Abstract
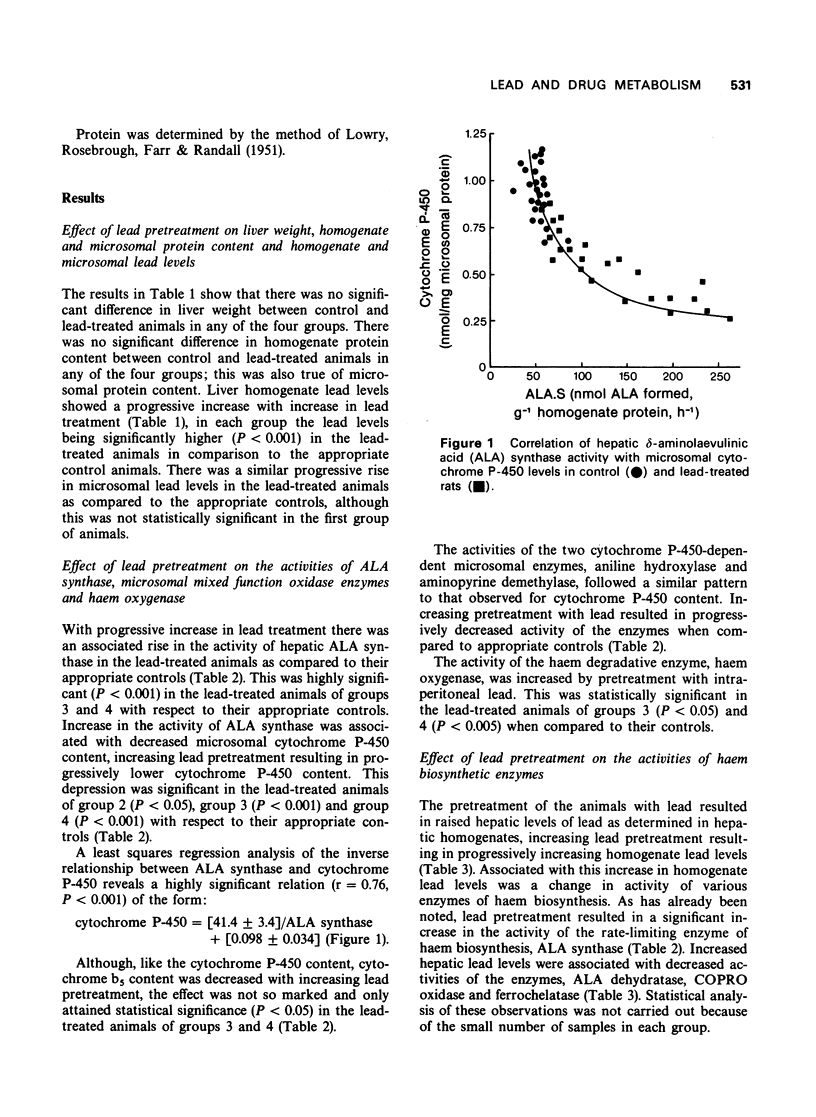
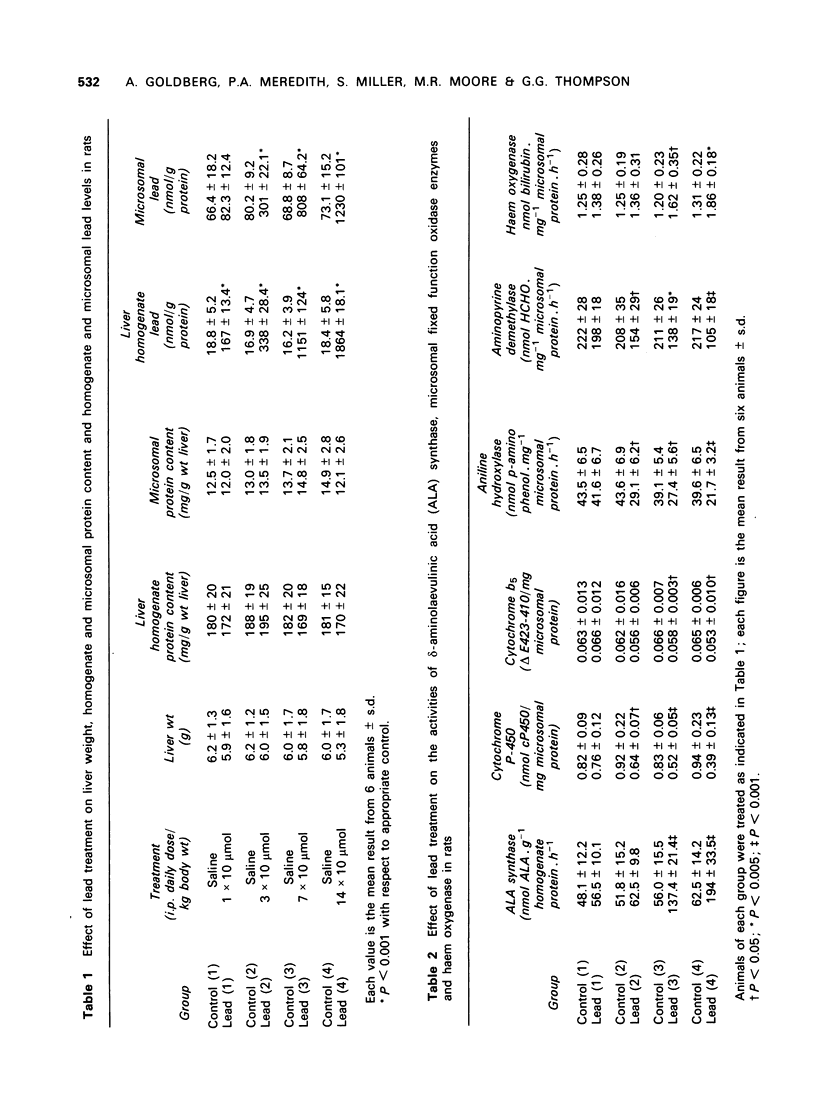
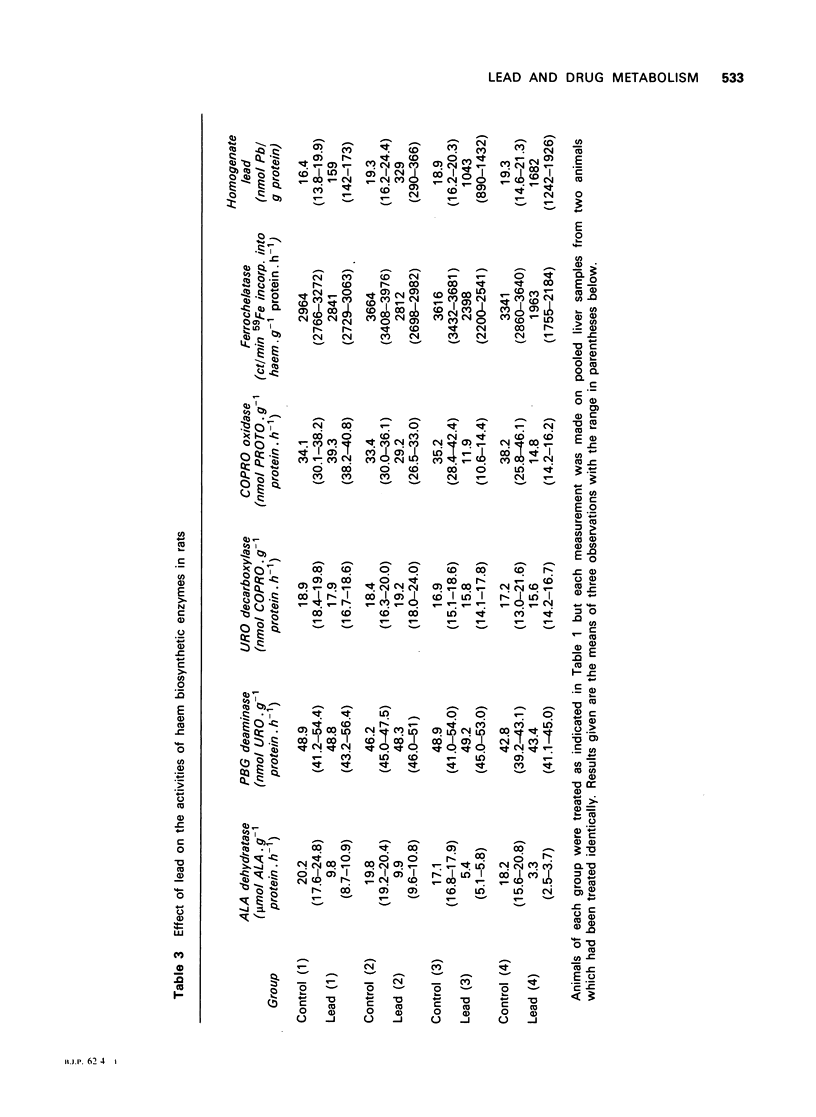
1 Pretreatment of rats with intraperitoneal injections of lead was shown to result in a depression of the microsomal mixed function oxidase system, as assessed by a decrease in hepatic microsomal P-450 and b5 content and by a decrease in the activity of the enzymes aniline hydroxylase and aminopyrine demethylase. Lead had a more marked effect on cytochrome P-450 than b5. 2 The activity of the rate-limiting enzyme of haem biosynthesis, delta-aminolaevulinic acid synthase, was inversely correlated with the microsomal cytochrome P-450 content. 3 The activity of the haem biosynthetic enzymes delta-aminolaevulinic acid dehydratase, coproporphyrinogen oxidase and ferrochelatase were decreased by increasing lead pretreatment. 4 The activity of the haem catabolic enzyme, haem oxygenase, was increased by lead pretreatment.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvares A. P., Kapelner S., Sassa S., Kappas A. Drug metabolism in normal children, lead-poisoned children, and normal adults. Clin Pharmacol Ther. 1975 Feb;17(2):179–183. doi: 10.1002/cpt1975172179. [DOI] [PubMed] [Google Scholar]
- Alvares A. P., Leigh S., Cohn J., Kappas A. Lead and methyl mercury: effects of acute exposure on cytochrome P-450 and the mixed function oxidase system in the liver. J Exp Med. 1972 Jun 1;135(6):1406–1409. doi: 10.1084/jem.135.6.