Abstract
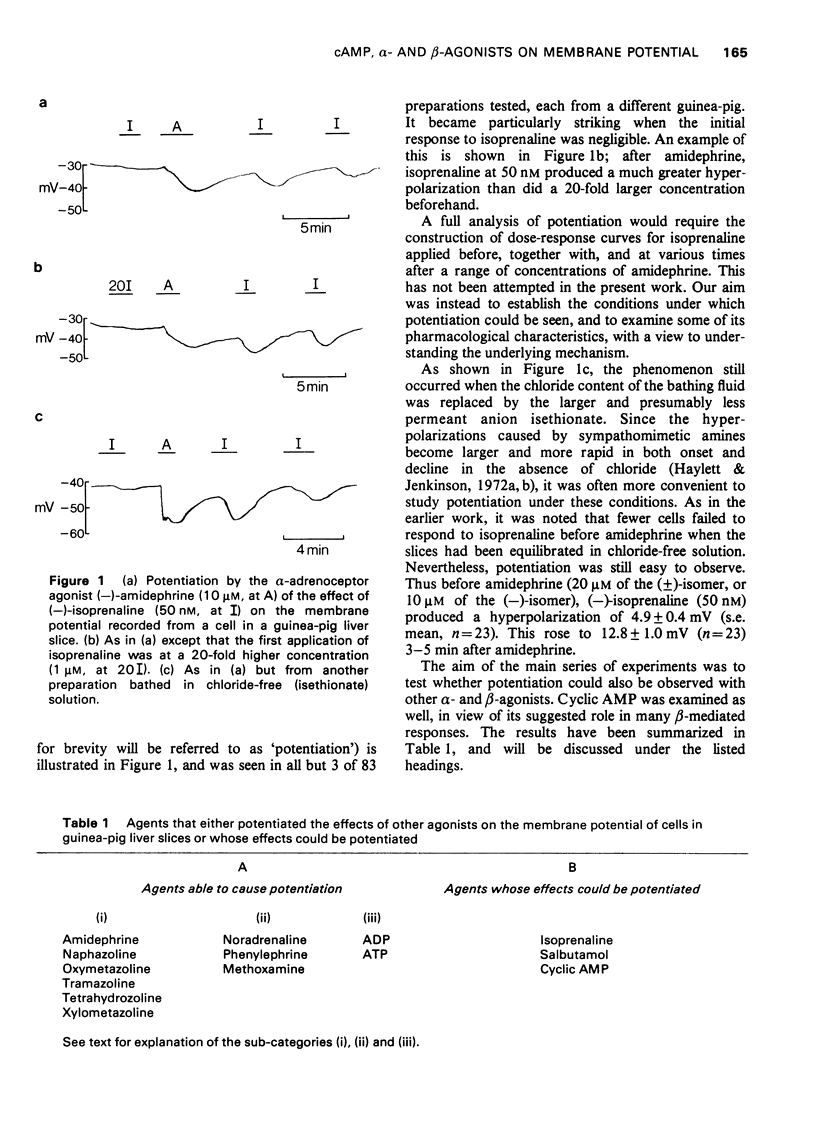
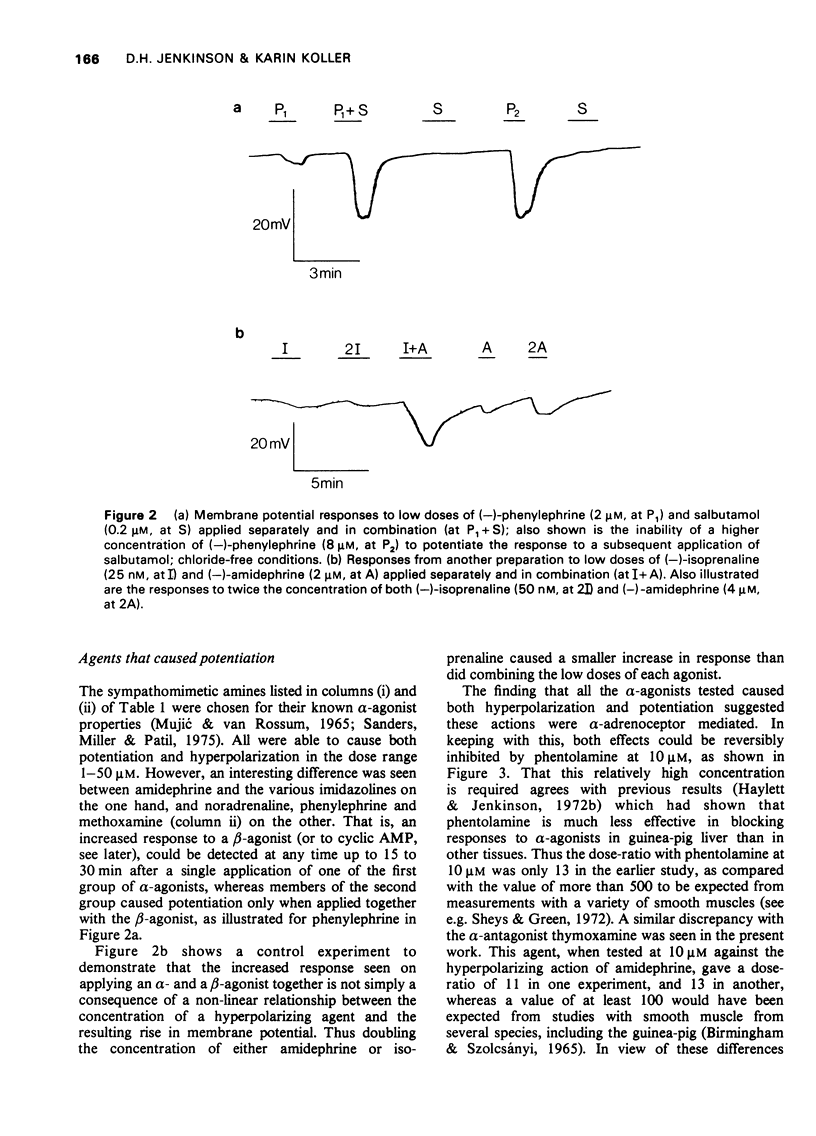
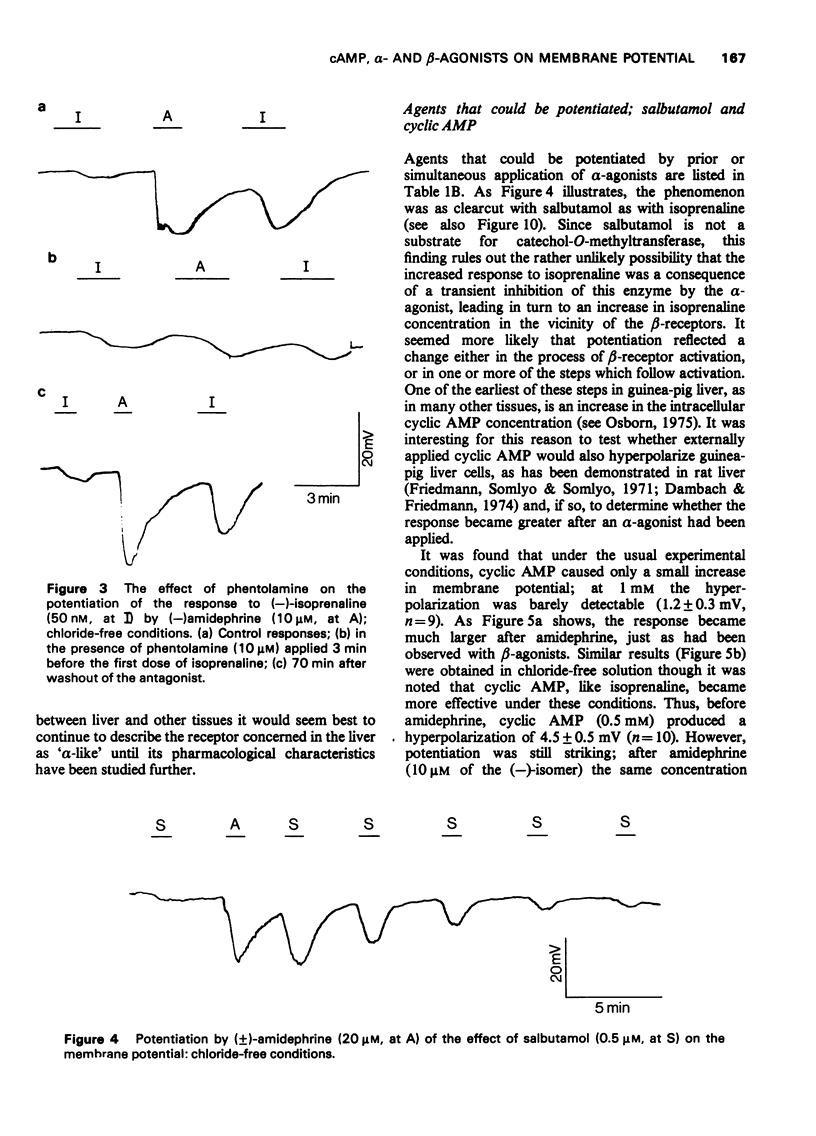
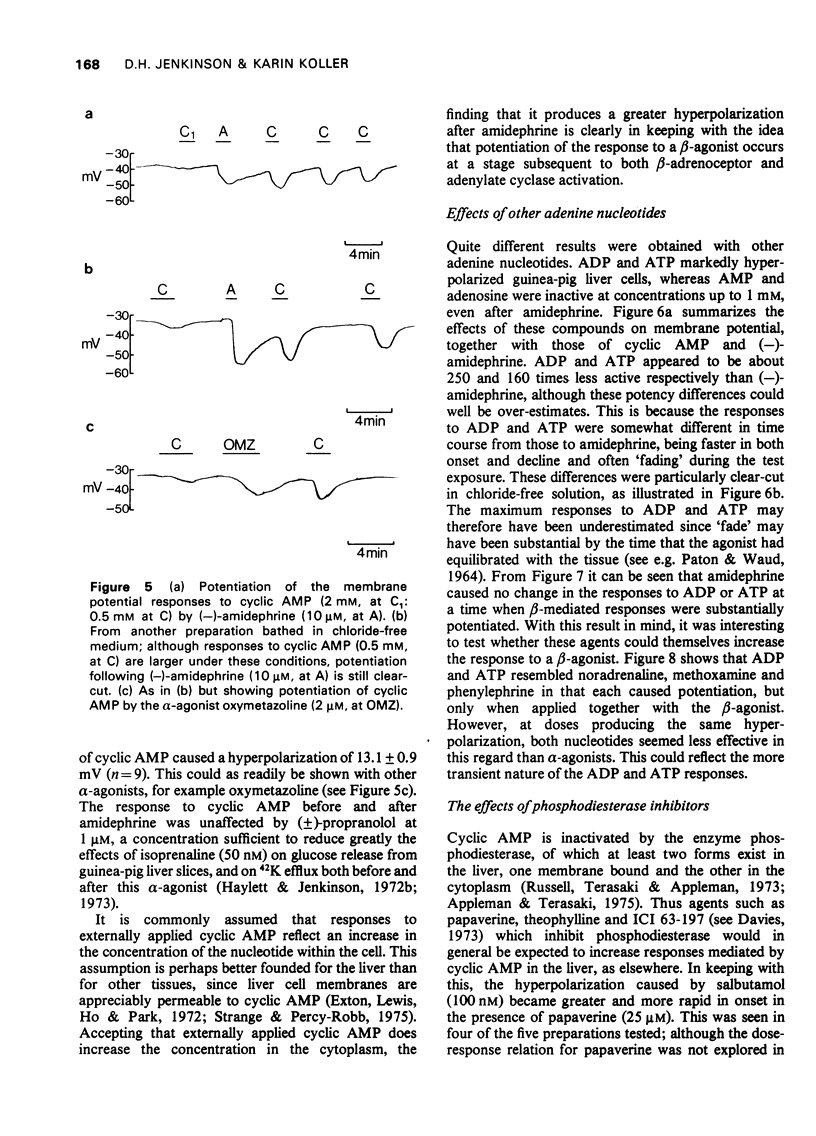
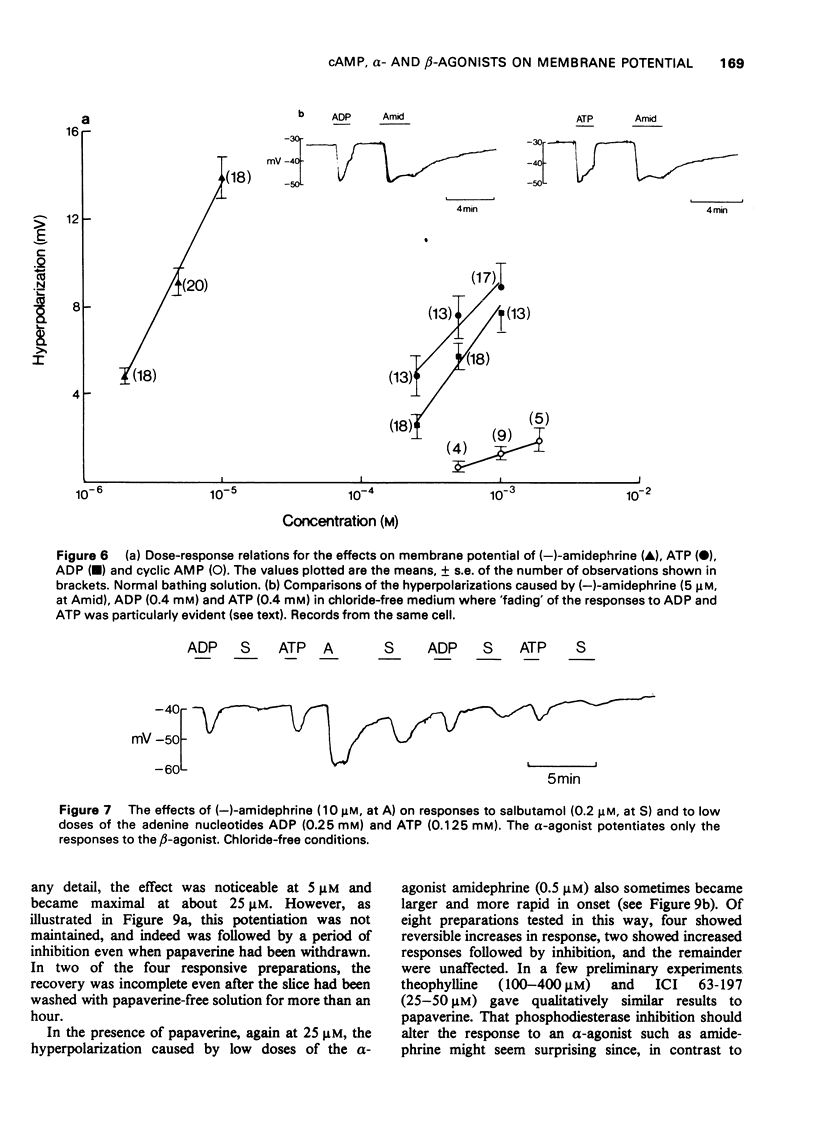
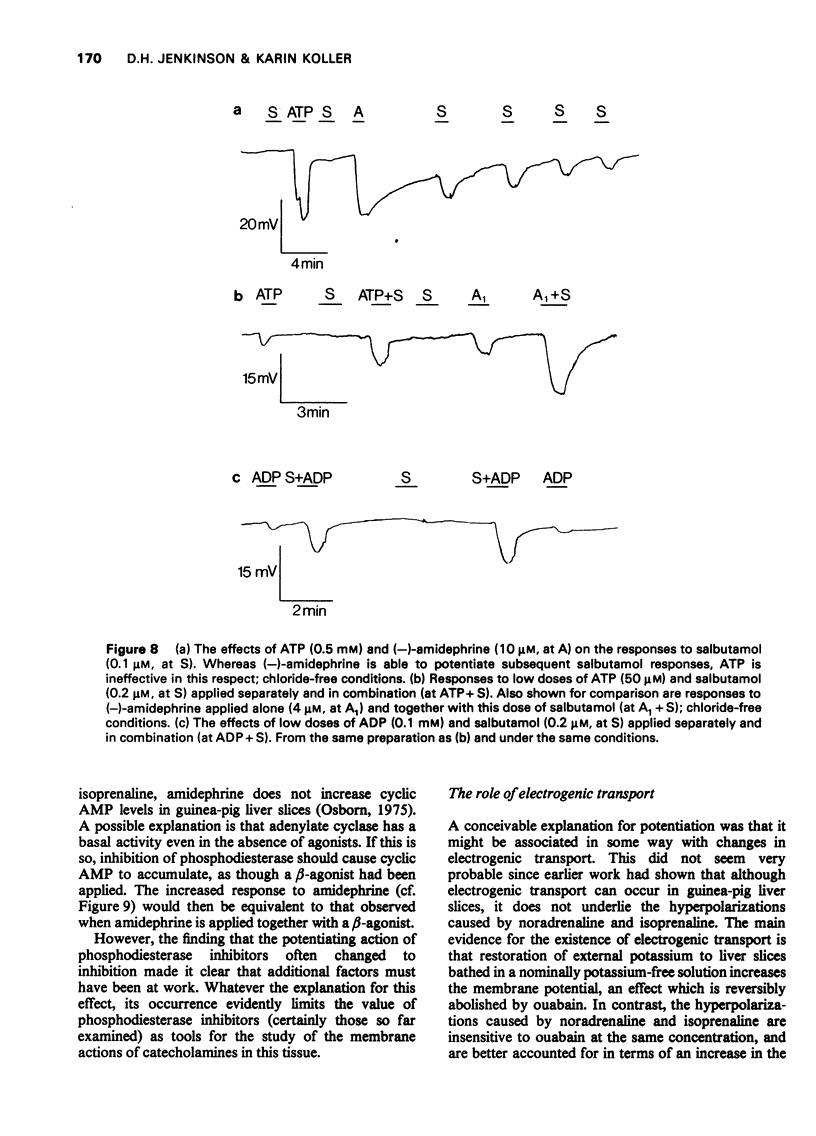
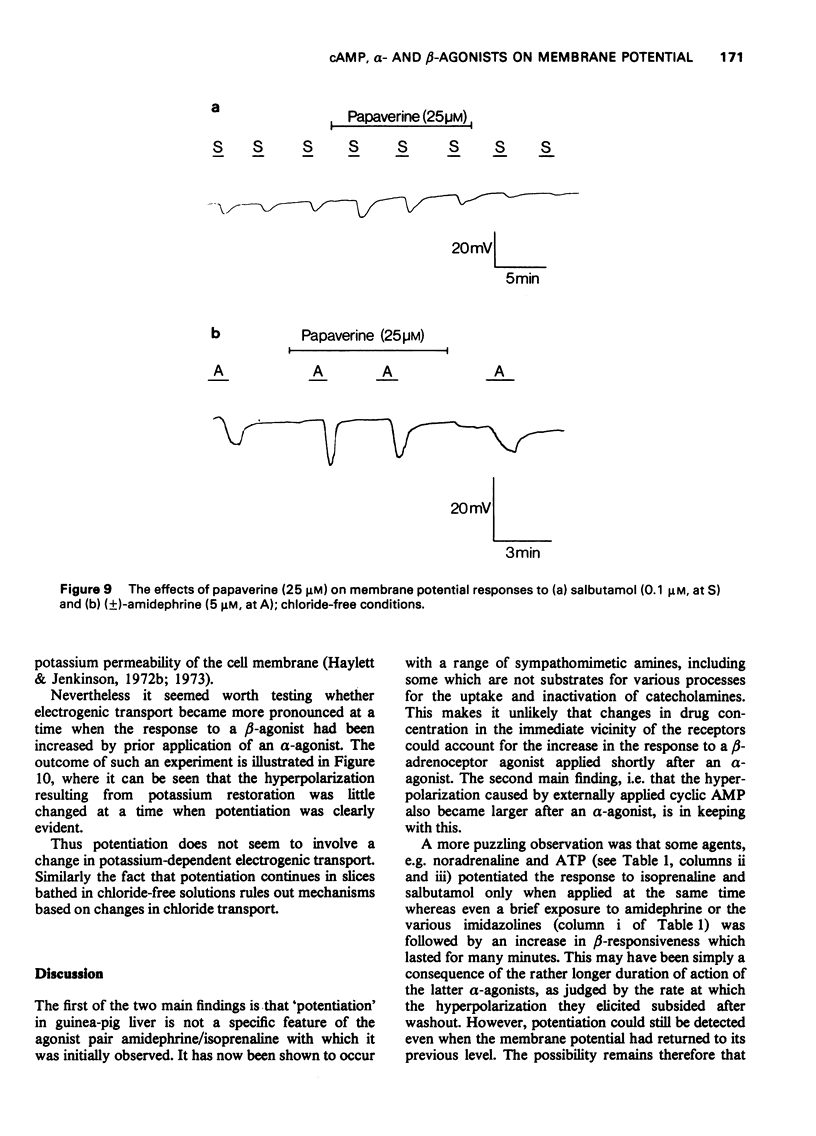
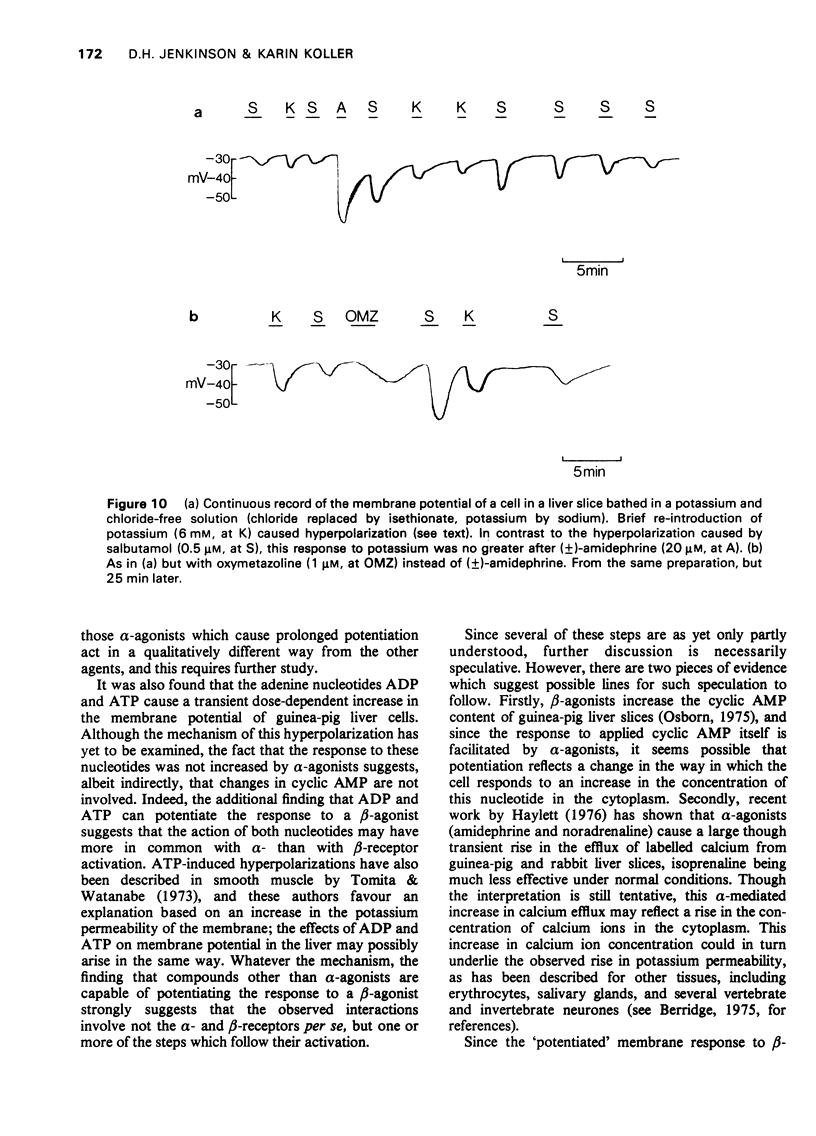
1 The beta-adrenoceptor agonist isoprenaline normally causes only a small and inconsistent increase in the membrane potential of cells in guinea-pig liver slices, in contrast to the large hyperpolarizations seen with alpha-agonists. However, after a selective alpha-adrenoceptor agonist has been applied, the response to isoprenaline becomes greatly enhanced. 2 Simultaneous application of small doses of an alpha- and beta-agonist produce hyperpolarizations larger than the sum of the responses to each agent alone. 3 These interactions occur with a range of sympathomimetic amines, including some which are not substrates for various processes for the uptake and inactivation of catecholamines. 4 Hyperpolarizations caused by externally applied cyclic adenosine-3',5'-monophosphate (cyclic AMP) also become larger after application of an alpha-agonist. 