Abstract
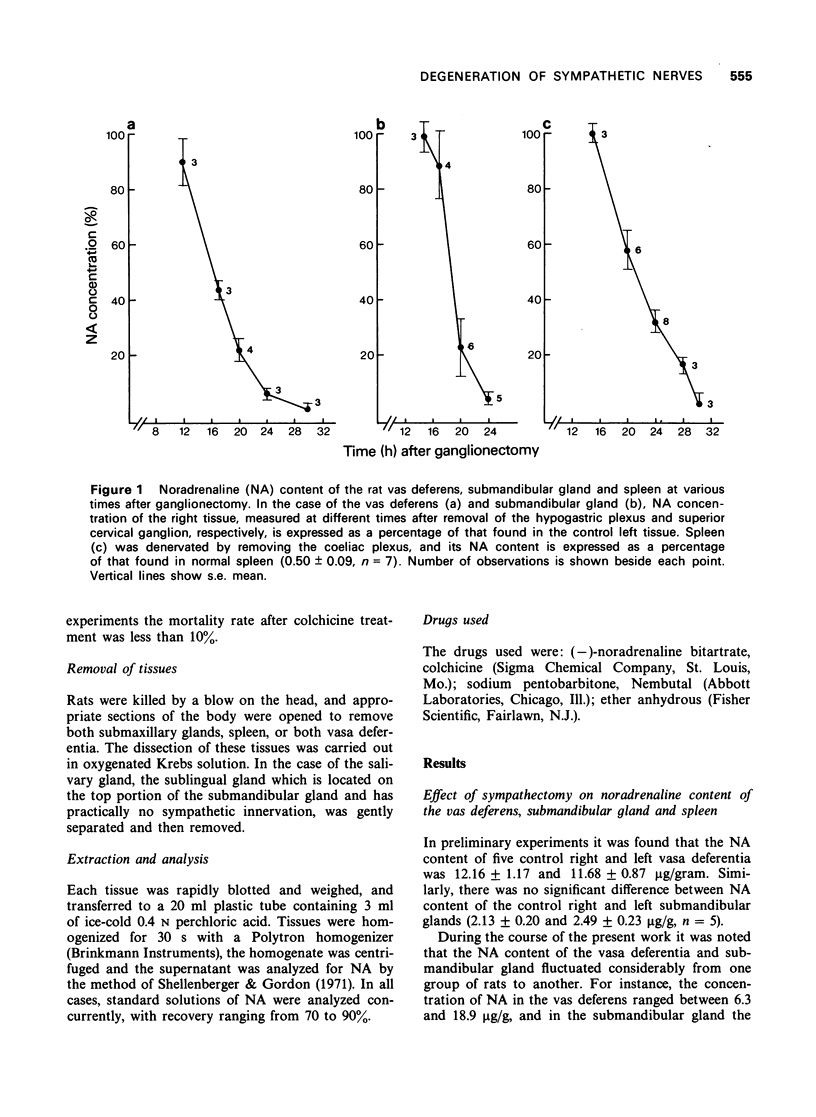
1 The time-course of degeneration of sympathetic nerves was investigated by measurement of the endogenous noradrenaline content of the rat vas deferens, submandibular gland and spleen following sympathectomy.
2 Extirpation of the hypogastric plexus, superior cervical ganglion and coeliac plexus under pentobarbitone anaesthesia caused 50% depletion of the noradrenaline content of the vas deferens, submandibular gland and spleen in approximately 16, 19 and 21 h, respectively.
