Abstract
Different lengths of the promoter of grape (Vitis vinifera) VvHT1 (Hexose Transporter 1) gene, which encodes a putative hexose transporter expressed during the ripening of grape, have been transcriptionally fused to the β-glucuronidase reporter gene. In transgenic tobacco (Nicotiana tabacum) transformed with these constructs, VvHT1 promoters were clearly responsible for the sink organ preferential expression. The potential sugar effectors of VvHT1 promoter were studied in tobacco cv Bright-Yellow 2 cells transformed with chimeric constructs. Glucose (56 mm), sucrose (Suc; 58 mm), and the non-transported Suc isomer palatinose doubled the β-glucuronidase activity conferred by the VvHT1 promoter, whereas fructose did not affect it. These effects were the strongest with the 2.4-kb promoter, which contains all putative sugar-responsive elements (activating and repressing), but they were also significant with the 0.3-kb promoter, which contains only activating sugar boxes. The induction of VvHT1 expression by both Suc and palatinose was confirmed in the homologous grape berry cell culture. The data provide the first example of a putative sugar transporter, which is induced by both glucose and Suc in higher plants. Although induction of VvHT1 expression by Suc does not require transport, the presence of glucosyl moiety is necessary for Suc sensing. These results provide new insights into sugar sensing and signaling in plants.
After the identification of the first eukaryotic sugar transporter in Chlorella kessleri (Sauer and Tanner, 1989), many monosaccharides transporters have been cloned from a variety of species (for review, see Büttner and Sauer, 2000). From a functional point of view, these transporters may vary in substrate specificity and in their affinity for monosaccharides. Thus, in C. kessleri, CkHUP1 and CkHUP2 are high-affinity transporters for Glc and Gal, respectively (Stadler et al., 1995). In Arabidopsis, AtSTP1 and AtSTP3 display high or low affinity for Glc, respectively (Sauer et al., 1990; Büttner and Sauer, 2000). In addition to these functional differences, there are marked differences in the pattern of expression. Some of these transporters are expressed in various organs of the plant, whereas others are only expressed in specific cell types. For example, AtSTP1 transcripts are present in leaves, stems, flowers, and roots (Sauer et al., 1990), whereas AtSTP2 is expressed in developing pollen (Truernit et al., 1999). Likewise, the PMt1 monosaccharide transporter from Petunia hybrida is expressed only in the male gametophyte (Ylstra et al., 1998). The expression also differs in sensitivity to environmental factors, such as pathogen attack or sugar concentration. AtSTP4 is strongly induced by wounding and bacterial and fungal elicitors (Truernit et al., 1996).
The data dealing with sugar control of gene expression of monosaccharide transporters, and also Suc transporters, are scarce and sometimes contradictory. In C. kessleri, the HUP1 hexose transporter and the HUP2 Gal transporter are co-induced when Glc is added to the medium (Stadler et al., 1995). The Suc transporter from sugar beet (Beta vulgaris; Chiou and Bush, 1998) is repressed by Suc, whereas a companion cell-specific Suc transporter of rice (Oryza sativa; Matsukura et al., 2000) is up-regulated by its own substrate. Nevertheless, Suc is able to induce genes implicated in sink-source regulation, such as the gene coding an apoplastic invertase of Chenopodium rubrum (Roitsch et al., 1995) or other cotransport related genes, such as two ATPase isotypes in tomato (Lycopersicon esculentum; Mito et al., 1996).
In suspension-cultured cells of C. rubrum, the expression of several homologs of monosaccharide transporters does not depend on the presence of Glc (Roitsch and Tanner, 1994). The VfSUT1 Suc transporter, which is normally expressed during seed development in broad bean (Vicia faba), is strongly repressed by high concentrations (150 mm) of either Suc or Glc (Weber et al., 1997). Infiltration of sugar beet leaves with Suc decreases the amounts of Suc transporter transcripts, as well as the proton-driven Suc transport activity measured in plasma membrane vesicles. Transport activity drops to 35% to 50% and to 20% to 25% of the control after infiltration for 24 h with 100 and 250 mm Suc, respectively (Chiou and Bush, 1998).
In addition to their role as major nutrients for cell growth and function, sugars may be involved in plant development and act as potential signals for the regulation of various genes controlling key processes (Jang and Sheen, 1994; Koch, 1996; Smeekens and Rook, 1997; Gibson, 2000; Smeekens, 2000). The understanding of sugar sensing and signaling in yeast (Saccharomyces cerevisiae) has made important advances in recent years and has become a strong base for the elucidation of nutrient-sensing mechanisms in other eukaryotic organisms (Rolland et al., 2001, 2002). In yeast, two particular members of the family of Glc transporters RGT2 and SNF3 have been proved to act as low- and high-affinity Glc sensors, respectively (Özcan et al., 1996). They both share limited sequence homology and a large cytosolic C-terminal part that is probably implicated in signal transduction. In Arabidopsis, two of 26 genes of monosaccharide transporters known, AtSUGTRPR and F23E12.140,encode proteins with large intracellular loops, which may play a role similar to the RGT2 and SNF3 sensor proteins in yeast (Lalonde et al., 1999). To date, the most plausible candidate, proposed as a sugar sensor in plants, is the Suc transporter SUT2 cloned from tomato and Arabidopsis. It is induced by 100 mm Suc, mainly in sink leaves, and displays an N-terminal cytosolic region and a central cytosolic loop longer than those of usual transporters (Barker et al., 2000).
Recently, we have cloned a putative hexose transporter cDNA and the corresponding gene from ripening grape (Vitis vinifera) berries (Fillion et al., 1999). A computer analysis of the promoter of grape VvHT1 (Hexose Transporter1) revealed the presence of several sugar boxes. Therefore, the possibility that the expression of this gene was partially under the control of sugars was worth testing, especially in the context of sugar sensing described above. This is the aim of the present study, which analyzes the sensitivity of VvHT1 promoter to different mono- and disaccharides. The activity of different lengths of VvHT1 promoter was tested either for organ-preferential expression in transgenic tobacco (Nicotiana tabacum) plants or for regulation by sugars in tobacco cv Bright-Yellow 2 (BY2) cells after stable transformation. This approach was completed by expression studies in the homologous grape cell suspension model.
