Abstract
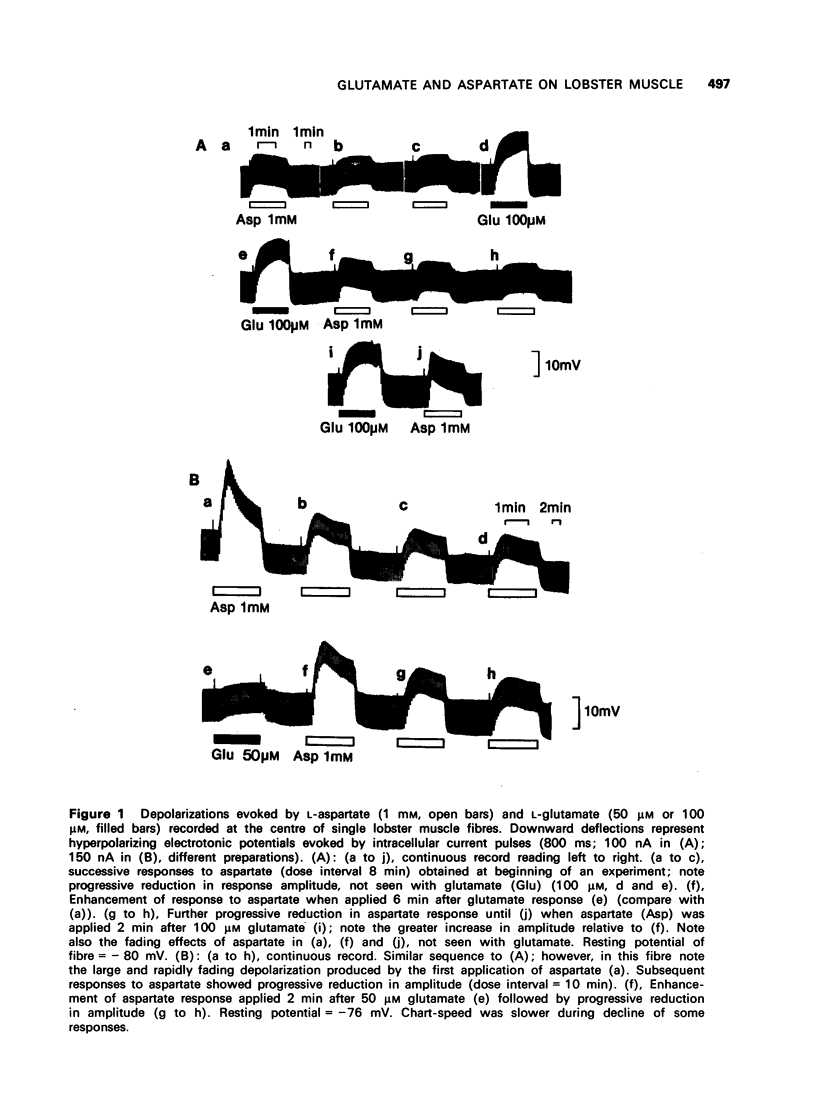
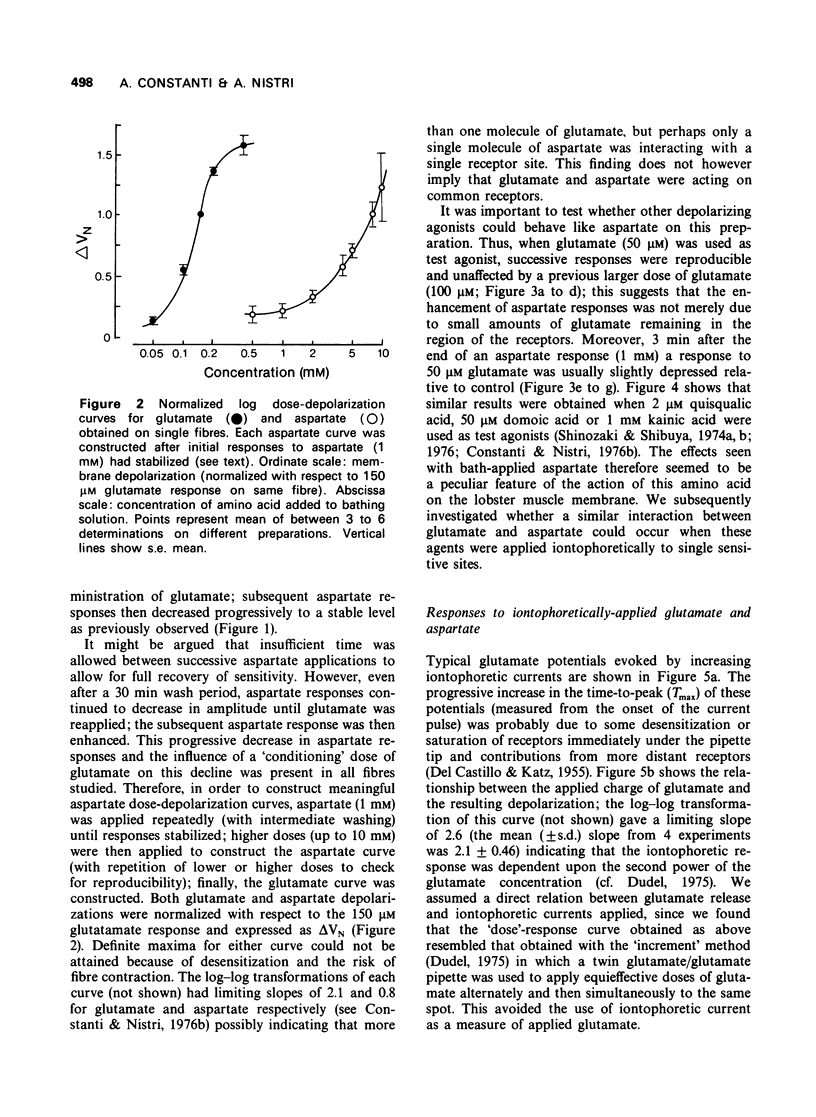
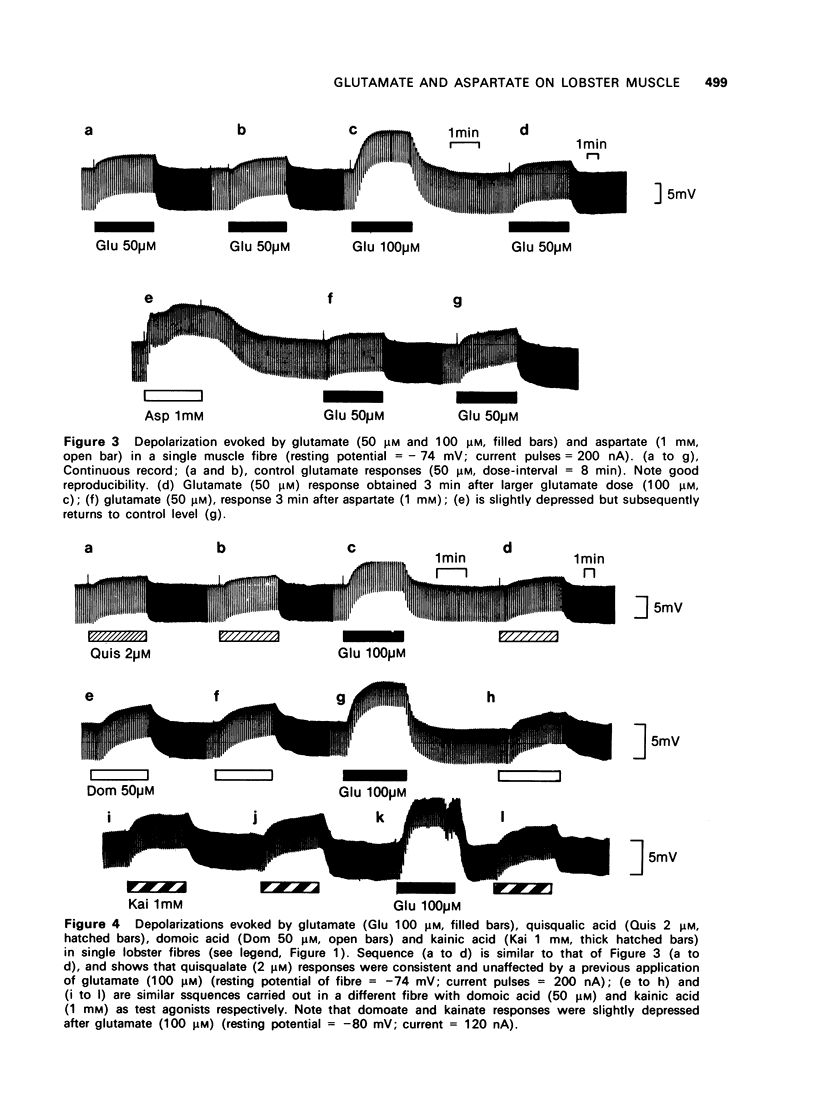
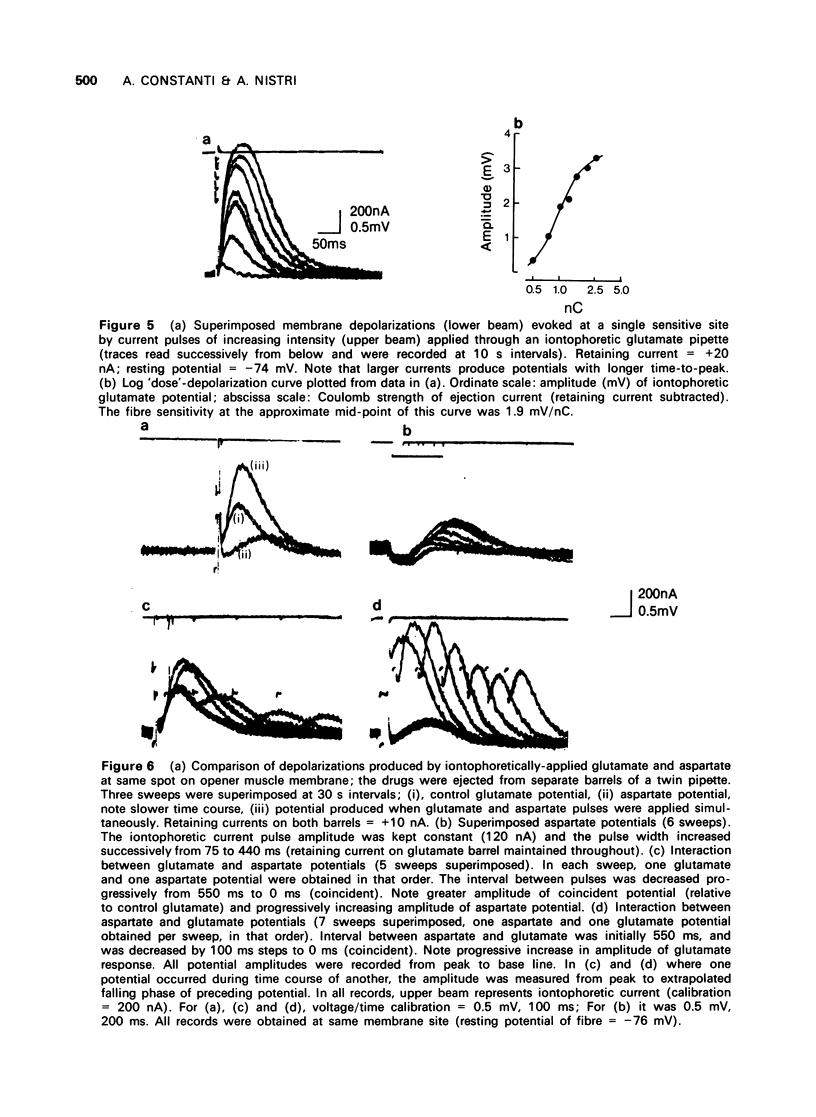
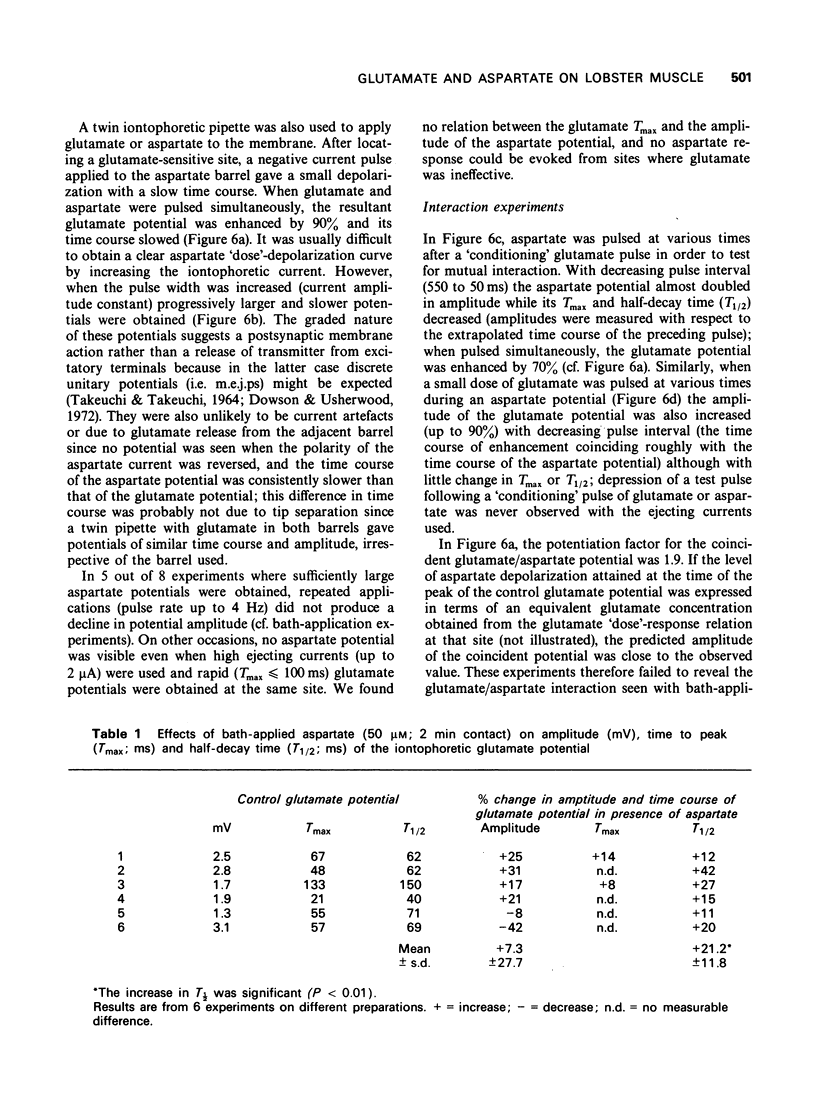
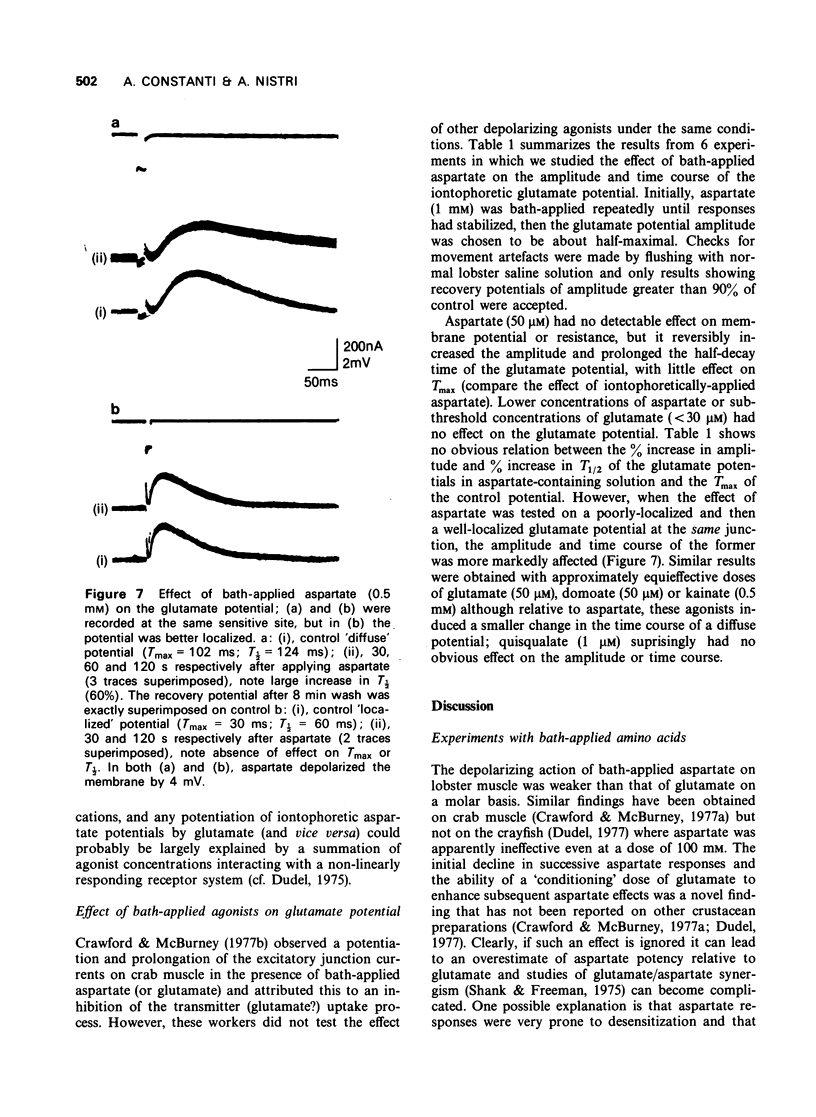
The depolarization produced by bath-applied or iontophoretically applied glutamate and aspartate were recorded from lobster muscle fibres by means of intracellular microelectrodes. 2 Bath-applied glutamate or aspartate evoked reversible, membrane depolarizations; however, responses to repeated applications of aspartate decreased progressively in amplitude until a plateau level was attained. Repeated applications of glutamate, kainate, domoate or quisqualate did not produce a similar effect. 3 After a dose of glutamate, responses to bath-applied aspartate were enhanced. Responses to other depolarizing agonists were little affected by previous administration of glutamate. Aspartate dose-depolarization curves were therefore constructed after initial aspartate responses had stabilized. The log-log transforms of the aspartate and glutamate curves had limiting slopes of 0.8 and 2.1 respectively. 