Abstract
1 The effects of isoprenaline, propranolol and phosphodiesterase inhibitors on 3H-transmitter overflow elicited by low frequency nerve stimulation were determined in the isolated perfused spleen of the cat.
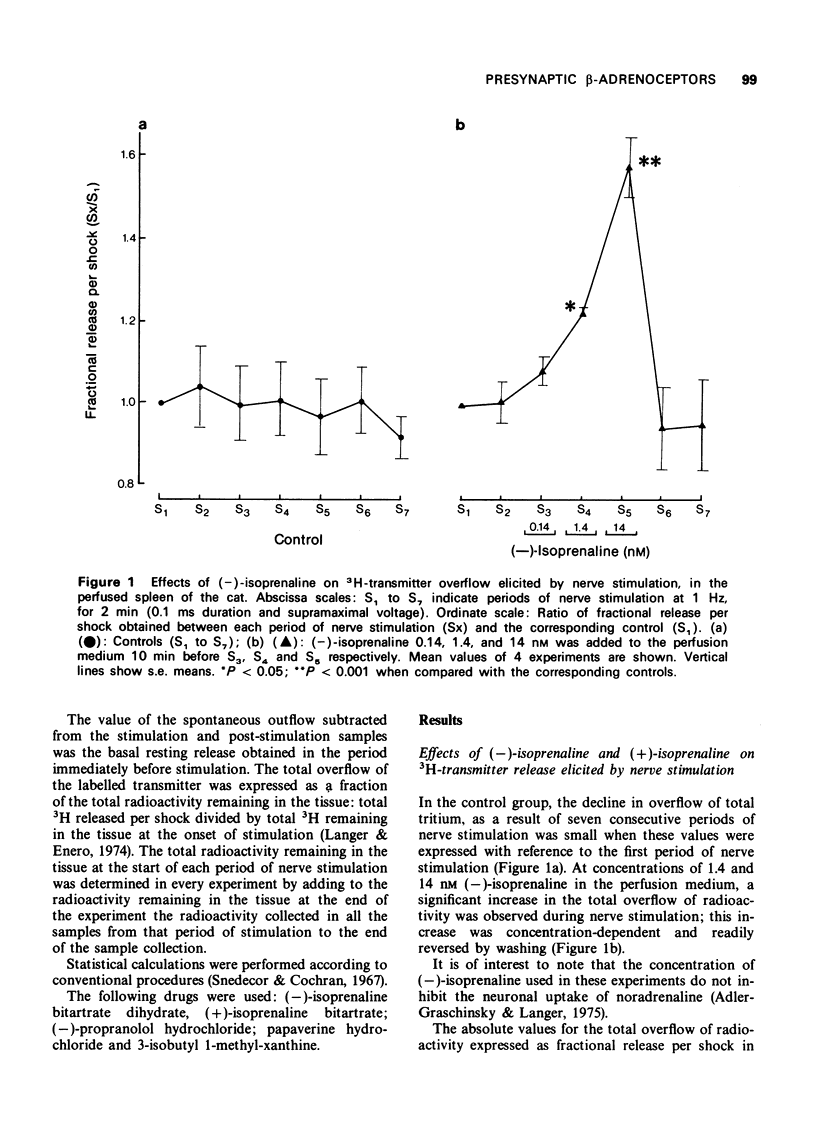
2 (-)-Isoprenaline (0.14, 1.4, and 14 nM) produced a concentration-dependent increase in [3H]-transmitter overflow evoked by nerve stimulation at 1 Hz and was more effective at 1 Hz than at 2 hertz.
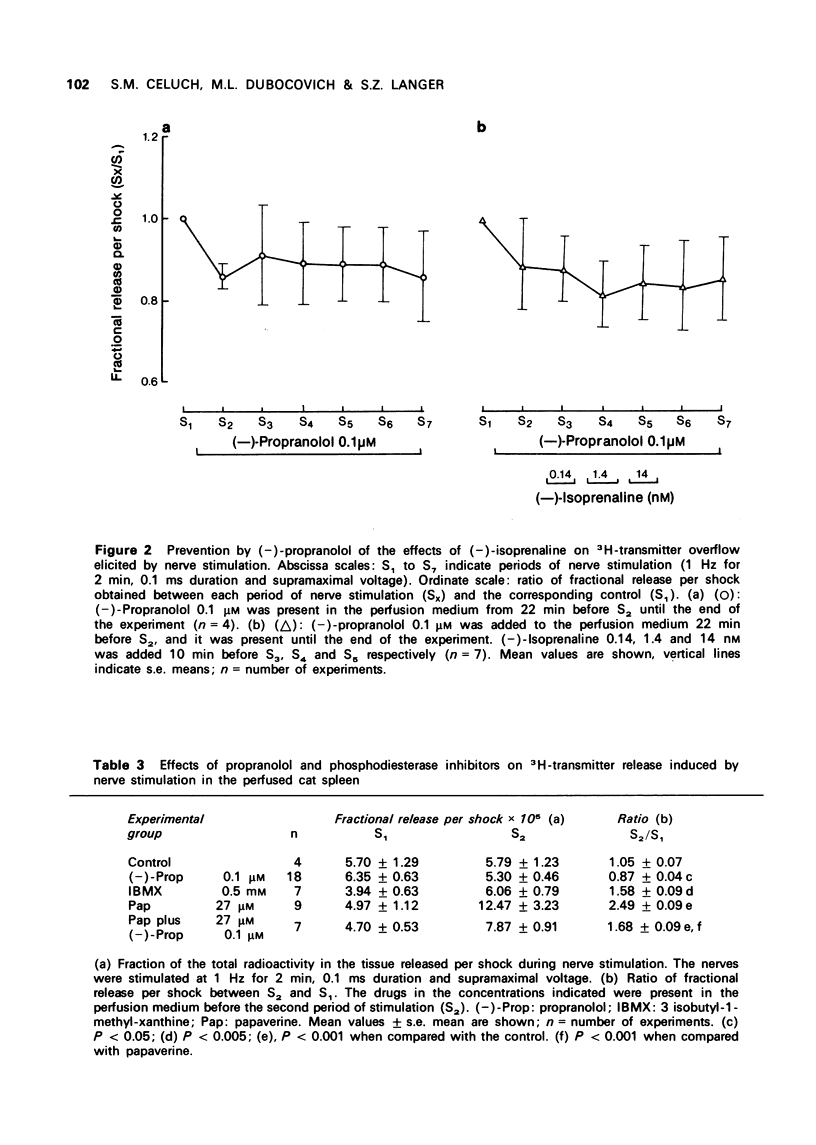