RESULTS
Deletions of VvHT1 Promoter Regions
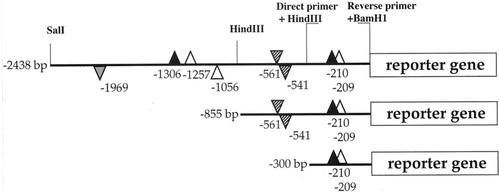
Transcriptional fusions of three lengths (300, 855, and 2,438 bp) of the VvHT1 promoter in front of the uidA coding region encoding β-glucuronidase (GUS) were prepared (Fig. 1). These regions encompass various potential sugar-responsive cis-elements, including three AATAGAAAA sequences named SURE1 (Suc-Responsive Element 1) and described as a prerequisite for the positive sugar control in up-regulated genes, such as the patatin gene of potato (Solanum tuberosum; Grierson et al., 1994). The first SURE1 copy is located at position −1,257 (+ strand), the second one is at position −1,056 (− strand), and the third one (an imperfect SURE1 box lacking just one A at each end) starts from position −209 (+ strand). Two Suc boxes 3 (AAAATCA-------TAA) described in sporamin (Hattori et al., 1990) and chalcone synthase genes (Tsukaya et al., 1991) are present at positions −1,306 and −210 (+ strand). A TATCCAT sequence, known as AMYBOX2 (Huang et al., 1990) or sugar starvation enhancer element in α-amylase 5′ region (Lu et al., 1998), is present in two copies in the middle part of VvHT1 promoter at −561 (+ strand) and at −541 (− strand). Finally, an AMYBOX1 motif (TAACAAA), conserved in α-amylase promoters of rice, wheat (Triticum aestivum), and barley (Hordeum vulgare) as an essential element of negative regulation by sugar (Huang et al., 1990), is located in the distal part of the promoter at position −1,969 (+ strand). For comparison, the strong constitutive 35S CaMV promoter fused to the uidA gene was also used.
Figure 1.
Schematic representation of VvHT1 promoter regions used for the constructs. Successive deletions of the distal part of the promoter (p2.4VvHT1, p0.8 VvHT1, and p0.3VvHT1), with specific sites for restriction enzymes and primers used for chimeric pVvHT1/GUS fusions, are shown. The positions of putative sugar-responsive elements are shown with respect to the translation initiation start. Suc box 3, black triangles; SURE1 motif, white triangles; AMYBOX1, gray triangle; AMYBOX2, hatched triangles. Position over or under the promoter line stands for plus or minus strand, respectively. Triangles corresponding to positive sugar response elements are presented upside up, and those corresponding to negative sugar response elements are presented upside down.
Organ Specificity of VvHT1 Promoter Expression
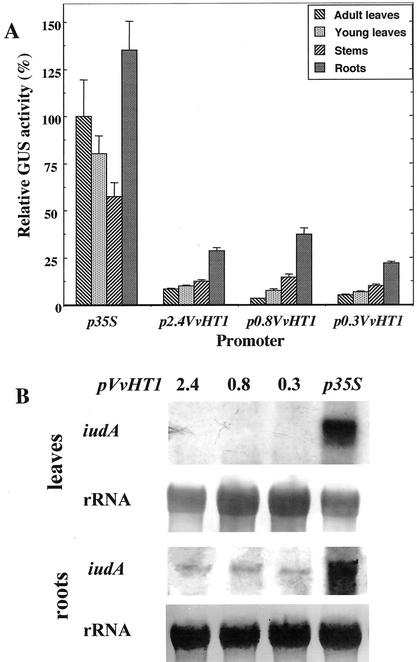
To study the organ specificity of VvHT1 promoter, transgenic tobacco plants were obtained after transformation with the different constructs and analyzed for GUS activity (Fig. 2A). In plants transformed with the p35S-GUS construct, reporter gene activity was very strong in the various organs tested: adult leaves, young leaves, stems, and roots. The expression pattern obtained under the control of 35S promoter was quite different from this corresponding to the expression conducted by VvHT1 promoter. There was not sink organ specificity of p35S-GUS activity and the highest activity was obtained in roots, whereas the lowest activity was observed in stems. Conversely, VvHT1 promoter conferred preferential expression of GUS reporter gene in sink organs (roots, stems, and young leaves), whereas the lowest activity was detected in adult leaves. This organ-specific pattern of expression was conserved for all truncated VvHT1 promoters.
Figure 2.
GUS activity and uidA expression in different organs of tobacco plants expressing the reporter gene under the control of different lengths of VvHT1 promoter. A, GUS activity of roots, stems, and young leaves was expressed relative to the activity measured in mature leaves for each independent transformant (13–20 per construct). The p35S/GUS activity in mature leaves was taken as 100%. Data are given ± se. B, Northern-blot analysis of reporter gene transcripts in source and sink organs under control of VvHT1 and 35S promoters hybridized with a probe corresponding to iudA coding region. Twenty micrograms (leaves) and 25 μg (roots) of total RNA were loaded on the gel.
These data were further confirmed by RNA gel-blot analysis. Two opposite types of organs, adult leaves (source), and roots (sink), were chosen to study the steady-state level of GUS transcripts. Total RNA from two independent transformants for each construct (p35S-GUS, p2.4VvHT-GUS, p0.8VvHT1-GUS, and p0.3VvHT1-GUS) were probed with the radiolabeled GUS probe. In adult leaves, the only clear signal corresponded to p35S-conferred reporter gene expression (Fig. 2B). No signal was detected in mature leaves for GUS gene expression driven by different VvHT1 promoters. In contrast, GUS messengers were detected in roots for all the promoters studied. The amount of uidA transcripts was the most important in the p35S-GUS transformants, but all pVvHT1-GUS constructs conferred detectable levels of reporter gene expression in roots. Taken together, the data lend support to the notion that VvHT1 promoter is clearly responsible for the preferential expression in sink organs, and the proximal 0.3-bp VvHT1 promoter conserves this organ specificity.
Activity of VvHT1 Promoter in BY2 Cell Suspension
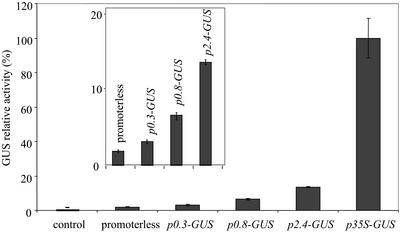
The same constructions were introduced into BY2 tobacco cells. The BY2 cell culture is a heterotrophic system because of the lack of photosynthetic activity, and the carbon source in the medium is Suc (88 mm). This experimental system was chosen because the homogeneity of the cell suspension allows a direct access of the exogenously supplied effectors to all cells. Figure 3 shows that the grape promoter was active in transgenic tobacco BY2 cells. In three independent transformation procedures, the activity of the p2.4 VvHT1-GUS-transformed cells accounted for 15% to 22% of the activity of p35S-GUS-transformed cells. The strongest activity was obtained with the p2.4VvHT1-GUS construct. This activity progressively decreased with the successive 5′ deletions of the promoter, but always remained significantly higher than that of control cells and cells transformed with the promoterless construct.