4 Iontophoretic application of aspartate to single glutamate-sensitive sites produced small depolarizations with slow time course, compared with the glutamate potentials. When aspartate and glutamate were pulsed simultaneously from a twin-barrelled pipette, the resultant glutamate potential was enhanced. It is suggested that this potentiation was due to summation of agonist concentrations in the receptor region interacting with a second-order dose-response relationship. 5 Bath-applied aspartate increased the amplitude and prolonged the half-decay time of the glutamate potential. This effect was particularly noticeable when the glutamate potential was of slow time course. 6 It is proposed that bath-applied aspartate has an agonist effect whose magnitude is possibly exaggerated by concomitant release of glutamate and/or inhibition by glutamate of aspartate uptake. This agonist action of aspartate is thought to be exerted mainly on extrajunctional areas of the glutamate-sensitive sites.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balcar V. J., Borg J., Mandel P. High affinity uptake of L-glutamate and L-aspartate by glial cells. J Neurochem. 1977 Jan;28(1):87–93. doi: 10.1111/j.1471-4159.1977.tb07712.x. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Headley P. M., Lodge D., Martin M. R., Watkins J. C. The sensitivity of rat spinal interneurones and renshaw cells to L-glutamate and L-aspartate. Exp Brain Res. 1976 Dec 22;26(5):547–551. doi: 10.1007/BF00238827. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Brown D. A., Collins G. G., Galvan M., Marsh S., Yamini G. Indirect effects of amino-acids on sympathetic ganglion cells mediated through the release of gamma-aminobutyric acid from glial cells. Br J Pharmacol. 1976 May;57(1):73–91. doi: 10.1111/j.1476-5381.1976.tb07658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A., Nistri A. A comparative study of the action of gamma-aminobutyric acid and piperazine on the lobster muscle fibre and the frog spinal cord. Br J Pharmacol. 1976 Jul;57(3):347–358. doi: 10.1111/j.1476-5381.1976.tb07673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A., Nistri A. A comparative study of the effects of glutamate and kainate on the lobster muscle fibre and the frog spinal cord. Br J Pharmacol. 1976 Jul;57(3):359–368. doi: 10.1111/j.1476-5381.1976.tb07674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constati A. A quantitative study of the gamma-aminobutyric acid (GABA) dose/conductance relationship at the lobster inhibitory neuromuscular junction. Neuropharmacology. 1977 May;16(5):357–366. doi: 10.1016/0028-3908(77)90073-9. [DOI] [PubMed] [Google Scholar]
- Crawford A. C., McBurney R. N. The synergistic action of L-glutamate and L-aspartate at crustacean excitatory neuromuscular junctions. J Physiol. 1977 Jul;268(3):697–709. doi: 10.1113/jphysiol.1977.sp011877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., McBurney R. N. The termination of transmitter action at the crustacean excitatory neuromuscular junction. J Physiol. 1977 Jul;268(3):711–729. doi: 10.1113/jphysiol.1977.sp011878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A., Teb ecis A. K., Watkins J. C. Excitation of mammalian central neurones by acidic amino acids. Brain Res. 1972 Jun 22;41(2):283–301. doi: 10.1016/0006-8993(72)90503-3. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. On the localization of acetylcholine receptors. J Physiol. 1955 Apr 28;128(1):157–181. doi: 10.1113/jphysiol.1955.sp005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoud A., Miller R. Release of glutamate and other amino acids from arthropod nerve-muscle preparations. J Neurochem. 1976 Jan;26(1):119–123. [PubMed] [Google Scholar]
- Dowson R. J., Usherwood P. N. The effect of low concentrations of L-glutamate and L-aspartate on transmitter release at the locust excitatory nerve-muscle synapse. J Physiol. 1973 Feb;229(1):13P–14P. [PubMed] [Google Scholar]
