Abstract
Improving plant nitrogen (N) use efficiency or controlling soil N requires a better knowledge of the regulation of plant N metabolism. This could be achieved using Arabidopsis as a model genetic system, taking advantage of the natural variation available among ecotypes. Here, we describe an extensive study of N metabolism variation in the Bay-0 × Shahdara recombinant inbred line population, using quantitative trait locus (QTL) mapping. We mapped QTL for traits such as shoot growth, total N, nitrate, and free-amino acid contents, measured in two contrasting N environments (contrasting nitrate availability in the soil), in controlled conditions. Genetic variation and transgression were observed for all traits, and most of the genetic variation was identified through QTL and QTL × QTL epistatic interactions. The 48 significant QTL represent at least 18 loci that are polymorphic between parents; some may correspond to known genes from the N metabolic pathway, but others represent new genes controlling or interacting with N physiology. The correlations between traits are dissected through QTL colocalizations: The identification of the individual factors contributing to the regulation of different traits sheds new light on the relations among these characters. We also point out that the regulation of our traits is mostly specific to the N environment (N availability). Finally, we describe four interesting loci at which positional cloning is feasible.
Nitrogen (N) is often considered to be one of the most important factors limiting plant growth in natural ecosystems and in most agricultural systems. In modern agricultural systems where plants rely on fertilizers to meet their demand in N, inadequate practices still cause environmental problems (Bacon, 1995; Lawlor et al., 2001), mainly linked to nitrate loss in the environment. At the same time, a part of research efforts has been devoted to develop genotypes that use N more efficiently. This highly complex objective requires a deep understanding of the genetic basis of N assimilation and N use at different stages of plant development.
The main structural elements of N assimilation pathway in higher plants are well known. Nitrate or ammonium uptake represents the first step in this pathway, and a large number of putative transporters have been identified (for review, see Orsel et al., 2002). The reduction of nitrate to nitrite and the subsequent reduction of nitrite to ammonium are catalyzed by the well-known enzymes nitrate reductase (NR) and nitrite reductase (NiR), respectively (Meyer and Stitt, 2001). This primary assimilation mainly takes place in leaves, and ammonium produced by this process or by others (photorespiration or atmospheric N2 fixation) is then incorporated into organic molecules by the Gln synthetase (GS)/Glu synthase (GOGAT) pathway (Hirel and Lea, 2001). Individual enzyme regulation profiles (transcriptional and posttranscriptional regulations) together with whole-plant physiology studies suggest that the N assimilation pathway is strongly integrated (Stitt, 1999). Although the structural components of this pathway are rather well characterized, the signals and the transduction pathway that govern their activities are far from being identified. Attempts to isolate regulatory mutants by genetic approaches led to the isolation of mutants affected either in nitrate transporters (Tsay et al., 1993), NR apoenzyme (Hoff et al., 1995), NR cofactor, and NiR gene expression (Leydecker et al., 2000), or in light-response, such as the Arabidopsis cop1 (Deng et al., 1991) and cr88 mutants (Lin and Cheng, 1997). This approach failed to isolate new genes that could be involved in the regulation of at least one step of the pathway. In fact, different alleles of those genes may cause subtle changes rather than strong global modifications.
Quantitative trait loci (QTL) mapping consists of identifying (through linked genetic markers) the individual genetic factors influencing the value of a quantitative trait. This approach is then particularly interesting when the different sources of variation mask the individual mechanisms leading to a phenotype. Understanding the complexity of the N metabolism network through QTL analysis could lead to the cloning of regulatory loci or factors interacting with them. This new approach of whole-plant N physiology has been performed on maize using field trials (Agrama et al., 1999; Bertin and Gallais, 2000; Hirel et al., 2001). It often leads to a discussion of the concept of N use efficiency, which represents the quantity of N used to build up a certain amount of biomass (or yield). The study of well-chosen traits allows the discussion of the relationship between processes corresponding to different levels of organization, through the identified QTL (Lebreton et al., 1995; Prioul et al., 1997). For example, in a maize study, coincidences were detected between QTL for yield (and its components) and QTL for GS enzyme activity, both of which colocalize with genes encoding cytosolic GS (Hirel et al., 2001). GS- and GOGAT-related QTL were also mapped in rice (Oryza sativa) in a recent study (Obara et al., 2001). The size of the maize (or even rice) genome, however, does not facilitate the fine-mapping of these QTL and the cloning of the corresponding genes.
Arabidopsis offers unequivocal genetic advantages for QTL mapping and cloning purposes: Among them, complete and dense genetic maps and the availability of the ultimate physical map (the complete sequence) are certainly decisive in this case (Alonso-Blanco and Koornneef, 2000; Lukowitz et al., 2000; Yano, 2001). Concerning N metabolism, at least 25 genes directly involved in this pathway are positioned on the Arabidopsis physical map, including nitrate transporter (NRT), NR, NiR, GS, GOGAT, and amino acid transporter (AAP) genes. Moreover, Arabidopsis accessions (natural populations) represent a resource of particular interest for QTL analysis, because they reflect genetic adaptations to their specific habitat, which are known to be diverse (Pigliucci, 1998; Alonso-Blanco and Koornneef, 2000). In Arabidopsis, so far, QTL analysis has only been used to study a limited number of quantitative traits, mostly flowering time (Jansen et al., 1995; Alonso-Blanco et al., 1998), but also, for example, seed dormancy (van der Schaar et al., 1997), disease resistance (Buell and Somerville, 1997; Wilson et al., 2001), circadian rhythm (Swarup et al., 1999), and floral morphology (Juenger et al., 2000). Very few studies concern plant growth (Mitchell-Olds, 1996), and only Mitchell-Olds and Pedersen (1998) have tried to dissect the genetic basis of some physiological traits involved in carbon metabolism. Rauh et al. (2002) recently reported the analysis of growth response to varying N sources.
Most of these studies have been performed using two recombinant inbred line (RIL) populations, namely Landsberg erecta (Ler)/Columbia and Ler/Cape Verde Islands populations. We previously described a new RIL population dedicated to QTL analysis that is derived from the cross between two genetically distant ecotypes, Bay-0 and Shahdara (Loudet et al., 2002; http://www.inra.fr/qtlat). The cross between a Central-Asian accession and an European accession should maximize interesting phenotypic variation reflecting the genetic distance between them (Loridon et al., 1998; Breyne et al., 1999; Sharbel et al., 2000). Moreover, this population is likely to ensure a high power of QTL mapping, because of the population size (Loudet et al., 2002).
In this paper, we describe the genetic analysis of several traits classically used to describe whole-plant N physiology and growth, at a vegetative stage. The study, conducted in controlled growth conditions, is aimed at comparing two different N environments. We identify and discuss several loci explaining the variability of growth and total N, nitrate, and free-amino acid contents. This represents, to our knowledge, the first extensive study of N metabolism in Arabidopsis using QTL mapping.
RESULTS
Experimental Strategy
The production of homogeneous plant material for a large number of lines was certainly the most challenging and limiting step of our work. The design of the experimental display was therefore essential. From our personal observations, we know that uncontrolled environmental effects can affect the evaluation of the quantitative traits and that environmental heterogeneity can occur between two different cultivation repetitions (even in the same growth chamber), as well as within one single growth chamber during a repetition. For these reasons, we chose (a) to always study all of the lines in the same cultivation repetition and (b) to compare different N environments in the same cultivation repetition. Moreover, by choosing only homogeneous plants 6 d after sowing, we tried to suppress the heterogeneity appearing at the very early stages of plant development. Intra-RIL heterogeneity was strongly reduced in comparison with what is obtained without seedling selection (O. Loudet, unpublished data). Figure 1 shows a typical experimental display 34 d after sowing.
Figure 1.
The experimental display in both N environments (N+ and N−) 34 d after sowing. One pot contains six plants from the same RIL. Each RIL is represented by a single pot in each N environment.
The names of the traits obtained in the N+ environment (10 mm nitrate) are suffixed with “10” (for example, DM10), whereas the names of the traits obtained in the N− environment (3 mm nitrate) are suffixed with “3” (for example, DM3). Nitrate content in plants cultivated in the N− environment was extremely low (very close to zero) and could not be correctly estimated by our analysis. Therefore, this trait was not studied here.
