptor density measured in the chronic patients after endogenous dopamine depletion might be a side effect of previous neuroleptic exposure.
Dopamine Levels and Baseline Symptomatology.
In patients, baseline global severity of positive symptoms (r2 < 0.01, P = 0.76) or negative symptoms (r2 = 0.10, P = 0.20) was not predictive of the magnitude of α-MPT effect on D2 receptor availability. Among positive symptoms, only severity of suspiciousness was associated at trend level with α-MPT effect on D2 receptor availability (r2 = 0.19, P = 0.07).
α-MPT Effect on Symptoms.
α-MPT exposure induced a significant reduction (−20% ± 18%) in the severity of positive symptoms (baseline PANSS positive subscale scores 18.2 ± 6.0; post-α-MPT, 14.5 ± 5.9, repeated-measure ANOVA, P = 0.001). High synaptic dopamine levels predicted good response of positive symptoms to α-MPT (relationship between relative decrease in positive symptoms and α-MPT-induced increase in D2 receptor BP, r2 = 0.54, P = 0.0005). α-MPT induced a nonsignificant increase in negative symptoms (baseline, 13.8 ± 5.4; post-α-MPT, 16.6 ± 8.3, repeated-measure ANOVA, P = 0.11). This effect was unrelated to the α-MPT effect on D2 receptor availability (r2 = 0.003, P = 0.84).
Dopamine Levels and Prediction of Treatment.
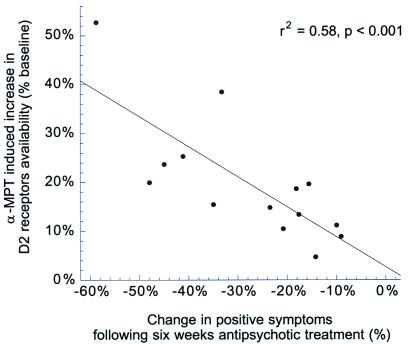
Fourteen of 18 patients agreed to complete the 6-week period of antipsychotic medication as inpatients (4 patients elected to be treated as outpatients and were excluded from the treatment phase of the study). To optimize clinical benefit, the choice and doses of antipsychotic medication were decided by the clinician on an individual basis. Thirteen of 14 patients were treated with atypical antipsychotic drugs (olanzapine, n = 8; clozapine, n = 1; risperidone, n = 2; and quetiapine, n = 2). One patient was treated with haloperidol. Benzodiazepine medications were added as needed. Table 4 presents the clinical ratings at baseline, during α-MPT-induced dopamine depletion, after 1 week of antipsychotic treatment, and after 6 weeks of antipsychotic treatment in this subset of 14 patients. Compared with baseline, a significant decrease in positive symptoms was measured at each time point (P < 0.0001). Changes in negative symptoms were not significant. A large between subject variability was observed in the positive symptoms improvement at 6 weeks (28% ± 16%). Higher synaptic levels of dopamine at baseline, as measured by the α-MPT effect on D2 receptor BP, were significantly associated with greater improvement of positive symptoms after 6 weeks of antipsychotic treatment (r2 = 0.58, P = 0.0029, Fig. 3).
Table 4.
Effect of α-MPT and antipsychotic medications on positive and negative symptoms
| Status | Clinical rating
|
|
|---|---|---|
| Positive symptoms | Negative symptoms | |
| Baseline | 18.6 ± 5.8 | 14.1 ± 5.2 |
| Post-α-MPT | 15.0 ± 6.4* | 17.7 ± 8.5 |
| Week 1 | 15.1 ± 6.4* | 14.6 ± 10.7 |
| Week 6 | 13.3 ± 5.1* | 13.8 ± 7.2 |
*, Significantly different from baseline, repeated-measure ANOVA, P < 0.001 (n = 14).
Figure 3.
Relationship between baseline dopamine levels, as estimated by the α-MPT effect on [123I]IBZM BP, and the decrease in positive symptoms measured after 6 weeks of antipsychotic treatments.
Discussion
The results of this study suggest that dopamine occupies a greater proportion of striatal D2 receptors in patients with schizophrenia compared with matched control subjects during first episode of illness and subsequent episodes of illness exacerbation. The dysregulation of dopamine transmission revealed by the imaging study was predictive of better response of positive symptoms to antipsychotic treatment. The significance of this result stems from the fact that the paradigm used here reveals D2 receptor occupancy by dopamine during the baseline scan—i.e., in the absence of any pharmacological intervention.