- Dudel J. Aspartate and other inhibitors of excitatory synaptic transmission in crayfish muscle. Pflugers Arch. 1977 May 6;369(1):7–16. doi: 10.1007/BF00580803. [DOI] [PubMed] [Google Scholar]
- Dudel J. Potentiation and desensitization after glutamate induced postsynaptic currents at the crayfish neuromuscular junction. Pflugers Arch. 1975;356(4):317–327. doi: 10.1007/BF00580005. [DOI] [PubMed] [Google Scholar]
- Duggan A. W. The differential sensitivity to L-glutamate and L-aspartate of spinal interneurones and Renshaw cells. Exp Brain Res. 1974 Mar 29;19(5):522–528. doi: 10.1007/BF00236115. [DOI] [PubMed] [Google Scholar]
- Evans P. D. Amino acid distribution in the nervous system of the crab, Carcinus maenas (L.). J Neurochem. 1973 Jul;21(1):11–17. doi: 10.1111/j.1471-4159.1973.tb04221.x. [DOI] [PubMed] [Google Scholar]
- Freeman A. R. Polyfunctional role of glutamic acid in excitatory synaptic transmission. Prog Neurobiol. 1976;3(2):137–153. doi: 10.1016/0301-0082(76)90012-5. [DOI] [PubMed] [Google Scholar]
- Gent J. P., Morgan R., Wolstencroft J. H. Determination of the relative potency of two excitant amino acids. Neuropharmacology. 1974 Jun;13(6):441–447. doi: 10.1016/0028-3908(74)90132-4. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol Rev. 1973 Jan;53(1):1–119. doi: 10.1152/physrev.1973.53.1.1. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Kravitz E. A. The metabolism of gamma-aminobutyric acid (GABA) in the lobster nervous system--uptake of GABA in the nerve-muscle preparations. J Neurochem. 1968 Jul;15(7):609–620. doi: 10.1111/j.1471-4159.1968.tb08960.x. [DOI] [PubMed] [Google Scholar]
- Kerkut G. A., Wheal H. V. Proceedings: The excitatory effects of aspartate and glutamate on the crustacean neuromuscular junction. Br J Pharmacol. 1974 May;51(1):136P–137P. [PMC free article] [PubMed] [Google Scholar]
- Roberts P. J., Watkins J. C. Structural requirements for the inhibition for L-glutamate uptake by glia and nerve endings. Brain Res. 1975 Feb 21;85(1):120–125. doi: 10.1016/0006-8993(75)91016-1. [DOI] [PubMed] [Google Scholar]
- Shank R. P., Freeman A. R. Cooperative interaction of glutamate and aspartate with receptors in the neuromuscular excitatory membrane in walking limbs of the lobster. J Neurobiol. 1975 May;6(3):289–303. doi: 10.1002/neu.480060305. [DOI] [PubMed] [Google Scholar]
- Shank R. P., Freeman A. R., McBride W. J., Aprison M. H. Glutamate and aspartate as mediators of neuromuscular excitation in the lobster. Comp Biochem Physiol C. 1975 Jan 1;50(1):127–131. [PubMed] [Google Scholar]