Figure 3.
Relative GUS activity under the control of different VvHT1 promoters, 35S promoter, or for the promoterless construct, in BY2 transgenic cell suspensions at the 7th d of subculture. Inset shows at a larger scale the activity conferred by different truncated VvHT1 promoters. Data are the mean values of three independent replicates ± se. GUS activity in the p35S-GUS-transformed cells was 386 pmol methyl-umbelliferone (MU) min−1 mg protein−1.
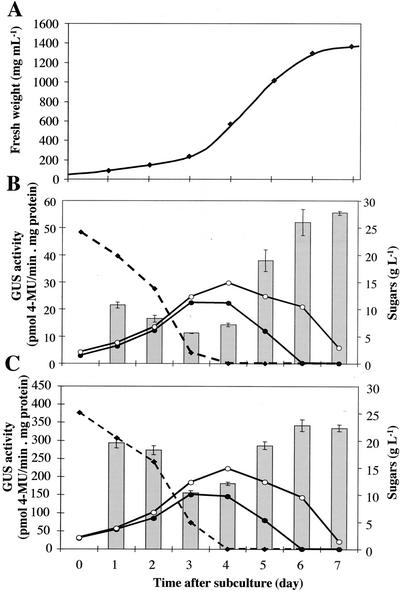
To determine the most favorable moment for the addition of different effectors, sugar concentration and GUS activity after subculture in normal conditions were measured. Under the experimental conditions used, cell growth was exponential from d 2 to 5, and then declined when entering in the stationary phase from d 5 to 7 (Fig. 4A). Suc initially added in the medium disappeared progressively, and this was correlated with a rise in hexose (Glc and Fru) concentrations, suggesting that cultured cells possess an active extracellular invertase. At d 4, Suc was completely depleted from the medium. Starting from this moment, Glc concentration decreased more rapidly than Fru concentration. This suggests that Glc is taken up preferentially over Fru. Similar sugar contents were found in the medium with cells transformed either with the p2.4VvHT1-GUS construct (Fig. 4B) or with the p35S-GUS construct (Fig. 4C).
Figure 4.
Cell growth (A), sugar content of the medium and GUS activity of BY2 transgenic cell suspensions expressing p2.4VvHT1-GUS (B), or p35S-GUS fusion (C). Suc (black squares), Glc (black circles), and Fru (white circles) content of the medium.
The general pattern of expression of the reporter gene during BY2 cells subculture was similar under the control of VvHT1 and 35S promoters (Fig. 4, B and C). The residual GUS activity measured before d 3 reflects mainly the slow turnover of this protein and not the induction of uidA gene expression during the early phase of cell proliferation. The reporter gene activity increased strongly from d 4 to 6. The constant GUS activity measured between d 5 and 7 in p2.4VvHT1-GUS- and p35S-GUS-transformed cells seems in contrast with the lack of any detectable uidA transcripts at the stationary phase (data not shown). This discrepancy is possibly because of the stability of the GUS protein already mentioned (Shaul et al., 1999). These results led us to choose the late exponential phase (i.e. d 4) as the most suitable time to add the different effectors.
Effectors of VvHT1 Promoter
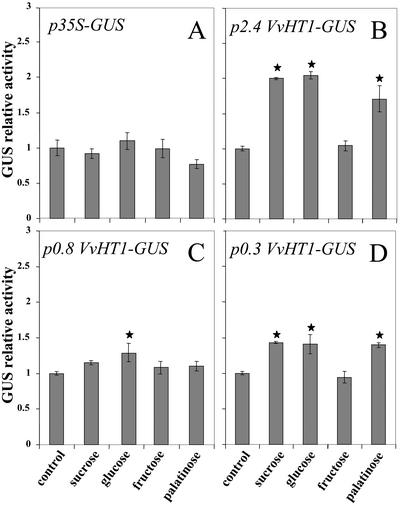
In further experiments, different sugars (Suc, Glc, and Fru) were added to the medium at the late exponential phase of cell growth. Suc (58 mm) or Glc (55.6 mm) doubled the activity measured in the p2.4VvHT1-GUS-transformed cells, compared with the untreated control (Fig. 5B), whereas Fru did not induce any rise in GUS activity. Palatinose, a non-transported analog of Suc (M'Batchi and Delrot, 1988) was as effective as Suc and Glc in promoting GUS activity (Fig. 5B). The same sugar sensitivity was found for the GUS activity of p0.3VvHT1-GUS transformed cells (Fig. 5D), although the extent of stimulation was slighter than in p2.4VvHT1-transformed cells. In the p0.8VvHT1-GUS-transformed cells, no significant effect was observed with Suc and palatinose, and the only slight increase in GUS activity was obtained with Glc (Fig. 5C). Sugars did not affect GUS activity in cells expressing the constitutive p35S-GUS construct (Fig. 5A).
Figure 5.
GUS relative activity in suspension cells carrying p0.3VvHT1/GUS, p0.8VvHT1/GUS, p2.4VvHT1/GUS, or p35S/GUS in response to the addition of different sugars. For each construct, the control is the same transformed cell suspension without treatment. The different compounds were added 4 d after the beginning of subculture and measurements were made 2 d later. Data are representative for three independent transformation procedures of BY2 cells for each construct and treatments produced in each of the transgenic cultures. Values differing significantly (Student's t test, P = 0.05) from the control are indicated by asterisks.
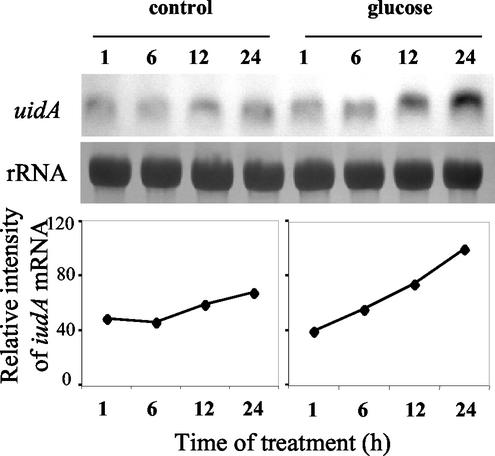
To check the effect of Glc on 2.4VvHT1-GUS transcripts, their steady-state level was studied in cells collected 1, 6, 12, and 24 h after Glc addition. RNA gel-blot analysis revealed that GUS messengers accumulated up to 24 h after Glc treatment, which induced a 2.6-fold increase of GUS mRNA pool (Fig. 6). Only a slight (1.3-fold) increase in transcript amount was observed in the untreated suspension. These results confirmed the appropriate choice of the moment of treatment. At this late exponential phase, it is possible to give evidence for a positive correlation between the steady-state level of uidA messengers and GUS activity, the latter being just delayed in time because of the stability of GUS protein.