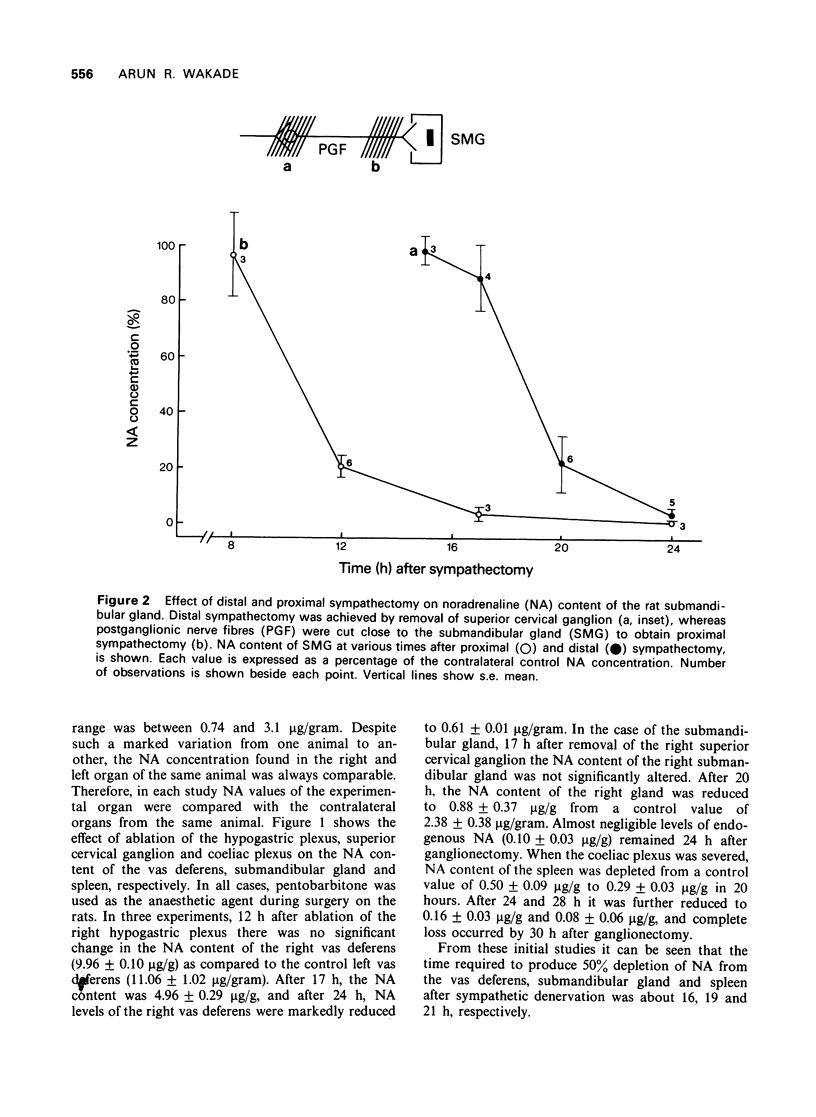
3 Under pentobarbitone anaesthesia, proximal sympathectomy (i.e., close to the end organ) produced depletion of the noradrenaline content of the submandibular gland 8 h earlier than that caused by distal sympathectomy. Under ether anaesthesia, the time difference in obtaining the same degree of depletion after the two procedures of sympathectomy was only 2 hours.
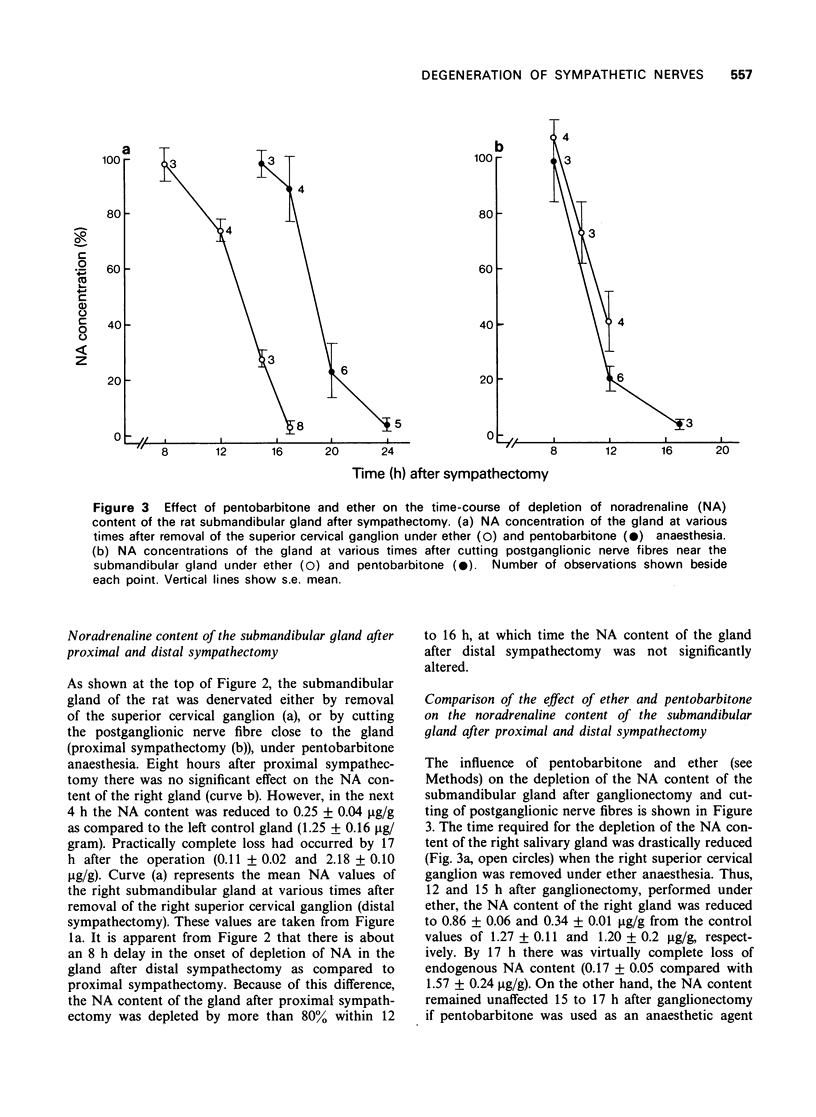
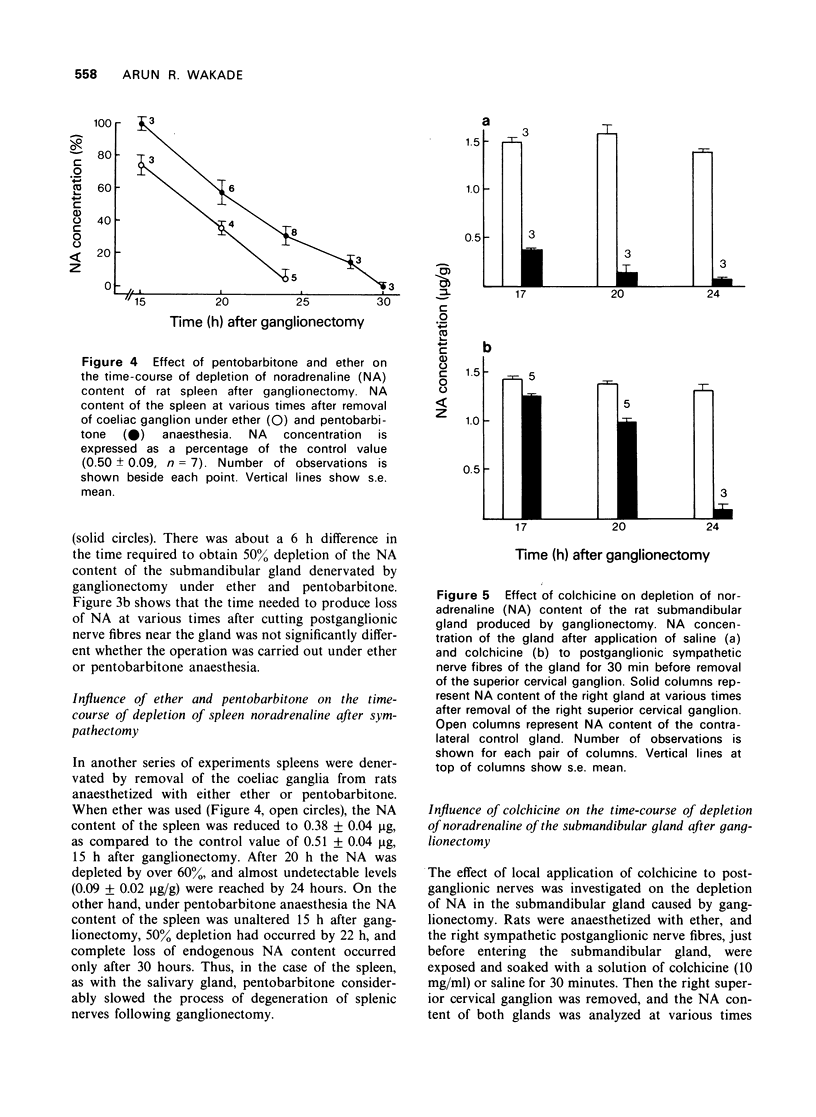
4 Removal of the superior cervical ganglion under ether anaesthesia resulted in almost complete depletion of noradrenaline content of the submandibular gland in 17 h, whereas when a similar operation was performed under pentobarbitone anaesthesia, nearly 24 h were required for the same degree of depletion. Similarly, the noradrenaline content of the spleen was depleted 4 h earlier if the coeliac plexus was ablated under ether as compared to pentobarbitone anaesthesia.