Decomposition of the Variance and Heritability
Table I indicates the sum of squares associated with the genotype (RIL) effect over the whole experiment. It is always highly significant (P(f) <0.001), revealing the high level of genetic variation for all traits in both environments. Another factor introduces strong variation in the phenotypic estimations: the cultivation repetition. For most traits, its effect is highly significant (P(f) <0.001), except for NP10 where it is only significant (P(f) <0.05; Table I). A part of the variation observed for each trait corresponds to a specific response to some environmental condition(s) that could not be controlled between the different cultivation repetitions. Nevertheless, this effect seems to affect all of the lines similarly, because the genotype × repetition interaction (as estimated across both environments) is globally not significant (data not shown). Only total N percentage (NP) shows a significant genotype × repetition interaction (P(f) <0.01), but we were able to verify that only a small number of lines were responsible for this interaction. For these reasons, we chose to perform all subsequent QTL analyses on unadjusted mean values across the different repetitions, which should represent a good estimation of the mean behavior of a genotype in a specific N environment.
Table I.
Traits, heritability, and sum of squares decomposition
| Name | Trait | Unit | Heritability | Genotype Effecta | Repetition Effectb | N Environment Effectc |
|---|---|---|---|---|---|---|
| DM10 | Shoot Dry Matter (N+) | mg plant−1 | 0.45 | 7,839*** | 982*** | 62,152*** |
| DM3 | Shoot Dry Matter (N−) | mg plant−1 | 0.20 | 1,289*** | 276*** | |
| NP10 | Total N Percentage (N+) | % (DM) | 0.45 | 82.5*** | 0.5 * | 9,929*** |
| NP3 | Total N Percentage (N−) | % (DM) | 0.55 | 69.9*** | 1.8*** | |
| NO10 | Nitrate content (N+) | nmol mg−1 DM | 0.40 | 57 × 106*** | 19 × 106*** | n.a. |
| AA10 | Free amino acids content (N+) | nmol mg−1 DM | 0.60 | 24 × 104*** | 3.8 × 104*** | 417 × 104*** |
| AA3 | Free amino acids content (N−) | nmol mg−1 DM | 0.60 | 9.8 × 104*** | 0.7 × 104*** |
***, Significant at the 0.1% level. *, Significant at the 5% level. n.a., Data not available.
Sum of squares associated with the genotype effect and significance.
Sum of squares associated with the repetition (cultivation) effect and significance.
Sum of squares associated with the N environment effect and significance.
Heritabilities of the different traits are presented in Table I. Most of these heritabilities fall around 0.5, indicating that one-half of the phenotypic variation observed for these traits is attributable to genetic factors, potentially QTL. A notable exception is DM3, whose variability is mostly (80%) controlled by environmental effects. In contrast, it must be stressed that a trait like free-amino acid content, which is less integrative than dry matter (DM), is more heritable (0.60) in both N environments (AA10 and AA3).
As expected, the N environment effect is strong for all traits (P(f) <0.001; Table I). The limitation of N availability has a systematic decreasing effect on the traits studied. Moreover, the genotype × N environment interaction is always highly significant (P(f) <0.001; data not shown), indicating that this population shows different responses to N stress.
Phenotypic Variation and Correlations among Traits
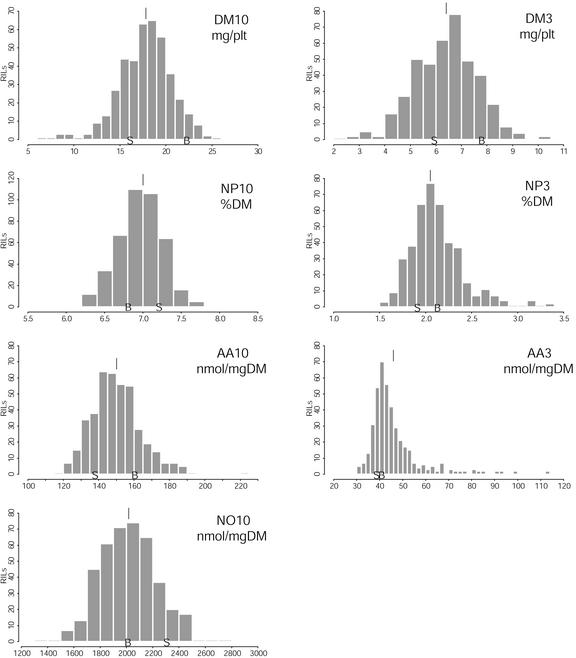
A histogram showing the distribution of the phenotypic variation in the 415 RIL is presented for each trait on Figure 2. Transgressive variation, i.e. the fact that the variation among the lines exceeds the variation between the parental accessions (Bay-0 and Shahdara), is obvious for all traits. On average, 50% of the lines participate in the transgression (in one or the other direction), whereas the other 50% have intermediate phenotypes. DM10 and NO10 both show an unbalanced transgression, with only a few lines (approximately 30–40) exceeding, respectively, Bay-0 plant weight and Shahdara nitrate content. AA3 represents, by far, the strongest transgressive segregation observed in this work: The parental phenotypes are nearly the same (39 nmol mg−1), and lines can be found with phenotypes as extreme as 30 or 115 nmol mg−1. The population mean of most traits lies between the parental values, except for AA3.
Figure 2.
Histograms of repartition of the phenotypic values in the Bay-0 × Shahdara population. For trait meanings, refer to Table I. B and S, Values obtained for parental accessions Bay-0 and Shahdara, respectively. The position of the vertical line above bars indicates the population mean value.
Phenotypic correlations among the traits and across N environments are presented in Table II. The strongest correlations are found in both environments between NP and one of its components, nitrate in the N+ environment (NP10 and NO10: + 0.74) and free amino acids in the N− environment (NP3 and AA3: + 0.84). Moreover, there is no significant correlation between nitrate and free-amino acid contents in the N+ environment. Another interesting positive correlation, although less strong, exists between DM10 and DM3 (+0.53). DM and NP are linked by a moderate negative correlation in both N environments (see Table II). The last three correlations will be analyzed in detail with Figures 3 and 4.
Table II.
Phenotypic correlations among traits
| N− N+ | DM | NP | NO | AA |
|---|---|---|---|---|
| DM | +0.53*** | −0.27*** | n.a. | −0.33*** |
| NP | −0.34*** | +0.12*** | n.a. | +0.84*** |
| NO | −0.23*** | +0.74*** | n.a. | n.a. |
| AA | NS | +0.10* | NS | +0.22*** |
***, Significant at the 0.1% level. *, Significant at the 5% level. NS, Not significant. n.a., Data not available. Values below the diagonal correspond to N+ environment correlations; values above the diagonal correspond to N− environment correlations; values on the diagonal correspond to across-N environment correlations. Explained variables for N+ and N− correlations are in column and line, respectively.
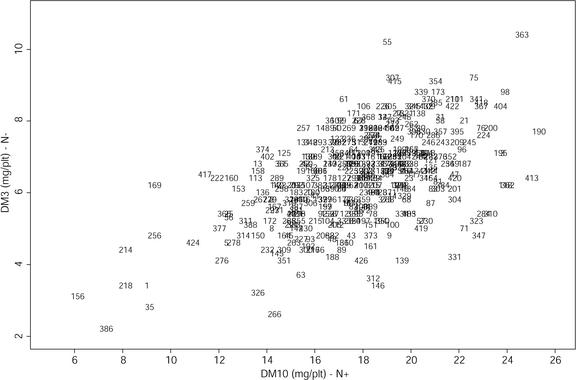
Figure 3.
Variation for shoot DM in both N environments in the Bay-0 × Shahdara population. Each one of the 415 RIL is represented by its number.
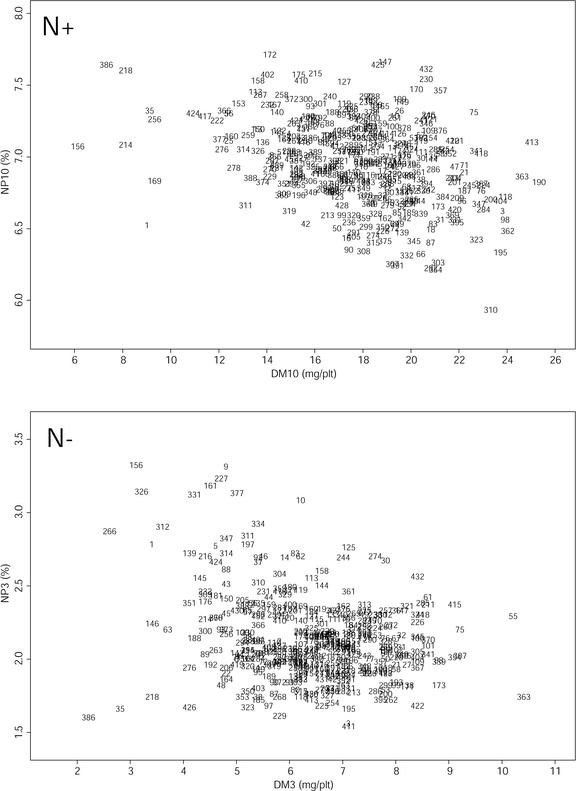
Figure 4.