5 The adenine nucleotides adenosine 5'-diphosphate (ADP) and adenosine 5'-triphosphate (ATP) hyperpolarize guinea-pig liver cells in the dose range 0.1-1.0 mM. This response is not increased after an alpha-agonist. However, ADP and ATP are themselves able to enhance the response to beta-agonists. 6 These interactions between alpha-agonists, beta-agonists and adenine nucleotides seem to involve steps subsequent to receptor activation. Changes in the intracellular actions of cyclic AMP may be concerned.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleman M. M., Terasaki W. L. Regulation of cyclic nucleotide phosphodiesterase. Adv Cyclic Nucleotide Res. 1975;5:153–162. [PubMed] [Google Scholar]
- Berridge M. J. The interaction of cyclic nucleotides and calcium in the control of cellular activity. Adv Cyclic Nucleotide Res. 1975;6:1–98. [PubMed] [Google Scholar]
- Birmingham A. T., Szolcsányi J. Competitive blockade of adrenergic alpha-receptors and histamine receptors by thymoxamine. J Pharm Pharmacol. 1965 Jul;17(7):449–458. doi: 10.1111/j.2042-7158.1965.tb07701.x. [DOI] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Suppression of spontaneous spike generation by catecholamines in the smooth muscle of the guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):103–119. doi: 10.1098/rspb.1969.0014. [DOI] [PubMed] [Google Scholar]
- Castro-Tavares J. A comparison between the influence of pindolol and propranolol on the response of plasma potassium to catecholamines. Arzneimittelforschung. 1976 Feb;26(2):238–241. [PubMed] [Google Scholar]
- Castro-Tavares J. Effects of isoprenaline and phenylephrine on plasma potassium: role of the liver. Arch Int Pharmacodyn Ther. 1975 Nov;218(1):110–119. [PubMed] [Google Scholar]
- D'Silva J. L. Action of adrenaline on the serum potassium. J Physiol. 1937 Aug 17;90(3):303–309. doi: 10.1113/jphysiol.1937.sp003515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Silva J. L. The action of adrenaline on serum potassium. J Physiol. 1936 Feb 8;86(2):219–228. doi: 10.1113/jphysiol.1936.sp003356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G. E. Antibronchoconstrictor activity of two new phosphodiesterase inhibitors, a triazolopyrazine (ICI 58 301) and a triazolopyrimidine (ICI 63 197). J Pharm Pharmacol. 1973 Sep;25(9):681–689. doi: 10.1111/j.2042-7158.1973.tb10048.x. [DOI] [PubMed] [Google Scholar]