Figure 6.
RNA gel-blot analysis of GUS transcripts accumulation under Glc induction of VvHT1 promoter activity in tobacco BY2 transgenic cell suspension and mRNA quantification by using a Bio-Imaging Analyzer. For each lane, 20 μg of total RNA was loaded on gel and membranes were hybridized with iudA-specific probe and rRNA probe.
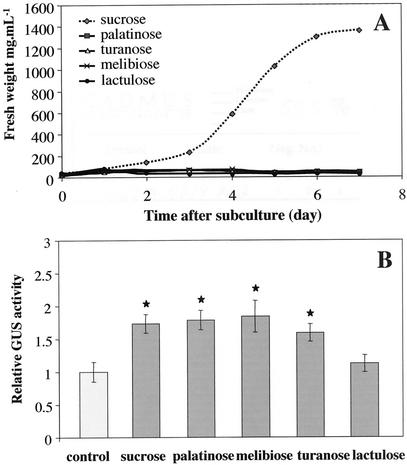
The ability of the different sugars to be metabolized was studied by monitoring the growth of cell suspensions supplemented with different sources of carbon. Among Suc, palatinose, melibiose, turanose, and lactulose, the only sugar allowing normal cell growth was Suc (Fig. 7A). This provided evidence that the non-cleavable disaccharides used in this study were also non-metabolizable (Fig. 7A).
Figure 7.
Growth of tobacco BY2 cells and GUS activity of BY2 cells in the presence of different disaccharides. A, Effect of different disaccharides provided as the unique source of carbon on cell growth. B, Effects of different disaccharides on 2.4VvHT1 promoter-conferred GUS activity. Values differing significantly (Student's t test, P = 0.05) from the control are indicated by asterisks.
To determine the sugar moieties that are necessary for pVvHT1 induction, different non-cleavable disaccharides were applied. Tobacco BY2 cell suspensions, transformed with pVvHT1-GUS or p35S-GUS constructs, were treated with palatinose (Glcα[1–6]Fru), melibiose (Galα[1–6]Glc), turanose (Glcα[1–3]Fru), and lactulose (Galβ[1–4]Fru) at a final concentration of 58 mm. Palatinose, melibiose, and turanose, each carrying one-moiety glucosyl, induced VvHT1 promoter activity (Fig. 7B). Lactulose, which lacks a glucosyl component, did not affect pVvHT1-driven expression. None of these disaccharides affected the activity of 35S promoter (data not shown). Therefore, induction of pVvHT1 activity by non-cleavable disaccharides (palatinose, melibiose, and turanose) seems to require at least a glucosyl moiety.
Sugar Regulation of VvHT1 Expression in a Grape Cell Culture
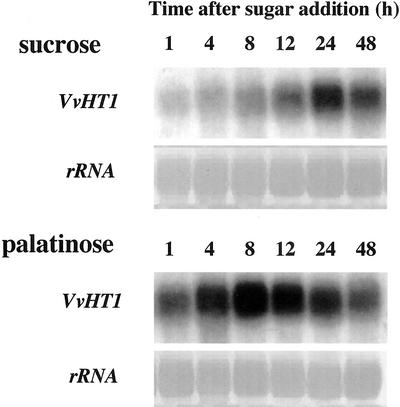
To study the regulation of VvHT1 gene expression in a homologous system, RNA gel-blot analyses were performed on a grape cell suspension, obtained from Cabernet Sauvignon berries (CSB). Both sugars were supplied at the same physiological concentration (58 mm). In Suc-treated cells, a gradual increase of VvHT1 transcripts level was observed from 4 to 24 h after medium change (Fig. 8). The VvHT1 mRNA reached a maximal level at 24 h, but a high expression was maintained up to 48 h after treatment. Palatinose also induced an increase in VvHT1 expression. Compared with Suc, palatinose induced a stronger and earlier accumulation of VvHT1 messengers, whose maximal level was reached 8 h after sugar addition. Therefore, the kinetics of VvHT1 induction by palatinose and Suc were quite different.
Figure 8.
Effect of Suc and palatinose treatment on VvHT1 expression in grape cell suspension culture. For each lane, 20 μg of total RNA was loaded on gel. Membranes were hybridized with VvHT1 and rRNA probes. Data are representative for three independent treatments of cell culture and for the corresponding RNA gel-blot experiments.
DISCUSSION
VvHT1 is a putative hexose transporter expressed in berries and in importing leaves of grape (Fillion et al., 1999). VvHT1 sequence is highly homologous to several plant monosaccharide transporters, especially to the tobacco MST1 monosaccharide transporter, which shares at least 75% homology with VvHT1 in its coding region. Overexpression of the VvHT1 cDNA in tobacco plantlets in vitro alters source/sink partitioning, and reduces Glc uptake capacity in leaf tissues, because of the silencing of endogenous monosaccharide transporter gene MST1 (M. Leterrier, R. Atanassova, L. Laquitaine, C. Gaillard, P. Coutos-Thévenot, and S. Delrot, unpublished data). In parallel to this study on the physiological function of VvHT1, the present work investigates regulation of its expression.
Previous studies have shown that sugar sensing and control of gene expression are involved in various physiological processes, including fruit development (Smeekens, 2000). In grape berries, VvHT1 is expressed after the induction of invertases (Davies et al., 1997) shortly after véraison, when sugars start to accumulate (Fillion et al., 1999). This expression profile and the presence of several sugar boxes in the promoter region of VvHT1 gene made it possible to hypothesize that VvHT1 expression is controlled by its own substrate. Therefore, special attention was paid to sugar regulation of VvHT1 expression.
In this context and because of the long time needed for grape transformation and regeneration, chimeric pVvHT1/GUS constructs were introduced in tobacco. Our data show that the grape promoter was active in tobacco, and that the GUS activity conferred by the promoter was always higher in sink organs than in source leaves (Fig. 2, A and B). In addition, the ortholog of VvHT1, the tobacco MST1 gene, displayed the same organ-specific regulation of expression that was nearly undetectable in source leaves, but strongly induced in roots (Sauer and Stadler, 1993). These data demonstrate the validity of the tobacco model system to study this promoter.