Relation between shoot DM and total NP in the Bay-0 × Shahdara population. The upper graph corresponds to N+ environment, the lower graph corresponds to N− environment. Each one of the 415 RIL is represented by its number.
Figure 3 illustrates the variation of reaction to N stress in the Bay-0 × Shahdara population, as can be inferred from the relative growth variability in N+ and N− environments. The range of variation in each N environment is wide: 35 d after sowing, the largest plants are more than four times heavier than the smallest ones. Globally, small lines in the N+ environment are small in the N− environment, and large lines in the N+ environment are also large in the N− environment, although there are some exceptions. Despite this correlation, a large part of the variation is still explained by some genotype-specific N stress reactions: Lines showing a mean growth in one environment cover the whole range of variation in the other environment. Figure 4 illustrates the negative correlation between growth and NP in each specific environment. However, in the Bay-0 × Shahdara population, a large variation of behavior is observed: Medium-size lines in the N+ environment can contain from 6.2% to 7.6% of N in the DM, and medium-size lines in the N− environment can contain from 1.5% to 3.0% of N in the DM. In the N+ environment, nitrate represents, on average, 40% of the total N content (data not shown), whereas it is almost zero in the N− environment. On average, free amino acids represent only 5% of the total N content in both environments (data not shown).
QTL Mapping
QTL mapping results are presented in Table III. QTL names are constructed using the trait name suffixed with an ordering number from the first chromosome. We found from four (DM3) to nine (AA10) QTL per trait and from zero to five (AA3) QTL × QTL epistatic interactions per trait. Individual QTL explain between 2% and 21% of the total phenotypic variation (R2) of the given trait, with only five QTL showing an R2 higher than 10%. For each of the seven traits, we mapped both positive and negative allelic effect QTL. A total of 48 QTL were found in this study. Table IV presents the estimated size of the confidence intervals obtained for each R2 class by both one-log of the odds (LOD) and bootstrap methods. One-LOD intervals represent anticonservative (generally too small) confidence intervals, as already shown in simulations (Visscher et al., 1996). Confidence intervals calculated by bootstrap method inversely seem to be highly conservative, especially when the contribution of the QTL is weak (Visscher et al., 1996; Walling et al., 1998). We will build hypotheses concerning the possible colocalization of QTL for different traits by studying the overlapping of one-LOD intervals.
Table III.
Results of QTL analyses for N traits in the Bay-0 × Shahdara population
| QTLa | Chromosome-Markerb | Positionc | LOD Score | Rd | 2ae | QTL × Ef |
|---|---|---|---|---|---|---|
| DM10.1 | Chrom 1-MSAT1.10 | 23.4 | 5.7 | 7 | +1.52 | S*** |
| DM10.2 | Chrom 1-T27K12 | 36.4 | 9.0 | 7 | +2.06 | S** |
| DM10.3 | Chrom 2-MSAT2.38 | 12.0 | 6.2 | 3 | −1.48 | NS |
| DM10.4 | Chrom 2-MSAT2.41 | 34.5 | 5.9 | 3 | +1.42 | NS |
| DM10.5 | Chrom 2-MSAT2.22 | 59.6 | 4.7 | 2 | −1.20 | S* |
| DM10.6 | Chrom 4-NGA8 | 19.0 | 7.9 | 4 | −1.52 | NS |
| DM10.7 | Chrom 5-NGA225 | 0.1 | 4.0 | 4 | +1.02 | S* |
| DM10.8 | Chrom 5-MSAT5.9 | 49.1 | 13.2 | 10 | +1.90 | S*** |
| DM10.7 × DM10.8 | 3 | |||||
| DM10 complete model | 43% | |||||
| DM3.1 | Chrom 1-NGA128 | 49.6 | 5.1 | 3 | +0.64 | S* |
| DM3.2 | Chrom 3-NGA172 | 0.1 | 2.6 | 4 | +0.40 | NS |
| DM3.3 | Chrom 3-MSAT3.21 | 49.5 | 2.4 | 4 | +0.38 | NS |
| DM3.4 | Chrom 4-NGA8 | 20.9 | 6.0 | 5 | −0.66 | NS |
| DM3 complete model | 16% | |||||
| NP10.1 | Chrom 1-NGA248 | 22.9 | 3.0 | 2 | −0.10 | NS |
| NP10.2 | Chrom 2-MSAT2.36 | 30.0 | 6.6 | 7 | −0.13 | S** |
| NP10.3 | Chrom 3-NGA172 | 2.2 | 27.2 | 21 | −0.29 | S** |
| NP10.4 | Chrom 3-MSAT3.18 | 62.6 | 3.6 | 2 | +0.11 | S*** |
| NP10.5 | Chrom 4-MSAT4.8 | 4.6 | 3.8 | 2 | +0.11 | NS |
| NP10.6 | Chrom 5-NGA249 | 2.6 | 6.8 | 8 | −0.14 | S* |
| NP10.7 | Chrom 5-MSAT5.9 | 52.5 | 3.1 | 2 | −0.10 | S** |
| NP10 complete model | 44% | |||||
| NP3.1 | Chrom 2-MSAT2.38 | 13.8 | 11.5 | 9 | +0.22 | S*** |
| NP3.2 | Chrom 2-MSAT2.41 | 34.5 | 2.8 | 2 | −0.11 | S** |
| NP3.3 | Chrom 2-MSAT2.10 | 53.2 | 4.0 | 2 | +0.14 | NS |
| NP3.4 | Chrom 3-NGA172 | 0.1 | 2.4 | 2 | −0.08 | S** |
| NP3.5 | Chrom 3-MSAT3.21 | 48.9 | 9.6 | 9 | −0.17 | S*** |
| NP3.6 | Chrom 4-NGA8 | 10.3 | 4.5 | 7 | +0.15 | NS |
| NP3.7 | Chrom 4-MSAT4.15 | 28.8 | 6.6 | 3 | +0.16 | S** |
| NP3.1 × NP3.5 | 4 | |||||
| NP3.1 × NP3.6 | 2 | |||||
| NP3 complete model | 40% | |||||
| NO10.1 | Chrom 1-NGA248 | 38.0 | 7.5 | 6 | −130 | n.a. |
| NO10.2 | Chrom 1-NGA128 | 49.1 | 9.4 | 7 | −166 | n.a. |
| NO10.3 | Chrom 1-MSAT1.13 | 73.6 | 5.9 | 4 | +102 | n.a. |
| NO10.4 | Chrom 2-MSAT2.38 | 22.6 | 4.5 | 6 | −112 | n.a. |
| NO10.5 | Chrom 2-MSAT2.41 | 29.5 | 6.7 | 5 | −116 | n.a. |
| NO10.6 | Chrom 3-NGA172 | 1.9 | 9.9 | 5 | −124 | n.a. |
| NO10.7 | Chrom 4-MSAT4.15 | 38.2 | 3.7 | 3 | −72 | n.a. |
| NO10.8 | Chrom 5-NGA249 | 3.1 | 4.6 | 2 | −60 | n.a. |
| NO10 complete model | 38% | |||||
| AA10.1 | Chrom 1-MSAT1.10 | 15.4 | 3.1 | 3 | −4.0 | NS |
| AA10.2 | Chrom 1-T27K12 | 45.7 | 24.9 | 20 | +13.2 | S*** |
| AA10.3 | Chrom 1-MSAT1.5 | 83.7 | 15.5 | 8 | +9.2 | S* |
| AA10.4 | Chrom 2-MSAT2.22 | 62.5 | 2.6 | 2 | −3.6 | S* |
| AA10.5 | Chrom 3-ATHCHIB2 | 5.1 | 14.9 | 13 | −9.0 | NS |
| AA10.6 | Chrom 3-MSAT3.18 | 65.8 | 2.5 | 2 | +3.4 | S*** |
| AA10.7 | Chrom 4-MSAT4.39 | 0.1 | 3.5 | 2 | −4.4 | S* |
| AA10.8 | Chrom 4-MSAT4.9 | 55.7 | 4.4 | 3 | +4.8 | NS |
| AA10.9 | Chrom 5-NGA249 | 3.1 | 2.5 | 2 | +3.4 | NS |
| AA10.1 × AA10.5 | 2 | |||||
| AA10 complete model | 57% | |||||
| AA3.1 | Chrom 1-MSAT1.5 | 76.1 | 2.7 | 2 | +3.6 | S* |
| AA3.2 | Chrom 2-MSAT2.38 | 13.7 | 13.0 | 11 | +7.8 | S*** |
| AA3.3 | Chrom 3-NGA172 | 0.1 | 2.7 | 3 | −3.2 | NS |
| AA3.4 | Chrom 3-MSAT3.21 | 49.0 | 12.8 | 14 | −7.4 | S*** |
| AA3.5 | Chrom 4-MSAT4.35 | 28.2 | 4.2 | 4 | +4.4 | NS |
| AA3.1 × AA3.4 | 3 | |||||
| AA3.2 × AA3.3 | 2 | |||||
| AA3.2 × AA3.4 | 6 | |||||
| AA3.3 × AA3.4 | 2 | |||||
| AA3.5 × AA3.4 | 4 | |||||
| AA3 complete model | 51% |
S***, Significant at the 0.1% level. S**, Significant at the 1% level. S*, Significant at the 5% level. NS, Not significant. n.a., Data not available.