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell D. M., Hammaker L. E. Cytochrome p-450 heme and the regulation of delta-aminolevulinic acid synthetase in the liver. Arch Biochem Biophys. 1976 Sep;176(1):103–112. doi: 10.1016/0003-9861(76)90145-4. [DOI] [PubMed] [Google Scholar]
- Brodie M. J., Thompson G. G., Moore M. R., Beattie A. D., Goldberg A. Hereditary coproporphyria. Demonstration of the abnormalities in haem biosynthesis in peripheral blood. Q J Med. 1977 Apr;46(182):229–241. [PubMed] [Google Scholar]
- COCHIN J., AXELROD J. Biochemical and pharmacological changes in the rat following chronic administration of morphine nalorphine and normorphine. J Pharmacol Exp Ther. 1959 Feb;125(2):105–110. [PubMed] [Google Scholar]
- Campbell B. C., Brodie M. J., Thompson G. G., Meredith P. A., Moore M. R., Goldberg A. Alterations in the activity of enzymes of haem biosynthesis in lead poisoning and acute hepatic prophyria. Clin Sci Mol Med. 1977 Oct;53(4):335–340. doi: 10.1042/cs0530335. [DOI] [PubMed] [Google Scholar]
- De Matteis F. Loss of haem in rat liver caused by the porphyrogenic agent 2-allyl-2-isopropylacetamide. Biochem J. 1971 Oct;124(4):767–777. doi: 10.1042/bj1240767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- Hayashi N., Kurashima Y., Kikuchi G. Mechanism of allylisopropylacetamide-induced increase of -aminolevulinate synthetase in liver mitochondria. V. Mechanism of regulation by hemin of the level of -aminolevulinate synthetase in rat liver mitochondria. Arch Biochem Biophys. 