Irrespectively of the length of VvHT1 promoter studied, the conferred sink organ preferential expression was always maintained (Fig. 2, A and B). This means that the cis-elements driving organ-specific expression of VvHT1 gene are contained in the 0.3-kb proximal region of the promoter, and among them there are at least the two sugar responsive elements, SURE1 and Suc box-3. Surprisingly, there was no significant difference in the level of expression among the three promoter lengths studied. A plausible explanation might be the great variability in GUS activity among independent clones obtained for each construct, due mainly to the positional effect of transgene insertion.
To circumvent these problems, the BY2 cell culture was chosen as an expression system in further study of VvHT1 promoter exogenous effectors. The BY2 transgenic cell suspension represents a large population of independent transformation events; thus, the cell culture gives a more statistical account of the effects observed. Figure 4, B and C, clearly demonstrates that Glc was taken up preferentially over Fru in both types of transgenic suspensions, containing either the pVvHT-GUS or the p35S-GUS construct. Moreover, in both cultures, the onset of the stationary phase was correlated with Glc depletion in the medium. This observation is of interest because Glc is considered as a low-Mr morphogen. In this context, Glc concentration is correlated to mitotic activity in sink organs, such as developing cotyledons of broad bean (Borisjuk et al., 1998).
After treatment with Glc, BY2 cells showed more than 2-fold higher pVvHT1-directed GUS activity than that of the same cell culture without sugar addition (Fig. 5B). A similar level of Glc induction was reported for the CHS promoter/GUS construct in Arabidopsis plantlets (Tsukaya et al., 1991). Under the same experimental conditions, Suc mimicked the effect of Glc in pVvHT1-GUS-transformed cells (Fig. 5B). All these effects were observed with physiological sugar concentrations (i.e. 58 mm) commonly found in sink tissues (Patrick, 1997). Two independent results clearly indicate that the effects of Glc and Suc are not simply osmotic. First, Fru did not affect pVvHT1-GUS expression (Fig. 5B, C and D). Second, palatinose, a non-cleavable (Roitsch et al., 2000; Fernie et al., 2001), non-transported (M'Batchi et al., 1985; M'Batchi and Delrot, 1988), and non-metabolizable (Loreti et al., 2000; Fernie et al., 2001; the present paper; Fig. 7) isomer of Suc, induced the same increase of GUS activity as Suc itself in pVvHT1-GUS cells. This is the first example of transcriptional regulation of a putative hexose transporter by its own substrate in higher plants.
Further experiments were conducted to get more insight into the different elements involved in Suc induction of VvHT1, the signal, the sensor, the signaling pathway, and promoter cis-elements. Because palatinose is not digested, this indicates that the Suc effect cannot be simply because of Glc generated by an extracellular invertase. Furthermore, because palatinose is not (or very poorly) transported, its effect is mediated through a sensor located at the plasma membrane, presumably a Suc sensor. These data also suggest that the VvHT1 promoter activity may be stimulated via two independent signaling pathways, i.e. a hexose pathway as well as a Suc pathway.
Other disaccharides, such as melibiose and turanose, which differ from Suc and palatinose by their monosaccharide composition, but also bear one glucosyl moiety, produced the same induction effect on pVvHT1-conferred GUS activity (Fig. 7B). Conversely, the disaccharide lactulose, which lacks a glucosyl component, did not affect VvHT1 promoter expression. Thus, in contrast to β-amylase repression by Suc in barley embryos, which requires a Fru moiety for disaccharide sensing (Loreti et al., 2000), the glucosyl moiety is necessary and sufficient for the induction of VvHT1. The perception of the Suc signal implies a receptor probably localized in the plasma membrane. Our data do not exclude the possibility of a common receptor for Glc and Glc-derived disaccharides.
When the p35S-GUS transgenic suspension was treated with exogenous sugars, there was no detectable change of GUS activity (Fig. 5A). This strongly suggests that the effects obtained with the pVvHT1-GUS constructs were specific for the studied promoter. Similarly, absence of responsiveness to sugars was observed for leaves of transgenic Arabidopsis plantlets carrying p35S-GUS fusion (Tsukaya et al., 1991). The VvHT1 promoter contains four different types of motifs potentially involved in sugar regulation of gene expression (Fig. 1). Interestingly, the effects of Suc and palatinose were not detectable with the p0.8VvHT1-GUS constructs, although they appeared with the p0.3VvHT1-GUS construct (Fig. 5C and D). Moreover, the effect of Glc was stronger with 0.3VvHT1 promoter than with 0.8VvHT1 one. Given the positions of the putative activating and repressing sugar boxes (see Fig. 1), it may be speculated that the two proximal-activating sugar boxes contained in the p0.3 VvHT1 promoter are responsive for both the Glc- and Suc-induced significant rise in GUS activity (Fig. 5D). It is likely that in the p0.8VvHT1-GUS construct, the effect of two sugar activation elements is antagonized by the presence of two AMYBOX2 sugar repression motifs. There may be a mutual compensation effect, which does not allow significant sugar modulation of 0.8VvHT1 promoter-driven GUS activity (Fig. 5C). In the entire 2.4VvHT1 promoter (Fig. 5B), the effect of sugar repression motifs is compensated by all proximal and distal positive sugar elements contained, thereby conferring the highest inductive effect of sugars. This interpretation will require the study of individual consensus sequences and the identification of transcription factors binding to the different cis elements. Recently, a DNA-binding protein inducing the expression of VvHT1 was identified by using the proximal 160-bp part of VvHT1 promoter, encompassing the two positive sugar response motifs, as a bait in the one-hybrid approach (B. Cakir, A. Agasse, S. Delrot, and R. Atanassova, unpublished data).
Induction of VvHT1 expression by Suc and palatinose in grape suspension cells (Fig. 8) confirms the data obtained with the reporter gene expressed in BY2 tobacco cells. Furthermore, the time courses of induction are very similar to those for the sink-specific extracellular invertase Lin6 and quite different from that of the source-specific RbcS (Alok et al., 2002). This sink-specific behavior of the VvHT1 gene under sugar control in cell culture is in agreement with its sink organ preferential expression in whole plants (Fig. 2). Palatinose induced a faster and stronger accumulation of VvHT1 messengers than Suc itself, thereby suggesting a differential regulation of VvHT1 by metabolizable and non-metabolizable sugars, as already revealed for Lin6 (Alok et al., 2002). The earlier effects of both palatinose and Suc on Lin6 messenger accumulation, than those on VvHT1 transcripts, may be explained by differences between the autotrophically growing tomato cell suspension and the heterotrophically growing grape cell suspension. In this context, it is noteworthy that palatinose has been shown to be able to induce mitogen-activated protein kinases (Roitsch et al., 2000; Alok et al., 2002).