The name given to a local LOD score peak contains the trait name suffixed with an order number.
The corresponding marker is the one used in composite interval mapping (CIM) model 6 and in ANOVA analysis.
The position of the QTL is expressed in cM from the first marker of the chromosome.
Percentage of variance explained by the QTL or by QTL × QTL interaction, when significant.
2a represents the mean effect (in trait unit, see Table I) of the replacement of the Shahdara allele by the Bay-0 allele at the QTL. By convention, the effect of the Bay-0 allele relative to the Shahdara allele at each locus then represents the sign of the allelic effect (positive or negative effect QTL).
QTL × Environment (N) interaction tested by ANOVA.
Table IV.
Confidence interval size estimated by both one-LOD and bootstrap methods
Mean support interval length, when constructed from one-LOD fall method.
Confidence interval length, when estimated from bootstrap simulation analysis.
Shoot dry matter in non-limiting N conditions (DM10) revealed eight significant QTL, distributed on all chromosomes, except chromosome 3. Most of them are small-effect QTL, except DM10.1 and DM10.2 on chromosome 1 and DM10.8 on the bottom of chromosome 5, which also shares an epistatic interaction with DM10.7, located on the same chromosome. These three medium-effect QTL have an interesting common feature: Bay-0 always carries the allele with a positive effect on DM10 (Table III; by convention, the effect of the Bay-0 allele relative to the Shahdara allele at each locus will represent the sign of the “QTL effect”). Fewer QTL were detected for DM3 (4 QTL) than for DM10. Their phenotypic contribution is quite small (between 3% and 5%), but this has to be balanced against the heritability of the trait (20%). Again, most of these QTL have positive allelic effects, except DM3.4. Only DM10.6 and DM3.4 seem to be potentially common to both N environments: Their most probable positions fall very close to each other (less than 2 centiMorgans apart), and the sign of the allelic effects are both negative. The concerned locus does not interact with N environment (Table III; tested by ANOVA, through the neighboring marker NGA8). All other QTL appear to be specific to one N environment or the other.
Total N percentage in the N+ environment is mostly controlled by one QTL (NP10.3), explaining more than 20% of the total phenotypic variation (Table III). The allelic effect of this major QTL is negative (the Bay-0 allele causes a 0.29-point fall of NP10 in comparison with the Shahdara allele). Only two small-effect QTL have positive allelic effects (NP10.4 and NP10.5) among the seven detected QTL. An equal number of QTL (seven) was detected in limiting N conditions (NP3). NP3.1 and NP3.5 both explain 9% of the total variation and have opposite allelic effect signs (respectively, positive and negative). Moreover, these QTL are linked by an epistatic interaction explaining 4% of the total variation. Three loci could potentially affect NP trait in both N conditions: NP10.2 and NP3.2, NP10.3 and NP3.4, and NP10.5 and NP3.6 could each correspond to a unique locus on chromosomes 2, 3, and 5, with negative, negative, and positive allelic effects, respectively. However, the first two loci show a highly significant interaction with N environment (Table III). All other QTL, and remarkably NP3.1 and NP3.5, appear specific to one N environment.
Nitrate content in non-limiting N conditions is controlled by at least eight QTL, which show no detectable epistatic interactions. All QTL but one (NO10.3) show a negative allelic effect, up to 166 nmol nitrate mg−1 shoot tissue. The phenotypic contributions of these QTL range from 2% to 7% (R2). Despite the use of composite interval mapping (CIM) analysis, the complexity of the QTL pattern on chromosome 1 certainly biased the estimations (especially R2 and allelic effects) on this chromosome.
Free-amino acid content is controlled by nine QTL in N+ conditions and five in N− conditions. Most of the N+ QTL have weak effects (<3%), except AA10.2 and AA10.5, which explain 20% and 13%, respectively, of the total variation, with an opposite allelic effect sign (Table III). The Bay-0 allele at AA10.2, when compared with the Shahdara allele, is responsible for a 13.2 nmol mg−1 increase of AA10, on the average. A unique epistatic interaction is significant in the N+ environment, between two negative-effect QTL (AA10.1 and AA10.5). The genetic decomposition of AA3 variation is very interesting: Five QTL are found, with either positive or negative effects. Two QTL essentially control amino acid content in these conditions: AA3.2 and AA3.4 have opposite effect signs and explain 11% and 14%, respectively, of the total variation. Moreover, they are linked by an epistatic interaction explaining 6% of the total variation. AA3.4 interacts also with all other QTL detected in the same conditions: As much as 15% of the total variance is explained by epistatic interactions involving AA3.4 (Table III). AA10.3 and AA3.1 colocalize to the same region and share the same allelic effect sign; however, if they correspond to the same locus, its effect is interacting with the N environment (Table III). AA10.5 and AA3.3 also potentially share the same genetic basis, with a negative effect on amino acid content in both N environments (no significant QTL × N interaction; Table III). The other QTL, particularly AA10.2, AA3.2, and AA3.4, are effective only in one N environment.
DISCUSSION
QTL mapping has been rarely used in Arabidopsis to dissect the genetic architecture of physiological traits and metabolic pathways (Mitchell-Olds and Pedersen, 1998; Bentsink et al., 2000; Kliebenstein et al., 2001). This study involves a new and large set of RIL dedicated to quantitative genetic studies, the Bay-0 × Shahdara population. The experimental display that we designed allowed us to measure different traits in interaction with N availability on 415 RIL grown in controlled conditions. The genetic variation described here is very large for each of the seven traits. The phenotypic description of this material identifies multiple and original sources of physiological variation, such as those leading to the variations of shoot DM and NP (see Fig. 4), which are particularly interesting for whole-plant physiologists (Lemaire and Gastal, 1997; Lawlor et al., 2001). The impact of N limitation is strong on all traits and leads, on the average, to a 65% decrease of DM in N− compared with N+. But the N stress effect is not equivalent for all lines, and the DM decrease varies from 33% to 84% among the RIL (illustrated on Fig. 3). This is expressed by the highly significant genotype × N interactions for all traits.
Global QTL Features
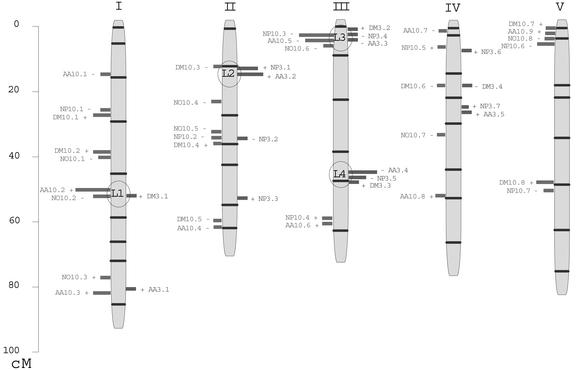
A summary of the QTL found for all traits together is given in Figure 5. The genetic dissection of these traits is very informative: For five of seven traits, we were able to distinctly map a large number of QTL (≥7). In Arabidopsis, for most of the studied traits, between two and four QTL are usually detected, even though a larger number of QTL has sometimes been revealed for highly heritable traits (Alonso-Blanco et al., 1998, 1999; Juenger et al., 2000). Epistatic interactions between our QTL seem to play an important role in the control of the phenotypic values, especially for AA3 and NP3. The interaction between AA3.4 and all other AA3 loci is original and unprecedented in Arabidopsis QTL analyses. The percentage of genetic variance identified through QTL and epistatic interactions between QTL (calculated as: complete model R2 sum from Table III/heritability from Table I) varies between 73% and 98% depending on the trait (data not shown). NP3 and DM3 are less “well dissected” than the other traits, possibly because a number of small non-detectable QTL are influencing them (DM3) and/or because of underestimation of the individual QTL contribution (NP3). The systematic presence of positive- and negative-effect QTL provides a genetic basis for the transgression observed through Figure 2. In some cases (NP3 and AA3), the unbalanced transgression can be explained (data not shown) by the effect of strong QTL × QTL interactions. NO10 also shows an unbalanced transgression that we cannot simply explain genetically (one positive-effect QTL but no epistatic interactions). Then structural and/or osmotic constraints could be proposed to explain why very few lines show more than 2,500 nmol nitrate mg−1 DM.