1972 Jan;148(1):10–21. doi: 10.1016/0003-9861(72)90109-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Cobalt stimulation of heme degradation in the liver. Dissociation of microsomal oxidation of heme from cytochrome P-450. J Biol Chem. 1975 Jun 10;250(11):4171–4177. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. The induction of heme oxidation in various tissues by trace metals: evidence for the catabolism of endogenous heme by hepatic heme oxygenase. Ann Clin Res. 1976;8 (Suppl 17):39–46. [PubMed] [Google Scholar]
- Marver H. S., Schmid R., Schützel H. Heme and methemoglobin: naturally occurring repressors of microsomal cytochrome. Biochem Biophys Res Commun. 1968 Dec 30;33(6):969–974. doi: 10.1016/0006-291x(68)90408-7. [DOI] [PubMed] [Google Scholar]
- Maxwell J. D., Meyer U. A. Effect of lead on hepatic delta-aminolaevulinic acid synthetase activity in the rat: a model for drug sensitivity in intermittent acute porphyria. Eur J Clin Invest. 1976 Sep 10;6(5):373–379. doi: 10.1111/j.1365-2362.1976.tb00531.x. [DOI] [PubMed] [Google Scholar]
- Meredith P. A., Campbell B. C., Moore M. R., Goldberg A. The effects of industrial lead poisoning on cytochrome P450 mediated phenazone (antipyrine) hydroxylation. Eur J Clin Pharmacol. 1977 Nov 14;12(3):235–239. doi: 10.1007/BF00609867. [DOI] [PubMed] [Google Scholar]