In conclusion, these data provide additional insight into the mechanism of sugar sensing in plants. They describe the first example of induction of a putative monosaccharide transporter from higher plants, induced by its own substrate (i.e. Glc). Furthermore, this transporter is also sensitive to Suc via a pathway, which does not seem to require Suc uptake and metabolism in the cell. These results demonstrate the regulation of VvHT1 expression by Glc and glucosyl-containing disaccharides. They lend further support to the notion that in different experimental systems Suc and its isomer palatinose may involve common sugar-sensing mechanisms but differential sugar-signaling pathways.
MATERIALS AND METHODS
Chimeric Constructs
All constructs of the promoter of grape (Vitis vinifera) VvHT1 in front of the uidA reporter gene were generated in the binary vector pBI101.1 (Jefferson et al., 1987). Two parts of VvHT1 5′ region were obtained as restriction fragments of 2,438 bp (SalI–BamHI) for the entire length promoter, and 855 bp (HindIII–BamHI) for the middle length promoter. Although the SalI (−2,438) and HindIII (−855) restriction sites were naturally present in the promoter, a BamHI site was introduced by PCR at the 3′ end, to eliminate the translation initiation sequence of VvHT1 gene. The proximal promoter region of 300 bp (HindIII–BamHI) was amplified by PCR, based on sequence-specific primers introducing both these restriction sites. The PCR products obtained were subcloned in the pGEM-T easy vector. The different VvHT1 truncated promoters produced were transcriptionally fused in front of uidA gene coding region. The 35S promoter/uidA fusion was used for the constitutive control (the pBI121 vector), and the promoterless-uidA construct was used as a negative control (the pBI101.1 vector). Cloning in Escherichia coli was performed in strain DH5α. The integrity of the vectors was confirmed by restriction analysis and sequencing. The constructs were introduced in Agrobacterium tumefaciens strain LBA4404.
Tobacco (Nicotiana tabacum cv Samsun NN) Plant Transformation
Tobacco plants were transformed by the modified leaf disc method of Horsch et al. (1985). Murashige and Skoog medium was used with one-half NH4NO3 concentration and supplemented with kanamycin at 150 μg mL−1 and cefotaxime at 350 μg mL−1 (Duchefa, Haarlem, The Netherlands) as selective agents during the in vitro regeneration and propagation of transgenic plants. Calli formation and shoot regeneration were allowed to proceed in the presence of 6-benzylaminopurine (2 mg L−1), 1-naphthalene acetic acid (0.05 mg L−1), and Suc (30 g L−1). Rooting was obtained on hormone-free Murashige and Skoog medium, at Suc concentration of 15 g L−1. For each construct, from 13 to 20 independent primary transformants were produced. Transformed plants were grown in vitro under a light/dark regime of 16/8 h at 22°C.
Cell Suspension Cultures and Transformation Procedures
Tobacco BY2 cells were transformed via A. tumefaciens by 48 h of coculture on solid Linsmaier and Skoog modified medium at 24°C (Nagata et al., 1992). After that, cells were washed three times with a medium complemented with the antibiotics kanamycin (100 μg mL−1) and carbenicillin (500 μg mL−1), both purchased from Duchefa. Cells were further transferred and grown on a three-layer medium consisting of a low layer of Linsmaier and Skoog solid medium, a middle layer of 1% (w/v) low-melting agarose in Linsmaier and Skoog medium containing the treated cells, and an upper layer of liquid Linsmaier and Skoog medium. This three-layer medium was supplemented with the same antibiotics at the above-mentioned concentrations. Thus, immersed BY2 transformed cells were grown and selected at 27°C in the dark for 3 to 4 weeks. The formed calli were resuspended in liquid Linsmaier and Skoog-modified medium, at the same antibiotic concentrations, and cultured at 27°C and 170 rpm in the dark to form a homogenous cell culture. This culture was maintained by weekly dilution of the cells by the medium (1.5:80 [v/v]).
The grape cell suspension was obtained from CSB by Mérillon and coworkers and was maintained at 25°C on an orbital shaker (100 rpm) by weekly subculture on medium supplemented with 58 mm Suc as previously described (Decendit et al., 1996). To avoid the stress because of dilution, 3 d after the beginning of the subculture, grape cells were let to settle, washed with fresh medium supplemented either with Suc or palatinose (58 mm) and suspended again in these new corresponding media.
Fluorimetric GUS Assay
GUS fluorimetric assays were carried out on BY2 transgenic suspension after different treatments and on different organs of 6-week-old transformed tobacco plantlets, according to the procedure of Jefferson et al. (1987). GUS enzyme activity was determined by measuring the kinetics of appearance of MU, produced by cleavage of methylumbelliferyl-β-d-glucuronide. Fluorescence was read on FluoroCount Microplate Fluorometer (Packard Instruments, Meriden, CT). GUS enzyme activity was expressed as picomoles of MU produced per minute per milligram of protein. Protein content was determined by the dye binding method of Bearden (1978), using bovine serum albumin as a standard. All GUS measurements presented were confirmed in three independently transformed suspensions and obtained on a total of six to 12 measurements coming from two to four independent experiments.
Determination of Sugar Contents
To determine sugar contents during BY2 cell suspension proliferation, 1-mL aliquots of culture medium were harvested every day of the subculture period, cleared by centrifugation, and the cell-free supernatant was stored frozen at −80°C. The enzymatic method of sugars content determination was applied according to Jones et al. (1977). Twenty-five microliters of each sample, diluted 50-fold with water, were mixed with 30 μL (16 units) of invertase (dissolved in 320 mm citrate buffer, pH 4.6) and 145 μL of water. After 30 min of incubation at 55°C, the samples were spectrophotometrically assayed in 1 mL of buffer (0.1 m HEPES [pH 7.6], 0.4 mm NADP, 1 mm ATP, 5 mm MgCl2, and 0.5 mm dithioerythreitol), at 340 nm. The contents of Suc, Glc, and Fru were determined under the same conditions after addition of hexokinase (0.5 units), phospho-Glc isomerase (2 units), and Glc-6-phosphate dehydrogenase (2.5 units), and incubation time of 30 min at 28°C. To obtain the values of Fru and Glc, invertase was deliberately omitted. To determine Glc content only, both invertase and phospho-Glc isomerase were omitted. All enzymes were purchased from Sigma (St. Louis).