- ELLIS S., BECKETT S. B. MECHANISM OF THE POTASSIUM MOBILIZING ACTION OF EPINEPHRINE AND GLUCAGON. J Pharmacol Exp Ther. 1963 Dec;142:318–326. [PubMed] [Google Scholar]
- Exton J. H., Lewis S. B., Ho R. J., Park C. R. The role of cyclic AMP in the control of hepatic glucose production by glucagon and insulin. Adv Cyclic Nucleotide Res. 1972;1:91–101. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975 Aug;249(3):469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann N., Somlyo A. V., Somlyo A. P. Cyclic adenosine and guaosine monophosphates and gucagon: effect on liver membrane potentials. Science. 1971 Jan 29;171(3969):400–402. doi: 10.1126/science.171.3969.400. [DOI] [PubMed] [Google Scholar]
- Gardner J. D., Mensh R. S., Kiino D. R., Aurbach G. D. Effects of beta-adrenergic catecholamines on potassium transport in turkey erythrocytes. J Biol Chem. 1975 Feb 25;250(4):1155–1163. [PubMed] [Google Scholar]
- Grassi A. O., de Lew M. F., Cingolani H. E., Blesa E. S. Adrenergic beta blockade and changes in plasma potassium following epinephrine administration. Eur J Pharmacol. 1971 Jul;15(2):209–213. doi: 10.1016/0014-2999(71)90175-0. [DOI] [PubMed] [Google Scholar]
- Green R. D., Dale M. M., Haylett D. G. Effect of adrenergic amines on the membrane potential of guinea-pig liver parenchymal cells in short term tissue culture. Experientia. 1972 Sep 15;28(9):1073–1074. doi: 10.1007/BF01918681. [DOI] [PubMed] [Google Scholar]
- Haylett D. G. Effects of sympathomimetic amines on 45Ca efflux from liver slices. Br J Pharmacol. 1976 May;57(1):158–160. doi: 10.1111/j.1476-5381.1976.tb07668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haylett D. G., Jenkinson D. H. Effects of noradrenaline on potassium reflux, membrane potential and electrolyte levels in tissue slices prepared from guinea-pig liver. J Physiol. 1972 Sep;225(3):721–750. doi: 10.1113/jphysiol.1972.sp009966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haylett D. G., Jenkinson D. H. The receptors concerned in the actions of catecholamines on glucose release, membrane potential and ion movements in guinea-pig liver. J Physiol. 