- Meredith P. A., Moore M. R., Goldberg A. Effects of aluminium, lead and zinc on delta-aminolaevulinic acid dehydratase. Enzyme. 1977;22(1):22–27. doi: 10.1159/000458503. [DOI] [PubMed] [Google Scholar]
- Meyer U. A., Schmid R. Hereditary hepatic porphyrias. Fed Proc. 1973 Jun;32(6):1649–1655. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Sassa S., Granick S. Induction of -aminolevulinic acid synthetase in chick embryo liver cells in cluture. Proc Natl Acad Sci U S A. 1970 Oct;67(2):517–522. doi: 10.1073/pnas.67.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoppa P., Roumengous M., Penning W. Hepatic drug metabolizing activity in lead-poisoned rats. Experientia. 1973 Aug 15;29(8):970–972. doi: 10.1007/BF01930408. [DOI] [PubMed] [Google Scholar]
- Sholnick P. L., Hammaker L. E., Marver H. S. Soluble hepatic delta-aminolevulinic acid synthetase: end-product inhibition of the partially purified enzyme. Proc Natl Acad Sci U S A. 1969 May;63(1):65–70. doi: 10.1073/pnas.63.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand L. J., Manning J., Marver H. S. The induction of -aminolevulinic acid synthetase in cultured liver cells. The effects of end product and inhibitors of heme synthesis. J Biol Chem. 1972 May 10;247(9):2820–2827. [PubMed] [Google Scholar]
- Tschudy D. P., Bonkowsky H. L. Experimental porphyria. Fed Proc. 1972 Jan-Feb;31(1):147–159. [PubMed] [Google Scholar]
- Watson C. J. Editorial: Hematin and porphyria. N Engl J Med. 1975 Sep 18;293(12):605–607. doi: 10.1056/NEJM197509182931210. [DOI] [PubMed] [Google Scholar]