RNA Gel-Blot Analysis
Total RNAs from 5-mL samples of tobacco BY2 cell suspension or 10-mL samples of grape CSB suspension, collected after different treatments, were isolated with the Rneasy Plant Mini Kit (QIAGEN GmbH, Hilden, Germany). Total RNAs from frozen plant material, corresponding to different organs of 6-week-old transformed tobacco plantlets, were obtained by phenol extraction (Howell and Hull, 1978), followed by selective precipitation with 2 m LiCl (Verwoerd et al., 1989). Purified RNA samples, 20 μg each, were separated by formaldehyde-agarose gel electrophoresis, and transferred to Hybond N+ membranes (Amersham, Buckinghamshire, UK). RNA blots were hybridized with randomly primed [32P] probes and mRNA was quantified using a Storm Bio-Imaging Analyzer (Molecular Dynamics, Sunnyvale, CA).
ACKNOWLEDGMENTS
We thank Prof. Toshiyuki Nagata and the Tobacco Science Research Laboratory (Japan Tobacco Inc., Oyama) for allowing us to use the BY2 cell suspension.
Footnotes
This work was supported by the Conseil Régional Poitou-Charentes, France (to M.L.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.009522.
LITERATURE CITED
- Alok SK, Hofmann MG, Römer Ulrike, Köckenberger W, Elling L, Roitsch T. Metabolizable and non-metabolizable sugars activate different signal transduction pathways in tomato. Plant Physiol. 2002;128:1480–1489. doi: 10.1104/pp.010771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker L, Kühn C, Weise A, Schulz A, Gebhardt C, Hirner B, Hellmann H, Schulze W, Ward JM, Frommer WB. SUT2, a putative sucrose sensor in sieve elements. Plant Cell. 2000;12:1153–1164. doi: 10.1105/tpc.12.7.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden JC. Quantitation of submicrogram quantities of protein by an improved protein-dye binding assay. Biochim Biophys Acta. 1978;533:525–529. doi: 10.1016/0005-2795(78)90398-7. [DOI] [PubMed] [Google Scholar]
- Borisjuk L, Walenta S, Weber H, Mueller-Klieser W, Wobus U. High-resolution histographical mapping of glucose concentrations in developing cotyledons of Vicia faba in relation to mitotic activity and storage processes: glucose as a possible developmental trigger. Plant J. 1998;15:583–591. [Google Scholar]
- Büttner M, Sauer N. Monosaccharide transporters in plants: structure, function and physiology. Biochim Biophys Acta. 2000;1465:263–274. doi: 10.1016/s0005-2736(00)00143-7. [DOI] [PubMed] [Google Scholar]
- Chiou TJ, Bush DR. Sucrose is a signal molecule in assimilate partitioning. Proc Natl Acad Sci USA. 1998;95:4784–4788. doi: 10.1073/pnas.95.8.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Boss PK, Robinson SP. Treatment of grape berries, a non climacteric fruit with a synthetic auxin, retards ripening and alters the expression of developmentally regulated genes. Plant Physiol. 1997;115:1155–1161. doi: 10.1104/pp.115.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decendit A, Ramawat KG, Waffo P, Deffieux G, Badoc A, Mérillon JM. Anthocyanins, catechins, condensed tannins and piceid production in Vitis vinifera cell bioreactor cultures. Biotechnol Lett. 1996;18:659–662. [Google Scholar]
- Fernie AR, Roessner U, Geigenberger P. The sucrose analog palatinose leads to a stimulation of sucrose degradation and starch synthesis when supplied to discs of growing potato tubers. Plant Physiol. 2001;125:1967–1977. doi: 10.1104/pp.125.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillion L, Ageorges A, Picaud S, Coutos-Thévenot P, Lemoine R, Romieu C, Delrot S. Cloning and expression of a hexose transporter gene expressed during the ripening of grape berry. Plant Physiol. 1999;120:1083–1094. doi: 10.1104/pp.120.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson SJ. Plant sugar-response pathways. Part of complex regulatory web. Plant Physiol. 2000;124:1532–1539. doi: 10.1104/pp.124.4.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson C, Du JS, De Torres Zabala S, Beggs K, Smith C, Holsworth M, Bevan M. Separate cis sequences and trans factors direct metabolic and developmental regulation of a potato tuber storage protein gene. Plant J. 1994;5:815–826. doi: 10.1046/j.1365-313x.1994.5060815.x. [DOI] [PubMed] [Google Scholar]
- Hattori T, Nakagawa S, Nakamura K. High level expression of tuberous root storage protein genes of sweet potato in stems of plantlets grown in vitro on sucrose medium. Plant Mol Biol. 1990;14:595–604. doi: 10.1007/BF00027505. [DOI] [PubMed] [Google Scholar]
- Horsch RB, Fry JE, Hoffman NL, Eichloltz D, Rogers SG, Fraley RT. A simple and general method for transferring genes into plants. Science. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Howell SH, Hull R. Replication of cauliflower mosaic virus and transcription of its genome in turnip leaf protoplasts. Virology. 1978;88:468–481. doi: 10.1016/0042-6822(78)90086-7. [DOI] [PubMed] [Google Scholar]
- Huang N, Sutliff T, Litts J, Rodriguez R. Classification and characterization of the rice alpha-amylase multigene family. Plant Mol Biol. 1990;14:655–668. doi: 10.1007/BF00016499. [DOI] [PubMed] [Google Scholar]
- Jang JC, Sheen J. Sugar sensing in higher plants. Plant Cell. 1994;6:1665–1679. doi: 10.1105/tpc.6.11.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M, Outlaw W, Lowry O. Enzymatic assay of 10–7 to 10–14 moles of sucrose in plant tissues. Plant Physiol. 1977;60:379–383. doi: 10.1104/pp.60.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KE. Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Lalonde S, Boles E, Hellmann H, Barker L, Patrick JW, Frommer WB. The dual function of sugar carriers: transport and sugar sensing. Plant Cell. 1999;11:707–726. doi: 10.1105/tpc.11.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreti E, Alpi A, Pierdomenico P. Glucose and disaccharide-sensing mechanisms modulate the expression of α-amylase in barley embryos. Plant Physiol. 2000;123:939–948. doi: 10.1104/pp.123.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CA, Lim EK, Yu SM. Sugar response sequence in the promoter of a rice a-amylase gene serves as a transcriptional enhancer. J Biol Chem. 1998;273:10120–10131. doi: 10.1074/jbc.273.17.10120. [DOI] [PubMed] [Google Scholar]