Figure 5.
Summary of the QTL detected for N traits in the Bay-0 × Shahdara population. Each QTL is represented by a bar located at its most probable position (or nearby). QTL on the left-side of the chromosomes are those detected in the N+ environment; QTL on the right-side of the chromosomes are those detected in the N− environment. The length of the bar is proportional to the QTL contribution (R2). The sign of the allelic effect is indicated for each QTL. The framework genetic map (indicating markers position) is from Loudet et al. (2002).
QTL Stability across N Environments
The significant correlation among traits measured in both N environments (Table II) is genetically explained by the finding of potential common genetic factors influencing these traits in N+ and N− conditions (Fig. 5). Surprisingly, the most N+/N− correlated trait (DM) shows only one potential common genetic factor (DM10.6/DM3.4), corresponding to the unique negative-effect QTL detected in N− conditions. NP10/NP3 and AA10/AA3 correlations potentially rely on more common loci (3 and 2, respectively), but most of them interact with N environment. Finally, a great part of the variation is controlled by factors specifically expressed in one or the other N conditions and/or interacting with N environment (Table III). We postulate that the loci that are not stable across N environments reflect adaptation to this constraint and are certainly more likely directly linked to N metabolism. Nitrate-dependent genes, for example, could correspond to these loci; they may represent new examples of nitrate-regulated transcription factors, like ANR1 (Zhang and Forde, 1998), involved in nitrate sensing during lateral root development. Our postulate is parallel to the method used in previous QTL analyses to identify the position in a pathway of QTL detected in specific environments and QTL detected in all environments (for flowering time pathway, see Alonso-Blanco et al., 1998; for light signaling pathway, see Borevitz et al., 2002). Otherwise, it is noteworthy that Rauh et al. (2002) identified aerial mass QTL in the Ler/Columbia population in regions that could correspond to our major loci named L2 and L3 on Figure 5. These QTL were detected in experiments involving ammonium-fed plants.
QTL Involved in Different Traits
One of the major issues with this approach is derived from the interpretation of the colocalization of QTL from different traits. We point out that QTL colocalization can be theoretically explained in different ways (Lebreton et al., 1995), essentially linkage (two different closely linked genes influence two different traits independently) and pleiotropy (the same genetic factor controls both traits). Figure 5 clearly brings out the numerous colocalizations of QTL corresponding to two to six different traits from both N environments.
Growth and N Content
One particular feature that stands out concerns the links between DM and NP in each N environment. Numerous colocalizations between DM and NP QTL can be found, with systematic opposite effect signs, such as DM10.1/NP10.1 (chromosome 1), DM10.4/NP10.2 (chromosome 2), DM10.7/NP10.6, and DM10.8/NP10.7 (chromosome 5) in the N+ environment or DM3.2/NP3.4 and DM3.3/NP3.5 (chromosome 3) in the N− environment. The stability of allelic effects for this type of locus is remarkable: In all cases, Bay-0 carries the favorable (positive) allele for DM QTL and the negative one for NP QTL. These loci could account for the negative correlation found between DM and NP and illustrated on Figure 4. This link between the two traits is classically described in most species when the dynamics of N accumulation in the shoot is studied over a certain period of time (Greenwood et al., 1990; Justes et al., 1994; Plénet and Lemaire, 2000) and is referred to as “N dilution.” The origin of this phenomenon is to be found in the modification of the equilibrium between different tissues (metabolic and structural) with the increase of plant size (Lemaire and Gastal, 1997). In our conditions, we have verified that Arabidopsis was also subjected to N dilution during the vegetative stage (O. Loudet, unpublished data). One possible genetic origin of some DM/NP QTL could then be a genetic factor affecting plant development (rhythm of growth and development, shoot architecture, etc.) and having pleiotropic consequences on both traits. These loci, however, are not stable across N environments, which could lead to the conclusion that the genetic factors controlling plant development are specific to one N environment. As an alternative, some of these loci (particularly those from limiting N conditions) do correspond to a variation in N use efficiency: Concerning the locus on the top of chromosome 3 (named L3 on Fig. 5), for example, if all of these QTL reveal the same gene, the variation in N content exists in both N environments and has consequences on growth only when N availability is limited.
N Content Explained by Nitrate Pool in the N+ Environment
The relative fluctuations of the different N pools can be analyzed through the colocalization of the respective QTL, together with NP QTL. As expected in the N+ environment, we find several colocalizations between NP and NO QTL: NP10.2/NO10.5, NP10.3/NO10.6, and NP10.6/NO10.8 always share a negative allelic effect. If this colocalization is confirmed, for example, for the major NP QTL (NP10.3), then a large part of total N content variation could be genetically explained by variations in nitrate content, which is one of the major components of stored N (see “Results”; Scheible et al., 1997; van der Leij et al., 1998; Hirel et al., 2001). However, if we translate the allelic effect of NO10.6 in terms of total N effect (14 g N mol−1 nitrate), it explains only 60% of the NP10.3 allelic effect. We can speculate that the amino acid QTL AA10.5 has the same genetic origin as those QTL (the L3 locus), but its contribution represents only 10% of total N variation (given a mean of 23 g N mol−1 amino acids). Other N compounds such as soluble proteins are bound to participate in this N content decrease. This is not in contradiction with the signaling role attributed to nitrate in plants (Stitt, 1999) that could drive other N compounds variations, but the dynamic nature and compartmentation of the metabolism make it difficult to isolate the primary change (Scheible et al., 1997). The identification of the gene(s) explaining this locus (L3 on Fig. 5) would give very interesting information on the origin of this variation (regulation of the global N content and of the nitrate vacuolar storage) and would distinguish between real N use efficiency and storage capacity variation.
Nitrate and Amino Acid Pools in the N+ Environment
Nitrate and free-amino acid overlapping QTL mostly indicate variations in the same direction for these traits. However, an original relation between NO10 and AA10 seems to be revealed by L1 locus (QTL NO10.2 and AA10.2; Fig. 5). These QTL have opposite allelic effects, even though not balanced in term of total N. This locus represents a good candidate to learn more about the regulation of flux and equilibrium between different N compounds (particularly reduced and nonreduced) in the shoot, certainly involving regulations of key enzymes in N metabolism (NR, NiR, GS, and GOGAT). Interestingly, transcriptional and/or posttranscriptional deregulation of NR gene in wild tobacco (Nicotiana plumbaginifolia) leads to similar opposite variations of nitrate and free-amino acid pools without any consequences on total N content (Quilleré et al., 1994; Nussaume et al., 1995). Otherwise, amino acid variation could regulate NO through nitrate uptake (Stitt, 1999).
N Content Explained by Amino Acid Pool in the N− Environment
When N is limiting growth, we find at least four loci potentially explaining the correlation between NP3 and AA3, most of them specific to the N− environment: NP3.1/AA3.2 (positive effect, L2 locus), NP3.4/AA3.3 (negative effect, L3 locus), NP3.5/AA3.4 (negative effect, L4 locus), and NP3.7/AA3.5 (positive effect). If AA3 variation only explains on average 10% of NP3 variation, AA3.2 × AA3.4 and NP3.1 × NP3.5 parallel epistatic interactions constitute a strong element confirming these colocalizations (Table III). Both negative-effect loci on NP3 and AA3 (L3 and L4 on Fig. 5) seem to have positive consequences on growth (DM3.2 and DM3.3 QTL), but we cannot easily determine the origin of these variations (change in N use during growth or change in growth through carbon metabolism). Nevertheless, L2 and L4 effects on N or N compounds are specific to limiting N conditions, which is an element indicating that the source of the variation is probably to be found in N metabolism itself. Finally, the study of amino acid variations in this material reveals numerous implications of free-amino acid content in the metabolic regulations. Whether amino acids act as a player or a witness remains to be determined, but their role is certainly worth elucidating. It has already been hypothesized that the first steps of ammonia integration into amino acids (and the amino acid pool itself) could concentrate strong limitations and metabolic integrations (Lam et al., 1995; Fuentes et al., 2001; Hirel and Lea, 2001). The recent finding of putative Glu sensors in plants (Lam et al., 1998) supports this idea.