α-MPT-induced dopamine depletion resulted in 9% ± 7% and 19% ± 11% increases in D2 receptor availability in control subjects and patients with schizophrenia, respectively. If α-MPT-induced dopamine depletion was complete, this increase in D2 receptor availability would indicate that dopamine occupies 8% ± 6% of D2 receptors in controls versus 15% ± 7% in patients with schizophrenia. It should be noted, however, that the precise occupancy of D2 receptors by dopamine cannot be calculated, because the exact magnitude of dopamine depletion achieved in this study is unknown.
We estimate that about 70–80% depletion of striatal dopamine was achieved in this study. At 4 g per day, α-MPT induces 70% depletion in cerebrospinal fluid homovanillic acid (HVA), and it is expected that the magnitude of dopamine depletion is larger than HVA depletion (27). Furthermore, at least 80% dopamine depletion or 80% D2 receptor blockade is needed to produce extrapyramidal symptoms (28, 29). While most of the subjects presented extrapyramidal symptoms, the moderate level of these symptoms likewise suggested a depletion in the 70–80% range. Assuming that α-MPT treatment resulted in 70% depletion of endogenous dopamine, the occupancy of D2 receptors by dopamine would be 12% ± 9% in control subjects versus 22% ± 10% in patients with schizophrenia (as derived with equation 9 of ref. 19). The conclusion that occupancy of D2 receptors by dopamine is increased in patients with schizophrenia compared with controls is valid even if the depletion is incomplete.
Nevertheless, two assumptions are required to support this conclusion. First, the relative magnitude of dopamine depletion should be comparable between groups. This assumption is supported by the observation of similar plasma α-MPT levels in the two groups. However, a better penetration of α-MPT into the brains of patients with schizophrenia, although unlikely, cannot be ruled out a priori. Cerebrospinal fluid measurements of α-MPT would be needed to confirm this point. Similarly, we cannot rule out a larger sensitivity of tyrosine hydroxylase to α-MPT blockade in patients with schizophrenia compared with controls.
Second, this conclusion implies that no significant up-regulation of D2 receptors occurred during the acute dopamine depletion. This assumption is supported by the observation that, in rodents, no significant D2 receptor up-regulation is detectable after 1 week of dopamine depletion induced by 6-hydroxydopamine (30). We confirmed this observation by comparing [125I]IBZM Bmax measured in vitro in rodents treated for 48 h with a high dose (400 mg/kg per day s.c.) of α-MPT (256 ± 28 fmol/mg of protein, n = 7) and with saline (228 ± 63 fmol/mg of protein, n = 7, unpaired t test, P = 0.31) (19). However, it is unknown if the lack of detectable up-regulation of D2 receptors after short-term dopamine depletion (1 week or less) in rodents can be extrapolated to humans, and to patients with schizophrenia. We should also note that the mechanisms underlying changes in [125I]IBZM or [11C]raclopride BP measured in vivo after changes in synaptic dopamine levels are probably more complex than accounted for by a simple binding competition model, and might also involve changes in subcellular distribution of receptors [for review and discussion, see Laruelle (53)].
A larger occupancy of D2 receptors by dopamine in patients versus controls may be related to higher levels of free dopamine in the vicinity of D2 receptors, higher affinity of D2 receptors for dopamine, or some combination of both factors. The development of a radiolabeled D2 receptor agonist as a positron-emission tomography radiotracer is needed to address this issue. Until then, the interpretation of the results of this study as reflective primarily of increased dopamine levels remains tentative. Assuming that the Ki of dopamine for [123I]IBZM in vivo is the same as measured in vitro at room temperature (160 nM; ref. 31) and that it is similar in patients and controls, the temporal average synaptic dopamine concentration would be 15 ± 12 nM in controls versus 30 ± 18 nM in patients (at 100% depletion) or 23 ± 18 nM in controls versus 48 ± 18 nM in patients (at 70% depletion).