- Shank R. P., Wang M. B., Freeman A. R. Action of aspartate at lobster excitatory neuromuscular junctions. Brain Res. 1977 Apr 22;126(1):176–180. doi: 10.1016/0006-8993(77)90226-8. [DOI] [PubMed] [Google Scholar]
- Shinozaki H., Shibuya I. A new potent excitant, quisqualic acid: effects on crayfish neuromuscular junction. Neuropharmacology. 1974 Jul;13(7):665–672. doi: 10.1016/0028-3908(74)90056-2. [DOI] [PubMed] [Google Scholar]
- Shinozaki H., Shibuya I. Effects of kainic acid analogues on crayfish opener muscle. Neuropharmacology. 1976 Feb;15(2):145–147. doi: 10.1016/0028-3908(76)90052-6. [DOI] [PubMed] [Google Scholar]
- Shinozaki H., Shibuya I. Potentiation of glutamate-induced depolarization by kainic acid in the crayfish opener muscle. Neuropharmacology. 1974 Nov;13(10-11):1057–1065. doi: 10.1016/0028-3908(74)90096-3. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. THE EFFECT ON CRAYFISH MUSCLE OF IONTOPHORETICALLY APPLIED GLUTAMATE. J Physiol. 1964 Mar;170:296–317. doi: 10.1113/jphysiol.1964.sp007332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Onodera K. Effects of kainic acid on the glutamate receptors of the crayfish muscle. Neuropharmacology. 1975 Sep;14(9):619–625. doi: 10.1016/0028-3908(75)90084-2. [DOI] [PubMed] [Google Scholar]