CONCLUSIONS
In this study, we have been able to identify a large number of QTL, representing potentially at least 18 genes that are polymorphic between Bay-0 and Shahdara. CIM combined with a large number of RIL proved to be very efficient to understand intricate multi-QTL situations (for example, several linked opposite effect QTL). Multiple-trait methods could certainly help increase the accuracy of mapping of correlated traits and would provide more information to test pleiotropy versus linkage (Jiang and Zeng, 1995). Our work clearly points out how QTL analysis can enhance the physiological study of a variation by isolating the effect of genetic factors individually controlling the traits. Finally, four loci (Fig. 5, L1–L4) are identified as sources of considerable variation in one or both N environments. Each one represents a specific pattern of variation of several traits, as discussed above. Moreover, the positional cloning of the genes underlying these loci is possible, especially using NP and amino acid traits, which are highly heritable.
Candidate (structural) genes from N metabolism can be assigned to some of these loci (AAP5, an amino acid transporter, and GLN1.2, a gene coding for cytosolic GS, colocalize with L1 locus; NRT2.6, a putative high-affinity nitrate transporter, colocalizes with L4 locus), but we still lack precision in the estimation of the QTL positions to elaborate a hypothesis based on these candidate genes. However, it is interesting to note that a recent QTL study in maize also identified coincidences between QTL for leaf nitrate content and cytosolic GS genes (Hirel et al., 2001). More excitingly, some of our QTL (particularly loci L2 and L3) do not colocalize with known N metabolism genes. This will certainly lead us to the discovery of new genes involved in the regulation of these traits. The construction of near isogenic lines for each of these loci is under way in our laboratory as a necessary step for the achievement of fine mapping (Alonso-Blanco and Koornneef, 2000). Taking advantage of the residual heterozygosity in F6 plants, we follow the method of the heterogeneous inbred family (Tuinstra et al., 1997). The cloning of the gene then uses conventional positional cloning techniques (Lukowitz et al., 2000; Yano, 2001), as was already performed in tomato (Lycopersicon esculentum; Frary et al., 2000) or rice (Yano et al., 2000). Whether the identified genes are directly involved in N metabolism regulation or not, the functional analysis of their interaction with N availability will shed a new light on whole-plant N physiology. The Bay-0 × Shahdara population will also be used profitably to study other N stresses and environments and other traits linked to N metabolism and to analyze their relations with already known genetic variation. Moreover, an equivalent study performed on other RIL populations would possibly provide information on other new genetic regulations involved.
MATERIALS AND METHODS
Plant Material
The material used in this study has been developed in our laboratory and deposited in public Arabidopsis stock centers. The Bay-0 × Shahdara RIL population has been fully described in a recent publication (Loudet et al., 2002) and at http://www.inra.fr/qtlat. For this study, we used F7 seeds obtained from the last generation of single-seed descent for the 415 lines. These seeds were obtained in homogeneous conditions for all lines, thus minimizing the maternal environment effect.
Phenotyping Display
The production of homogeneous vegetative plant material for the 415 lines was performed in controlled conditions (growth chamber). The whole set of RIL was cultivated in each experiment (cultivation repetition) in two N environments. The experimental unit was a small pot (length = 60 mm, width = 65 mm, height = 60 mm) containing six plants positioned on a circle. With only one repetition per RIL (one pot, i.e. six plants) and 17 connecting controls (Bay-0 and Shahdara repetitions), the whole population studied in one N environment represented 432 experimental units, organized in 18 blocks of 24 pots. The RIL were completely and independently randomized in each cultivation repetition (performed successively in the same growth chamber). The blocks were rotated every other day, following a scheme that allows each block to move all around the growth chamber. Two N environments were compared in this study: The first one (N+) did not limit plant growth at any stage during our experiment, and the second one (N−) strongly limited growth (for details, see watering solutions below). The data from three cultivation repetitions of N+ environment and two repetitions of N− environment have been collected and analyzed in detail.
Growth Conditions
Pots were carefully filled with a homogeneous nonenriched compost composed of blond and brown peats (1:1) sifted at 2 to 3 mm (Basis Substrat II, Stender GmbH, Schermbeck, Germany). The pH of this compost was stabilized between 5.5 and 5.9, and it contained only very small amounts of nitrate (< 0.5 mm in the soil solution). Every other day, the pots were watered (by immersion of the base of the pots) in a solution containing either 10 mm (N+) or 3 mm (N−) nitrate. Phosphate and sulfate were present in both solutions at the same concentration (0.25 mm), as well as magnesium (0.25 mm) and sodium ions (0.20 mm). The difference between N+ and N− solutions concerned only potassium (5.25 and 2.75 mm, respectively, in N+ and N− solutions), calcium (2.50 and 0.50 mm, respectively) and chloride ions (0.20 and 0.70 mm, respectively), but all of these concentrations were supra-optimal for plant growth. The pH of the watering solutions remained between 5.1 and 5.5.
The seeds were stratified for 48 h in 0.1% (w/v) agar solution (in water) at 4°C in the dark. Then, six positions on a circle were determined in each pot and received approximately seven seeds per position, from the same RIL (with a pipetor, the distribution of a small volume of the stratification solution ensured the sowing of a steady number of seeds at each position). Homogeneous germination occurred 2 d after sowing. Six days after sowing, only one seedling per position was retained while the others were removed, resulting in six homogeneous seedlings per pot. The plants were maintained in short days during all of the culture with a photoperiod of 8 h. The day and night temperatures were regulated at 21°C and 17°C, respectively. The hygrometry fluctuated between 65% during the day and 90% during the night. Light was provided by 20 mercury-vapor bulbs, ensuring a photosynthetic photon flux density of approximately 160 μmol m−2 s−1. Plants (shoot) were harvested 35 d after sowing.
Measured Traits
The six plants harvested for each RIL were pooled for one cultivation repetition and one N environment and freeze-dried for 72 h. Shoot DM per plant was then estimated as the mean DM of these plants (in milligrams per plant). This dry material was finely ground in a vibrator using steel beads. NP was determined for an aliquot of this powder (5–7 mg) after weighing and analysis for total N content using the Dumas method on an NA 1500CN Fisons Instrument (Thermoquest, Runcorn, Cheshire, UK) analyzer. Another aliquot of the powder (between 8 and 10 mg) was weighed and extracted with a two-step ethanol-water procedure conducted in a 96-deep well plate. The first step consisted in a 25-min extraction at 80°C using 500 μL of 80% (v/v) ethanol, whereas the second step completed the extraction by using 500 μL of water at 80°C for 20 min. These extracts were diluted before analyzing nitrate concentration by HPLC on a DX-120 (Dionex, Sunnyvale, CA) for determination of the nitrate content (NO) in nanomoles per milligram of DM. The same extracts were also subjected to a Rosen (1957) evaluation of free-amino acid concentration conducted in a 96-deep well plate. We used this result to calculate the free-amino acid content in nanomoles per milligram of DM. Table I summarizes the traits measured.
Statistical Analysis and QTL Mapping
The complete set of data from each environment was involved in an analysis of variance (ANOVA) to determine the specific effects of genotype (i.e. the RIL) and repetition (i.e. the cultivation repetition) factors. This ANOVA allowed the quantification of the broad-sense heritability (genetic variance/total phenotypic variance). The genotype × repetition interaction could only be tested using grouped N+ and N− data (corresponding to common cultivation repetitions) in the same analysis. Using the same set of data, we performed a two-factor ANOVA to determine the significance of the N environment effect and the genotype × N interaction. Subsequent analyses involved unadjusted mean values from the different repetitions in each N environment. Phenotypic correlations were calculated for all combinations of traits in each N environment and across N environments for each trait. ANOVA and correlation estimations were performed using aov() and lm() functions of S-PLUS 3.4 statistical package (Statistical Sciences, Inc., Seattle).
The original set of markers (38 microsatellite markers) and the genetic map obtained with MAPMAKER 3.0, as previously described (Loudet et al., 2002), were used in this study. All QTL analyses were performed using the Unix version of QTL Cartographer (v1.14; Basten et al., 1994, 2000). We mostly used classical methods as previously described (Loudet et al., 2002), successively, interval mapping and CIM. First, interval mapping (Lander and Botstein, 1989) was used to determine putative QTL involved in the variation of the trait. CIM model 6 of QTL Cartographer (v1.14; Basten et al., 2000) was then performed on the same data: The closest marker to each local LOD score peak (putative QTL) was used as a cofactor to control the genetic background while testing at a position of the genome. When a cofactor was also a flanking marker of the tested region, it was excluded from the model. The number of cofactors involved in our models varied between 4 and 7. The walking speed chosen for all QTL analysis was 0.1 centiMorgan. The LOD significance threshold (2.3 LOD) was estimated from several permutation test analyses, as suggested by Churchill and Doerge (1994). One thousand permutations of phenotypic data were analyzed using the CIM model with the specific conditions described above for each trait and the maximum “experimentwise threshold” obtained (overall error level, 5%) was used for all traits.