Recent imaging studies clearly indicate that the competition between endogenous dopamine and radiolabeled tracers takes place at the intrasynaptic rather than extrasynaptic receptors. Drugs that enhance dopamine release without blocking dopamine transporters (nicotine, ketanserin) induce significant [11C]raclopride displacement without significantly affecting microdialysis measurements of extracellular dopamine (32, 33). Therefore, D2 receptors located in the synaptic space appear to be the receptors involved in these endogenous competition studies. This point is important, as it clarifies that these data are compatible with increased phasic activity of dopamine neurons. Grace (34, 35) proposed the term “phasic release” to characterize the intrasynaptic release of dopamine elicited by cell firing, and the term “tonic release” to characterize the low level of extrasynaptic release that occurs independent of cell firing. He also proposed that, in schizophrenia, increased phasic activity of dopamine neurons might be secondary to decreased tonic activity due to deficits in prefrontal-striatal glutamatergic drive, which controls tonic release. In that sense, our data are in accordance with the hypothesis advanced by Grace (34, 35).
The higher occupancy of D2 receptors by dopamine in patients with schizophrenia was not a long-term consequence of previous exposure to D2 receptor antagonists. Thus, this factor appears to be associated with schizophrenia per se. However, because patients studied here were experiencing an acute episode of illness (first episode or relapse), we cannot conclude that this dysregulation is also present during periods of illness remission. A study of patients during remission phase is needed to clarify this point.
After removal of dopamine, higher D2 receptor BP was measured in patients with schizophrenia compared with controls. This observation was consistent with the proposition that radiotracers sensitive to competition by endogenous dopamine may fail to detect an increase in density of D2 receptors in schizophrenia due to higher synaptic levels of dopamine (36, 37) and helps to clarify the discrepant results obtained when measuring D2 receptors in patients with schizophrenia by using radiotracers that are sensitive (i.e., [11C]raclopride) or insensitive (i.e., N-[11C]methylspiperone) to endogenous dopamine competition (38–41). However, previous antipsychotic treatment might have contributed to the increased density of D2 receptors measured in patients, an effect consistent with the observation that D2 receptor antagonists up-regulate D2 receptors (42).
Interestingly, in the schizophrenia group, global severity of positive symptoms did not correlate with occupancy of striatal D2 receptors by dopamine. This negative result might be due to the limited resolution of the SPECT camera. Considerable preclinical evidence from rodent studies supports that antipsychotic drug action is associated with D2 receptor antagonism in the mesolimbic (ventral striatal, including nucleus accumbens) rather than the nigrostriatal (dorso-striatal) dopamine systems (for review see ref. 43). The limited resolution of the SPECT camera prevented us from distinguishing the respective contributions of the ventral and dorsal striata to the SPECT signal. Studies with a high-resolution positron-emission tomography camera are needed to clarify this point.
On the other hand, this negative result might indicate that the severity of positive symptoms rated cross-sectionally by the PANSS depends mostly on factors located downstream from the mesolimbic dopaminergic synapses. The dysfunctional neuronal circuits that underlie the experience of positive symptoms are likely to involve dysregulated prefrontal–ventral striatal–ventral pallidal–mediodorsal thalamic–prefrontal loops, and their regulation by hippocampal and amygdalar afferents (54–56). The results of this and previous studies (13–15) directly confirm that these loops are under modulatory influence of subcortical dopamine. A sudden rise in subcortical dopamine (such as measured following amphetamine) will exacerbate these symptoms, whereas a sudden decline in dopamine (such as measured following α-MPT) will blunt their intensity. Thus, psychotic symptomatology includes both dopamine-dependent and dopamine-independent components, with the respective contribution of each component varying from patient to patient (and presumably varying with time within the same patient). Ellinwood (44) proposed that suspiciousness/paranoia is the most “dopamine dependent” dimension of psychosis. This hypothesis was supported by the fact that suspiciousness severity was associated at trend level with intensity of stimulation of D2 receptors by dopamine.
Schizophrenic patients who experienced positive symptoms in the presence of increased dopamine stimulation of D2 receptors showed a remarkable and rapid decline in these symptoms after treatment with antipsychotic drugs. On the other hand, subjects who experienced positive symptoms in the presence of apparently normal stimulation of D2 receptors by dopamine showed little improvement of these symptoms after 6 weeks of antipsychotic treatment. The fact that high levels of synaptic dopamine at baseline predicted better or faster response to atypical antipsychotic drugs (13 of 14 patients were treated with atypical drugs) also suggests that the D2 receptor blockade induced by these drugs remains a key component of their initial mode of action.