5 Local application of colchicine (10 mg/ml, 30 min) to postganglionic sympathetic nerve axons had no effect on the noradrenaline content of the submandibular gland up to 24 hours. However, removal of the superior ganglion following colchicine application considerably slowed the depletion of the noradrenaline content of the submandibular gland (at 17 and 20 h after ganglionectomy, 10 and 20% depletion, respectively, in the experimental gland, as compared to 70 and 80%, respectively, in the control gland).
6 To explain the results, it is proposed that injury to the sympathetic nerves at the site of sectioning triggers a signal (messenger substance) which travels down to the nerve endings to produce degeneration. Thus, the length of the extrinsic nerve fibre influences the time course of degeneration by changing the rate of transport of the messenger substance, whereas pentobarbitone and colchicine alter the synthesis and/or transport of the messenger substance to modify the time-course of degeneration.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almgren O., Dahlström A., Häggendal J. Degeneration secretion and noradrenaline disappearance in rat salivary glands following proximal or distal axotomy. Acta Physiol Scand. 1976 Dec;98(4):457–464. doi: 10.1111/j.1748-1716.1976.tb10336.x. [DOI] [PubMed] [Google Scholar]
- Bareggi S., Dahlström A., Häggendal J. Intra-axonal transport and degeneration of adrenergic nerve terminals after axotomy with long and short nerve stump. Med Biol. 1974 Oct;52(5):327–335. [PubMed] [Google Scholar]
- Birmingham A. T. Sympathetic denervation of the smooth muscle of the vas deferens. J Physiol. 1970 Mar;206(3):645–661. doi: 10.1113/jphysiol.1970.sp009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERTTING G., AXELROD J., KOPIN I. J., WHITBY L. G. Lack of uptake of catecholamines after chronic denervation of sympathetic nerves. Nature. 1961 Jan 7;189:66–66. doi: 10.1038/189066a0. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Thesleff S. Nerve stump length and membrane changes in denervated skeletal muscle. Nat New Biol. 1972 Mar 15;236(63):60–61. doi: 10.1038/newbio236060a0. [DOI] [PubMed] [Google Scholar]
- KIRPEKAR S. M., CERVONI P., FURCHGOTT R. F. Catecholamine content of the cat nicitating membrane following procedures sensitizing it to norepinephrine. J Pharmacol Exp Ther. 1962 Feb;135:180–190. [PubMed] [Google Scholar]
- Kirpekar S. M., Wakade A. R., Prat J. C. Regeneration of sympathetic nerves to the vas deferens and spleen of the cat. J Pharmacol Exp Ther. 1970 Oct;175(1):197–205. [PubMed] [Google Scholar]
- Lundberg D. Effects of colchicine, vinblastine and vincristine on degeneration transmitter release after sympathetic denervation studied in the conscious rat. Acta Physiol Scand. 1972 May;85(1):91–98. doi: 10.1111/j.1748-1716.1972.tb05238.x. [DOI] [PubMed] [Google Scholar]
- Shellenberger M. K., Gordon J. H. A rapid, simplified procedure for simultaneous assay of norepinephrine, dopamine, and 5-hydroxytryptamine from discrete brain areas. Anal Biochem. 1971 Feb;39(2):356–372. doi: 10.1016/0003-2697(71)90426-x. [DOI] [PubMed] [Google Scholar]
- VON EULER U. S., PURKHOLD A. Effect of sympathetic denervation on the noradrenaline and adrenaline content of the spleen, kidney, and salivary glands in the sheep. Acta Physiol Scand. 1951;24(2-3):212–217. doi: 10.1111/j.1748-1716.1951.tb00839.x. [DOI] [PubMed] [Google Scholar]
- Van Orden L. S., 3rd, Bensch K. G., Langer S. Z., Trendelenburg U. Histochemical and fine structural aspects of the onset of denervation supersensitivity in the nictitating membrane of the spinal cat. J Pharmacol Exp Ther. 1967 Aug;157(2):274–283. [PubMed] [Google Scholar]
- Wakade A. R., Kirpekar S. M. Chemical and histochemical studies on the sympathetic innervation of the vas deferens and seminal vesicle of the guinea pig. J Pharmacol Exp Ther. 1971 Sep;178(3):432–441. [PubMed] [Google Scholar]
- Watanabe M. Eine Modifikation des isolierten N.hypogastricus-Vas deferens-Präparates vom Meerschweinchen für die Prüfung von ganglionären Wirkungen. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1969;262(2):221–227. [PubMed] [Google Scholar]