Additive effects (Table III, 2a) of detected QTL were estimated from CIM results; 2a represents the mean effect of the replacement of the Shahdara allele by the Bay-0 allele at the studied locus. The contribution of each identified QTL to the total variance (R2) was estimated by variance component analysis. For each trait, the model involved the genotype at the closest marker to the corresponding detected QTL as random factors in ANOVA. Only homozygous genotypes were included in the ANOVA analysis. Significant QTL × QTL interactions were also added to the linear model via the corresponding marker × marker interactions, and their contribution to the total variance was also estimated. QTL × N environment interaction was assessed by a two-factor ANOVA, with the corresponding marker genotype and N environment as classifying factors. One-LOD support interval of the detected QTL gives information regarding the precision of the estimated position (Lander and Botstein, 1989). We analyzed the LOD score profile of almost 50 QTL and estimated a mean one-LOD support interval for each R2 class. Because they seem to represent anticonservative (generally too small) confidence intervals, we also estimated confidence intervals from a bootstrap simulation study as proposed by Visscher et al. (1996). Ten series of 1,000 resampling data sets were analyzed for each R2 class.
ACKNOWLEDGMENTS
We thank François Gosse for taking care of the plants; Roger Voisin and Jean-Paul Saint-Drenant for keeping the growth chamber operational; all of the Nutrition Azotée des Plantes laboratory for essential help during the huge harvests; Chris Basten for his kind help with QTL Cartographer; and Anne Krapp, Hoai-Nam Truong, Christian Meyer, and Jon Werner for careful reading of the manuscript.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.010785.
LITERATURE CITED
- Agrama HAS, Zakaria AG, Said FB, Tuinstra MR. Identification of quantitative trait loci for nitrogen use efficiency in maize. Mol Breed. 1999;5:187–195. [Google Scholar]
- Alonso-Blanco C, Blankestijn-de Vries H, Hanhart CJ, Koornneef M. Natural allelic variation at seed size loci in relation to other life history traits of Arabidopsis thaliana. Proc Natl Acad Sci USA. 1999;96:4710–4717. doi: 10.1073/pnas.96.8.4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Blanco C, El-Assal SED, Coupland G, Koornneef M. Analysis of natural allelic variation at flowering time loci in the Landsberg erecta and Cape Verde islands ecotypes of Arabidopsis thaliana. Genetics. 1998;149:749–764. doi: 10.1093/genetics/149.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Blanco C, Koornneef M. Naturally occurring variation in Arabidopsis: an underexploited resource for plant genetics. Trends Plant Sci. 2000;5:22–29. doi: 10.1016/s1360-1385(99)01510-1. [DOI] [PubMed] [Google Scholar]
- Bacon PE. Nitrogen fertilization in the environment. New York: Marcel Dekker; 1995. [Google Scholar]
- Basten CJ, Weir BS, Zeng Z-B. Zmap: a QTL cartographer. In: Smith C, Gavora JS, Burnside EB, editors. 5th World Congress on Genetics Applied to Livestock Production: Computing Strategies and Software. Ontario, Canada: Guelph; 1994. pp. 65–66. [Google Scholar]
- Basten CJ, Weir BS, Zeng Z-B. QTL Cartographer, Version 1.14. Raleigh: North Carolina State University; 2000. [Google Scholar]
- Bentsink L, Alonso-Blanco C, Vreugdenhil D, Tesnier K, Groot SP, Koornneef M. Genetic analysis of seed-soluble oligosaccharides in relation to seed storability of Arabidopsis. Plant Physiol. 2000;124:1595–1604. doi: 10.1104/pp.124.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin P, Gallais A. Physiological and genetic basis of nitrogen use efficiency in maize: II. QTL detection and coincidences. Maydica. 2000;45:67–80. [Google Scholar]
- Borevitz JO, Maloof JN, Lutes J, Dabi T, Redfern JL, Trainer GT, Werner JD, Asami T, Berry CC, Weigel D et al. Quantitative trait loci controlling light and hormone response in two accessions of Arabidopsis thaliana. Genetics. 2002;160:683–696. doi: 10.1093/genetics/160.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breyne P, Rombaut D, van Gysel A, van Montagu M, Gerats T. AFLP analysis of genetic diversity within and between Arabidopsis thaliana ecotypes. Mol Gen Genet. 1999;261:627–634. doi: 10.1007/s004380050005. [DOI] [PubMed] [Google Scholar]
- Buell CR, Somerville SC. Use of Arabidopsis recombinant inbred lines reveals a monogenic and a novel digenic resistance mechanism to Xanthomonas campestris pv campestris. Plant J. 1997;12:21–29. doi: 10.1046/j.1365-313x.1997.12010021.x. [DOI] [PubMed] [Google Scholar]
- Churchill GA, Doerge RW. Empirical threshold values for quantitative trait mapping. Genetics. 1994;138:963–971. doi: 10.1093/genetics/138.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng XW, Caspar T, Quail PH. cop1: a regulatory locus involved in light-controlled development and gene expression in Arabidopsis. Genes Dev. 1991;5:1172–1182. doi: 10.1101/gad.5.7.1172. [DOI] [PubMed] [Google Scholar]
- Frary A, Nesbitt TC, Grandillo S, Knaap E, Cong B, Liu J, Meller J, Elber R, Alpert KB, Tanksley SD. fw2.2: a quantitative trait locus key to the evolution of tomato fruit size. Science. 2000;289:85–88. doi: 10.1126/science.289.5476.85. [DOI] [PubMed] [Google Scholar]
- Fuentes SI, Allen DJ, Ortiz-Lopez A, Hernandez G. Over-expression of cytosolic glutamine synthetase increases photosynthesis and growth at low nitrogen concentrations. J Exp Bot. 2001;52:1071–1081. doi: 10.1093/jexbot/52.358.1071. [DOI] [PubMed] [Google Scholar]
- Greenwood DJ, Lemaire G, Gosse G, Cruz P, Draycott A, Neeteson JJ. Decline in percentage N of C3 and C4 crops with increasing plant mass. Ann Bot. 1990;66:425–436. [Google Scholar]
- Hirel B, Bertin P, Quilleré I, Bourdoncle W, Attagnant C, Dellay C, Gouy A, Cadiou S, Retailliau C, Falque M et al. Towards a better understanding of the genetic and physiological basis for nitrogen use efficiency in maize. Plant Physiol. 2001;125:1258–1270. doi: 10.1104/pp.125.3.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirel B, Lea PJ. Ammonia assimilation. In: Lea PJ, Morot-Gaudry J-F, editors. Plant Nitrogen. Berlin: Springer-Verlag; 2001. pp. 79–99. [Google Scholar]
- Hoff T, Schnorr KM, Meyer C, Caboche M. Isolation of two Arabidopsis cDNAs involved in early steps of molybdenum cofactor biosynthesis by functional complementation of Escherichia coli mutants. J Biol Chem. 1995;270:6100–6107. doi: 10.1074/jbc.270.11.6100. [DOI] [PubMed] [Google Scholar]
- Jansen RC, van Ooijen JW, Stam P, Lister C, Dean C. Genotype-by-environment interaction in genetic mapping of multiple quantitative trait loci. Theor Appl Genet. 1995;91:33–37. doi: 10.1007/BF00220855. [DOI] [PubMed] [Google Scholar]
- Jiang C, Zeng ZB. Multiple trait analysis of genetic mapping for quantitative trait loci. Genetics. 1995;140:1111–1127. doi: 10.1093/genetics/140.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juenger T, Purugganan MD, Mackay TFC. Quantitative trait loci for floral morphology in Arabidopsis thaliana. Genetics. 2000;156:1379–1392. doi: 10.1093/genetics/156.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justes E, Mary B, Meynard J-M, Machet J-M, Thelier-Huche L. Determination of a critical nitrogen dilution curve for winter wheat crops. Ann Bot. 1994;74:397–407. [Google Scholar]
- Kliebenstein DJ, Gershenzon J, Mitchell-Olds T. Comparative Quantitative trait loci mapping of aliphatic, indolic and benzylic glucosinolate production in Arabidopsis thaliana leaves and seeds. Genetics. 2001;159:359–370. doi: 10.1093/genetics/159.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam H-M, Chiu J, Hsieh M-H, Meisel L, Oliveira IC, Shin M, Coruzzi GM. Glutamate-receptor genes in plants. Nature. 1998;396:125–126. doi: 10.1038/24066. [DOI] [PubMed] [Google Scholar]