Contrary to widely accepted views, antipsychotic drugs have only partial efficacy against positive symptoms. A substantial proportion of schizophrenic patients, possibly a third, remain actively psychotic despite appropriate and prolonged blockade of D2 receptors (45, 46). The data presented in this study suggest that, in some patients, blockade of D2 receptors by antipsychotic drugs fails to significantly alter positive symptoms because these symptoms might not be related to excessive stimulation of these receptors by dopamine. Evidently, 6 weeks is too short to document treatment failure, and a study examining longer-term outcome is warranted to further assess this issue. Nevertheless, this observation suggests that even atypical antipsychotic drugs such as olanzapine might not provide immediate benefit in psychotic patients with unaltered dopamine levels. In these patients, psychotic symptoms may reflect a non-dopaminergic chemical imbalance, such as a deficiency in glutamatergic function (47–50). These data reinforce the need for alternative pharmacological approaches to the treatment of psychotic symptoms in schizophrenia.
In conclusion, this study provides direct in vivo evidence that schizophrenia is associated with excessive stimulation of D2 receptors by dopamine, and that this dysregulation is predictive of good treatment response to antipsychotic drugs. Both results warrant replication in a larger cohort. Dysregulation of subcortical dopamine function in schizophrenia may be secondary to a failure of prefrontal cortical control of subcortical dopaminergic function (43, 51, 52). Additional studies are needed to explore the relationship between prefrontal pathology and subcortical dopamine dysfunction in schizophrenia.
Acknowledgments
We thank the subjects who participated in this study, and we thank Mali Pratap, Ted Pozniakoff, Manuel DeLaNuez, Yolanda Rubino, and Suehee Chung and the staff of the Schizophrenia Research Unit at New York State Psychiatric Institute. This work was supported by the National Alliance for Research in Schizophrenia and Depression (NARSAD), the EJLB Foundation, the Stanley Foundation, and the U.S. Public Health Service (National Institute of Mental Health Grants RO1-MH54192, K02-MH01603-01, P20 MH50727-10, and NIH M01RR00645).
Abbreviations
- BP
binding potential
- [123I]IBZM
(S)-(−)-3-[123I]iodo-2-hydroxy-6-methoxy-N-[(1-ethyl-2-pyrrolidinyl)methyl]benzamide
- SPECT
single-photon computerized emission tomography
- α-MPT
α-methyl-para-tyrosine
- PANSS
Positive and Negative Symptom Scale
Footnotes
See commentary on page 7673.
References
- 1.Bromet E J, Fennig S. Biol Psychiatry. 1999;46:871–881. doi: 10.1016/s0006-3223(99)00153-5. [DOI] [PubMed] [Google Scholar]
- 2.Andreasen N C, Olsen S. Arch Gen Psychiatry. 1982;39:789–794. doi: 10.1001/archpsyc.1982.04290070025006. [DOI] [PubMed] [Google Scholar]
- 3.Gold J M, Harvey P D. Psychiatr Clin North Am. 1993;16:295–312. [PubMed] [Google Scholar]
- 4.Hegarty J D, Baldessarini R J, Tohen M, Waternaux C, Oepen G. Am J Psychiatry. 1994;151:1409–1416. doi: 10.1176/ajp.151.10.1409. [DOI] [PubMed] [Google Scholar]
- 5.Seeman P, Chau-Wong M, Tedesco J, Wong K. Proc Natl Acad Sci USA. 1975;72:4376–4380. doi: 10.1073/pnas.72.11.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Creese I, Burt D R, Snyder S H. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 7.Davis K L, Kahn R S, Ko G, Davidson M. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 8.Reith J, Benkelfat C, Sherwin A, Yasuhara Y, Kuwabara H, Andermann F, Bachneff S, Cumming P, Diksic M, Dyve S E, et al. Proc Natl Acad Sci USA. 1994;91:11651–11654. doi: 10.1073/pnas.91.24.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hietala J, Syvalahti E, Vuorio K, Rakkolainen V, Bergman J, Haaparanta M, Solin O, Kuoppamaki M, Kirvela O, Ruotsalainen U, et al. Lancet. 1995;346:1130–1131. doi: 10.1016/s0140-6736(95)91801-9. [DOI] [PubMed] [Google Scholar]