1972 Sep;225(3):751–772. doi: 10.1113/jphysiol.1972.sp009967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson D. H., Morton I. K. The role of alpha- and beta- adrenergic receptors in some actions of catecholamines on intestinal smooth muscle. J Physiol. 1967 Feb;188(3):387–402. doi: 10.1113/jphysiol.1967.sp008145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller K., Jenkinson D. H. Proceedings: An interaction between the alpha and beta actions of catecholamines in guinea-pig liver slices. Br J Pharmacol. 1976 Mar;56(3):362P–363P. [PMC free article] [PubMed] [Google Scholar]
- Kroeger E. A., Marshall J. M. Beta-adrenergic effects on rat myometrium: mechanisms of membrane hyperpolarization. Am J Physiol. 1973 Dec;225(6):1339–1345. doi: 10.1152/ajplegacy.1973.225.6.1339. [DOI] [PubMed] [Google Scholar]
- Mujić M., van Rossum J. M. Comparative pharmacodynamics of sympathomimetic imidazolines; studies on intestinal smooth muscle of the rabbit and the cardiovascular system of the cat. Arch Int Pharmacodyn Ther. 1965 Jun;155(2):432–449. [PubMed] [Google Scholar]
- Osborn D., Jenkinson D. H. Proceedings: Comparison of the effects of selective alpha and beta-receptor agonists on intracellular cyclic AMP levels and glycogen phosphorylase activity in guinea-pig liver. Br J Pharmacol. 1975 Oct;55(2):286P–287P. [PMC free article] [PubMed] [Google Scholar]
- PATON W. D., WAUD D. R. A QUANTITATIVE INVESTIGATION OF THE RELATIONSHIP BETWEEN RATE OF ACCESS OF A DRUG TO RECEPTOR AND THE RATE OF ONSET OR OFFSET OF ACTION. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1964 May 11;248:124–143. doi: 10.1007/BF00246668. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B. Calcium and cAMP as interrelated intracellular messengers. Ann N Y Acad Sci. 1975 Jun 30;253:789–796. doi: 10.1111/j.1749-6632.1975.tb19247.x. [DOI] [PubMed] [Google Scholar]
- Russell T. R., Terasaki W. L., Appleman M. M. Separate phosphodiesterases for the hydrolysis of cyclic adenosine 3',5'-monophosphate and cyclic guanosine 3',5'-monophosphate in rat liver. J Biol Chem. 1973 Feb 25;248(4):1334–1340. [PubMed] [Google Scholar]
- Sanders J., Miller D. D., Patil P. N. Alpha adrenergic and histaminergic effects of tolazoline-like imidazolines. J Pharmacol Exp Ther. 1975 Nov;195(2):362–371. [PubMed] [Google Scholar]
- Sheys E. M., Green R. D. A quantitative study of alpha adrenergic receptors in the spleen and aorta of the rabbit. J Pharmacol Exp Ther. 1972 Feb;180(2):317–325. [PubMed] [Google Scholar]
- Strange R. C., Robb I. W. Hepatic clearance of adenosine 3:5-cyclic monophosphate from plasma in the rat. Biochem J. 1975 Feb;146(2):509–512. doi: 10.1042/bj1460509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd E. P., Vick R. L. Kalemotropic effect of epinephrine: analysis with adrenergic agonists and antagonists. Am J Physiol. 1971 Jun;220(6):1964–1969. doi: 10.1152/ajplegacy.1971.220.6.1964. [DOI] [PubMed] [Google Scholar]
- Tomita T., Watanabe H. A comparison of the effects of adenosine triphosphate with noradrenaline and with the inhibitory potential of the guinea-pig taenia coli. J Physiol. 1973 May;231(1):167–177. doi: 10.1113/jphysiol.1973.sp010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick R. L., Todd E. P., Luedke D. W. Epinephrine-induced hypokalemia: relation to liver and skeletal muscle. J Pharmacol Exp Ther. 1972 Apr;181(1):139–146. [PubMed] [Google Scholar]