- Matsukura C, Saitoh T, Hirose T, Ohsugi R, Perata P, Yamaguchi J. Sugar uptake and transport in rice embryo. Expression of companion cell-specific sucrose transporter (OsSUT1) induced by sugar and light. Plant Physiol. 2000;124:85–93. doi: 10.1104/pp.124.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M'Batchi B, Delrot S. Stimulation of sugar exit from leaf tissues. Planta. 1988;174:340–348. doi: 10.1007/BF00959519. [DOI] [PubMed] [Google Scholar]
- M'Batchi B, Pichelin D, Delrot S. The effect of sugars on the binding of [203Hg]-p-chloromercuribenzene sulfonic acid to leaf tissues. Plant Physiol. 1985;79:537–542. doi: 10.1104/pp.79.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mito N, Wimmers LE, Bennett AB. Sugar regulates mRNA abundance of H+-ATPase gene family members in tomato. Plant Physiol. 1996;112:1229–1236. doi: 10.1104/pp.112.3.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T, Nemoto Y, Hasezawa S. Tobacco BY-2 cell line as the “HeLa” cell in the cell biology of higher plants. Int Rev Cytol. 1992;132:1–30. [Google Scholar]
- Özcan S, Dover J, Rosenwald AG, Wölfl S, Johnston M. Two glucose transporters in Saccharomyces cerevisiae are glucose sensors that generate a signal for induction of gene expression. Proc Natl Acad Sci USA. 1996;93:12428–12432. doi: 10.1073/pnas.93.22.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick JW. Phloem unloading: sieve elements unloading and post-sieve element transport. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:191–222. doi: 10.1146/annurev.arplant.48.1.191. [DOI] [PubMed] [Google Scholar]
- Roitsch T, Büttner M, Godt DE. Induction of apoplastic invertase of Chenopodium rubrum by d-glucose and a glucose analog and tissue-specific expression suggest a role in sink-source regulation. Plant Physiol. 1995;108:285–294. doi: 10.1104/pp.108.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitsch T, Ehness R, Goetz M, Hause B, Hofmann M, Sinha AK. Regulation and function of extracellular invertase from higher plants in relation to assimilate partitioning, stress responses and sugar signalling. Aus J Plant Physiol. 2000;27:815–825. [Google Scholar]
- Roitsch T, Tanner W. Expression of a sugar-transporter gene family in a photoautotrophic suspension culture of Chenopodium rubrum L. Planta. 1994;193:365–371. doi: 10.1007/BF00201814. [DOI] [PubMed] [Google Scholar]
- Rolland F, Winderickx J, Thevelein J. Glucose sensing mechanisms in eukaryotic cells. Trends Biochem Sci. 2001;26:310–317. doi: 10.1016/s0968-0004(01)01805-9. [DOI] [PubMed] [Google Scholar]
- Rolland F, Winderickx J, Thevelein J. Glucose sensing and signalling mechanisms in yeast. FEMS Yeast Res. 2002;2:183–201. doi: 10.1111/j.1567-1364.2002.tb00084.x. [DOI] [PubMed] [Google Scholar]
- Sauer N, Friedlander K, Graml-Wicke U. Primary structure, genomic organization and heterologous expression of a glucose transporter from Arabidopsis thaliana. EMBO J. 1990;9:3045–3050. doi: 10.1002/j.1460-2075.1990.tb07500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer N, Stadler R. A sink-specific H+/monosaccharide co-transporter from Nicotiana tabacum: cloning and heterologous expression in baker's yeast. Plant J. 1993;4:601–610. doi: 10.1046/j.1365-313x.1993.04040601.x. [DOI] [PubMed] [Google Scholar]
- Sauer N, Tanner W. The hexose carrier from Chlorella. cDNA cloning of a eukaryotic H+-cotransporter. FEBS Lett. 1989;259:43–46. doi: 10.1016/0014-5793(89)81489-9. [DOI] [PubMed] [Google Scholar]
- Shaul O, Mironov V, Van Montagu M, Inzé D. Tobacco cultures transformed with cyclin promoter-gus constructs reveal a discrepancy between gus mRNA levels and GUS protein activity upon leaving the stationary state. Plant Sci. 1999;141:67–71. [Google Scholar]
- Smeekens S. Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:49–81. doi: 10.1146/annurev.arplant.51.1.49. [DOI] [PubMed] [Google Scholar]
- Smeekens S, Rook F. Sugar sensing and sugar-mediated signal transduction in plants. Plant Physiol. 1997;115:7–13. doi: 10.1104/pp.115.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler R, Wolf K, Hilgarth C, Tanner W, Sauer N. Subcellular localization of the inducible Chlorella HUP1 monosaccharide-H+ symporter and cloning of a Co-induced galactose-H+ symporter. Plant Physiol. 1995;107:33–41. doi: 10.1104/pp.107.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truernit E, Schmid J, Epple P, Illig J, Sauer N. The sink-specific and stress-regulated Arabidopsis STP4 gene: enhanced expression of a gene encoding a monosaccharide transporter by wounding, elicitors, and pathogen challenge. Plant Cell. 1996;8:2169–2182. doi: 10.1105/tpc.8.12.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truernit E, Stadler R, Baier K, Sauer N. A male gametophyte-specific monosaccharide transporter in Arabidopsis. Plant J. 1999;17:191–201. doi: 10.1046/j.1365-313x.1999.00372.x. [DOI] [PubMed] [Google Scholar]
- Tsukaya H, Ohshima T, Naito S, Chino M, Komeda Y. Sugar-dependent expression of the CHS-A gene for chalcone synthase from petunia in transgenic Arabidopsis. Plant Physiol. 1991;97:1414–1421. doi: 10.1104/pp.97.4.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd TC, Dekker BMM, Hoekema A. A small scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989;17:2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Borisjuk L, Heim U, Sauer N, Wobus U. A role for sugar transporters during seed development: molecular characterization of a hexose and a sucrose carrier in fava bean seeds. Plant Cell. 1997;9:895–908. doi: 10.1105/tpc.9.6.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylstra B, Garrido D, Busscher J, Van Tunen AJ. Hexose transport in growing petunia pollen tubes and characterization of a pollen-specific, putative monosaccharide transporter. Plant Physiol. 1998;118:297–304. doi: 10.1104/pp.118.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]