- 10.Dao-Castellana M H, Paillere-Martinot M L, Hantraye P, Attar-Levy D, Remy P, Crouzel C, Artiges E, Feline A, Syrota A, Martinot J L. Schizophrenia Res. 1997;23:167–174. doi: 10.1016/S0920-9964(96)00102-8. [DOI] [PubMed] [Google Scholar]
- 11.Hietala J, Syvalahti E, Vilkman H, Vuorio K, Rakkolainen V, Bergman J, Haaparanta M, Solin O, Kuoppamaki M, Eronen E, et al. Schizophrenia Res. 1999;35:41–50. doi: 10.1016/s0920-9964(98)00113-3. [DOI] [PubMed] [Google Scholar]
- 12.Lindstrom L H, Gefvert O, Hagberg G, Lundberg T, Bergstrom M, Hartvig P, Langstrom B. Biol Psychiatry. 1999;46:681–688. doi: 10.1016/s0006-3223(99)00109-2. [DOI] [PubMed] [Google Scholar]
- 13.Laruelle M, Abi-Dargham A, van Dyck C H, Gil R, D'Souza C D, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi S S, et al. Proc Natl Acad Sci USA. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breier A, Su T P, Saunders R, Carson R E, Kolachana B S, deBartolomeis A, Weinberger D R, Weisenfeld N, Malhotra A K, Eckelman W C, et al. Proc Natl Acad Sci USA. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abi-Dargham A, Gil R, Krystal J, Baldwin R, Seibyl J, Bowers M, van Dyck C, Charney D, Innis R, Laruelle M. Am J Psychiatry. 1998;155:761–767. doi: 10.1176/ajp.155.6.761. [DOI] [PubMed] [Google Scholar]
- 16.Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Biol Psychiatry. 1999;46:56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 17.Spector S, Sjoerdsma A, Udenfriend S. J Pharmacol Exp Ther. 1965;147:86–95. [PubMed] [Google Scholar]
- 18.Udenfriend S, Nagatsu T, Zaltzman-Nirenberg P. Biochem Pharmacol. 1965;14:837–847. doi: 10.1016/0006-2952(65)90103-6. [DOI] [PubMed] [Google Scholar]
- 19.Laruelle M, D'Souza C D, Baldwin R M, Abi-Dargham A, Kanes S J, Fingado C L, Seibyl J P, Zoghbi S S, Bowers M B, Jatlow P, et al. Neuropsychopharmacology. 1997;17:162–174. doi: 10.1016/S0893-133X(97)00043-2. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Ed. Washington, DC: Am. Psychiatric Assoc.; 1994. [Google Scholar]
- 21.Laruelle M, Abi-Dargham A, van Dyck C H, Rosenblatt W, Zea-Ponce Y, Zoghbi S S, Baldwin R M, Charney D S, Hoffer P B, Kung H F, et al. J Nucl Med. 1995;36:1182–1190. [PubMed] [Google Scholar]
- 22.Zea-Ponce Y, Laruelle M. Nucl Med Biol. 1999;26:661–665. doi: 10.1016/s0969-8051(99)00031-1. [DOI] [PubMed] [Google Scholar]
- 23.Engelman K, Jequier E, Udenfriend S, Sjoerdsman A. J Clin Invest. 1968;47:568–576. doi: 10.1172/JCI105753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang A E, Marsden C D. Clin Neuropharmacol. 1982;5:375–387. doi: 10.1097/00002826-198212000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Woods R P, Grafton S T, Holmes C J, Cherry S R, Mazziotta J C. J Comput Assist Tomogr. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- 26.Kay S R, Fiszbein A, Opler L A. Schizophrenia Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 27.Brodie H K, Murphy D L, Goodwin F K, Bunney W., Jr Clin Pharmacol Ther. 1971;12:218–224. doi: 10.1002/cpt1971122part1218. [DOI] [PubMed] [Google Scholar]
- 28.Hornykiewicz O, Kish S J. Adv Neurol. 1987;45:19–34. [PubMed] [Google Scholar]