- Lam H-M, Coschigano KT, Schultz C, Melo-Oliveira R, Tjaden G, Oliveira IC, Ngai N, Hsieh M-H, Coruzzi GM. Use of Arabidopsis mutants and genes to study amide amino acid biosynthesis. Plant Cell. 1995;7:887–898. doi: 10.1105/tpc.7.7.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander ES, Botstein D. Mapping Mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics. 1989;121:185–199. doi: 10.1093/genetics/121.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DW, Gastal F, Lemaire G. Nitrogen, plant growth and crop yield. In: Lea PJ, Morot-Gaudry J-F, editors. Plant Nitrogen. Berlin: Springer-Verlag; 2001. pp. 343–367. [Google Scholar]
- Lebreton C, Lazic-Jancic V, Steed A, Pekic S, Quarrie SA. Identification of QTL for drought responses in maize and their use in testing causal relationships between traits. J Exp Bot. 1995;46:853–865. [Google Scholar]
- Lemaire G, Gastal F. N uptake and distribution in plant canopies. In: Lemaire G, editor. Diagnosis of the Nitrogen Status in Crops. Berlin: Springer-Verlag; 1997. pp. 3–43. [Google Scholar]
- Leydecker MT, Camus I, Daniel-Vedele F, Truong HN. Screening for Arabidopsis mutants affected in the Nii gene expression using the Gus reporter gene. Physiol Plant. 2000;108:161–170. [Google Scholar]
- Lin Y, Cheng CL. A chlorate-resistant mutant defective in the regulation of nitrate reductase gene expression in Arabidopsis defines a new HY locus. Plant Cell. 1997;9:21–35. doi: 10.1105/tpc.9.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loridon K, Cournoyer B, Goubely C, Depeiges A, Picard G. Length polymorphism and allele structure of trinucleotide microsatellites in natural accessions of Arabidopsis thaliana. Theor Appl Genet. 1998;97:591–604. [Google Scholar]
- Loudet O, Chaillou S, Camilleri C, Bouchez D, Daniel-Vedele F. Bay-0 × Shahdara recombinant inbred line population: a powerful tool for the genetic dissection of complex traits in Arabidopsis. Theor Appl Genet. 2002;104:1173–1184. doi: 10.1007/s00122-001-0825-9. [DOI] [PubMed] [Google Scholar]
- Lukowitz W, Gillmor CS, Scheible W-R. Positional cloning in Arabidopsis: why it feels good to have a genome initiative working for you. Plant Physiol. 2000;123:795–805. doi: 10.1104/pp.123.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C, Stitt M. Nitrate reduction and signalling. In: Lea PJ, Morot-Gaudry J-F, editors. Plant Nitrogen. Berlin: Springer-Verlag; 2001. pp. 37–59. [Google Scholar]
- Mitchell-Olds T. Genetic constraints on life-history evolution: quantitative trait loci influencing growth and flowering in Arabidopsis thaliana. Evolution. 1996;50:140–145. doi: 10.1111/j.1558-5646.1996.tb04480.x. [DOI] [PubMed] [Google Scholar]
- Mitchell-Olds T, Pedersen D. The molecular basis of quantitative genetic variation in central and secondary metabolism in Arabidopsis. Genetics. 1998;149:739–747. doi: 10.1093/genetics/149.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussaume L, Vincentz M, Meyer C, Boutin J-P, Caboche M. Post-transcriptional regulation of nitrite reductase by light is abolished by an N-terminal deletion. Plant Cell. 1995;7:611–621. doi: 10.1105/tpc.7.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara M, Kajiura M, Fukuta Y, Yano M, Hayashi M, Yamaya T, Sato T. Mapping of QTLs associated with cytosolic glutamine synthetase and NADH-glutamate synthase in rice (Oryza sativa L.) J Exp Bot. 2001;52:1209–1217. [PubMed] [Google Scholar]
- Orsel M, Filleur S, Fraisier V, Daniel-Vedele F. Nitrate transport in plants: which gene and which control? J Exp Bot. 2002;53:825–833. doi: 10.1093/jexbot/53.370.825. [DOI] [PubMed] [Google Scholar]
- Pigliucci M. Ecological and evolutionary genetics of Arabidopsis. Trends Plant Sci. 1998;3:485–489. [Google Scholar]
- Plénet D, Lemaire G. Relationships between dynamics of nitrogen uptake and dry matter accumulation in maize crops: determination of critical N concentration. Plant Soil. 2000;216:65–82. [Google Scholar]
- Prioul JL, Quarrie SA, Causse M, de Vienne D. Dissecting complex physiological functions through the use of molecular quantitative genetics. J Exp Bot. 1997;48:1151–1163. [Google Scholar]
- Quilleré I, Dufosse C, Roux Y, Foyer CH, Caboche M, Morot-Gaudry J-F. The effects of deregulation of NR gene expression on growth and nitrogen metabolism of Nicotiana plumbaginifolia plants. J Exp Bot. 1994;45:1205–1211. [Google Scholar]
- Rauh BL, Basten C, Buckler ES. Quantitative trait locus analysis of growth response to varying nitrogen sources in Arabidopsis thaliana. Theor Appl Genet. 2002;104:743–750. doi: 10.1007/s00122-001-0815-y. [DOI] [PubMed] [Google Scholar]
- Rosen H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957;67:10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- Scheible W-R, Lauerer M, Schulze E-D, Caboche M, Stitt M. Accumulation of nitrate in the shoot acts as a signal to regulate shoot-root allocation in tobacco. Plant J. 1997;11:671–691. [Google Scholar]
- Sharbel TF, Haubold B, Mitchell-Olds T. Genetic isolation by distance in Arabidopsis thaliana: biogeography and post-glacial colonization of Europe. Mol Ecol. 2000;9:2109–2118. doi: 10.1046/j.1365-294x.2000.01122.x. [DOI] [PubMed] [Google Scholar]
- Stitt M. Nitrate regulation of metabolism and growth. Curr Opin Plant Biol. 1999;2:178–186. doi: 10.1016/S1369-5266(99)80033-8. [DOI] [PubMed] [Google Scholar]
- Swarup K, Alonso-Blanco C, Lynn JR, Michaels SD, Amasino RM, Koornneef M, Millar AJ. Natural allelic variation identifies new genes in the Arabidopsis circadian system. Plant J. 1999;20:67–77. doi: 10.1046/j.1365-313x.1999.00577.x. [DOI] [PubMed] [Google Scholar]
- Tsay YF, Schroeder J, Feldmann K, Crawford NM. The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell. 1993;72:705–713. doi: 10.1016/0092-8674(93)90399-b. [DOI] [PubMed] [Google Scholar]
- Tuinstra MR, Ejeta G, Goldsbrough PB. Heterogeneous inbred family (HIF) analysis: a method for developing near-isogenic lines that differ at quantitative trait loci. Theor Appl Genet. 1997;95:1005–1011. [Google Scholar]
- van der Leij M, Smith SJ, Miller AJ. Remobilisation of vacuolar stored nitrate in barley root cells. Planta. 1998;205:64–72. [Google Scholar]
- van der Schaar W, Alonso-Blanco C, LeonKloosterziel KM, Jansen RC, van Ooijen JW, Koornneef M. QTL analysis of seed dormancy in Arabidopsis using recombinant inbred lines and MQM mapping. Heredity. 1997;79:190–200. doi: 10.1038/hdy.1997.142. [DOI] [PubMed] [Google Scholar]
- Visscher PM, Thompson R, Haley CS. Confidence intervals in QTL mapping by bootstrapping. Genetics. 1996;143:1013–1020. doi: 10.1093/genetics/143.2.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling GA, Visscher PM, Haley CS. A comparison of bootstrap methods to construct confidence intervals in QTL mapping. Genet Res. 1998;71:171–180. [Google Scholar]
- Wilson IW, Schiff CL, Hughes DE, Somerville SC. Quantitative trait loci analysis of powdery mildew disease resistance in the Arabidopsis thaliana accession Kashmir-1. Genetics. 2001;158:1301–1309. doi: 10.1093/genetics/158.3.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M. Genetic and molecular dissection of naturally occurring variation. Curr Opin Plant Biol. 2001;4:130–135. doi: 10.1016/s1369-5266(00)00148-5. [DOI] [PubMed] [Google Scholar]
- Yano M, Katayose Y, Ashikari M, Yamanouchi U, Monna L, Fuse T, Baba T, Yamamoto K, Umehara Y, Nagamura Y et al. Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell. 2000;12:2473–2484. doi: 10.1105/tpc.12.12.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HM, Forde BG. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science. 1998;279:407–409. doi: 10.1126/science.279.5349.407. [DOI] [PubMed] [Google Scholar]