- 29.Farde L, Wiesel F A, Nordström A L, Sedvall G. Psychopharmacology. 1989;99:S28–S31. doi: 10.1007/BF00442555. [DOI] [PubMed] [Google Scholar]
- 30.Narang N, Wamsley J K. J Chem Neuroanatomy. 1995;9:41–53. doi: 10.1016/0891-0618(95)00064-e. [DOI] [PubMed] [Google Scholar]
- 31.Brücke T, Tsai Y, McLellan C, Singhanyom W, Kung H, Cohen R, Chiueh C. Life Sci. 1988;42:2097–2104. doi: 10.1016/0024-3205(88)90123-3. [DOI] [PubMed] [Google Scholar]
- 32.Kim S E, Shin I, Oh S J, Kim S H, Choe Y S, Choi Y, Kim B T. J Nucl Med. 1998;39:54P. [Google Scholar]
- 33.Tsukada H, Nishiyama S, Kakiuchi T, Ohba H, Sato K, Harada N. Brain Res. 1999;841:160–169. doi: 10.1016/s0006-8993(99)01834-x. [DOI] [PubMed] [Google Scholar]
- 34.Grace A A. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- 35.Grace A A. J Neural Transm. 1993;91:111–134. doi: 10.1007/BF01245228. [DOI] [PubMed] [Google Scholar]
- 36.Seeman P, Guan H-C, Niznik H B. Synapse. 1989;3:96–97. doi: 10.1002/syn.890030113. [DOI] [PubMed] [Google Scholar]
- 37.Seeman P, Niznik H B, Guan H-C. Arch Gen Psychiatry. 1990;47:1170–1172. doi: 10.1001/archpsyc.1990.01810240090014. [DOI] [PubMed] [Google Scholar]
- 38.Wong D F, Wagner H N, Tune L E, Dannals R F, Pearlson G D, Links J M, Tamminga C A, Broussolle E P, Ravert H T, Wilson A A, et al. Science. 1986;234:1558–1563. doi: 10.1126/science.2878495. [DOI] [PubMed] [Google Scholar]
- 39.Andreasen N C, Carson R, Diksis M, Evans A, Farde L, Gjedde A, Hakim A, Lal S, Nair N, Sedvall G, et al. Schizophrenia Bull. 1988;14:471–484. doi: 10.1093/schbul/14.3.471. [DOI] [PubMed] [Google Scholar]
- 40.Farde L, Wiesel F, Stone-Elander S, Halldin C, Nordström A L, Hall H, Sedvall G. Arch Gen Psychiatry. 1990;47:213–219. doi: 10.1001/archpsyc.1990.01810150013003. [DOI] [PubMed] [Google Scholar]
- 41.Laruelle M. Q J Nucl Med. 1998;42:211–221. [PubMed] [Google Scholar]
- 42.Muller P, Seeman S. Life Sci. 1977;21:1751–1758. doi: 10.1016/0024-3205(77)90155-2. [DOI] [PubMed] [Google Scholar]
- 43.Deutch A Y. J Neural Transm. 1993;91:197–221. doi: 10.1007/BF01245232. [DOI] [PubMed] [Google Scholar]
- 44.Ellinwood E H, Sudilovsky A, Nelson L M. Am J Psychiatry. 1973;130:1088–1093. doi: 10.1176/ajp.130.10.1088. [DOI] [PubMed] [Google Scholar]
- 45.Huckle P L, Palia S S. Br J Hosp Med. 1993;50:467–471. [PubMed] [Google Scholar]
- 46.Weiden P, Aquila R, Standard J. J Clin Psychiatry. 1996;57, Suppl. 11:53–60. [PubMed] [Google Scholar]
- 47.Carlsson A. Neuropsychopharmacology. 1988;1:179–186. doi: 10.1016/0893-133x(88)90012-7. [DOI] [PubMed] [Google Scholar]
- 48.Moghaddam B. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:859–870. doi: 10.1016/0278-5846(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 49.Olney J W, Farber N B. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 50.Tamminga C A. Crit Rev Neurobiol. 1998;12:21–36. doi: 10.1615/critrevneurobiol.v12.i1-2.20. [DOI] [PubMed] [Google Scholar]
- 51.Pycock C J, Kerwin R W, Carter C J. Nature (London) 1980;286:74–77. doi: 10.1038/286074a0. [DOI] [PubMed] [Google Scholar]
- 52.Weinberger D R. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 53.Laruelle M. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 54.O'Donnell P, Grace A A. Schizophrenia Bull. 1998;24:267–283. doi: 10.1093/oxfordjournals.schbul.a033325. [DOI] [PubMed] [Google Scholar]
- 55.Grace A A, Moore H, O'Donnell P. Adv Pharmacol. 1998;42:721–724. doi: 10.1016/s1054-3589(08)60849-2. [DOI] [PubMed] [Google Scholar]
- 56.Laruelle M, Abi-Dargham A. J Psychopharmacol. 1999;13:358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]