3 A concentration of propranolol (0.1 μM), devoid of neurone blocking activity, blocked this effect of (-)-isoprenaline. These results are compatible with the presence of β-adrenoceptors in the noradrenergic nerve endings of the cat spleen.
4 (+)-Isoprenaline (140 nM) failed to increase the release of radioactivity induced by nerve stimulation, indicating that the β-adrenoceptor mediating the facilitation of transmitter release was stereospecific.
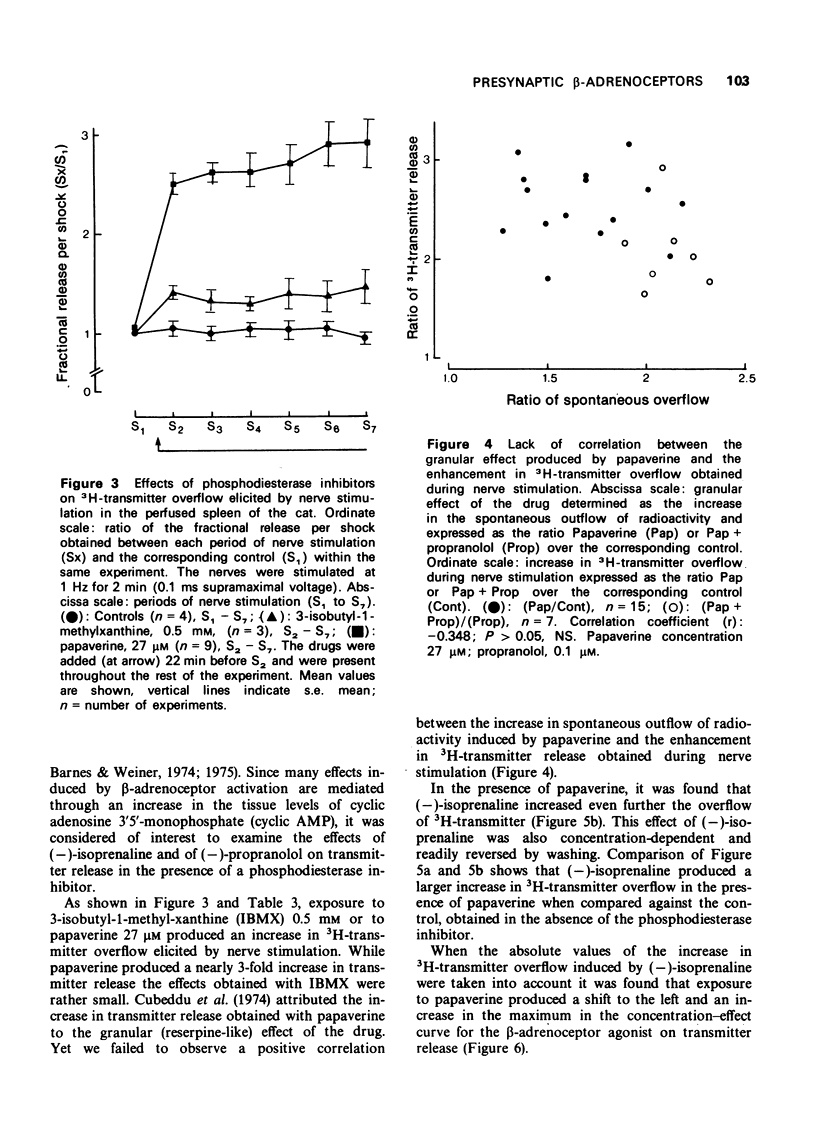
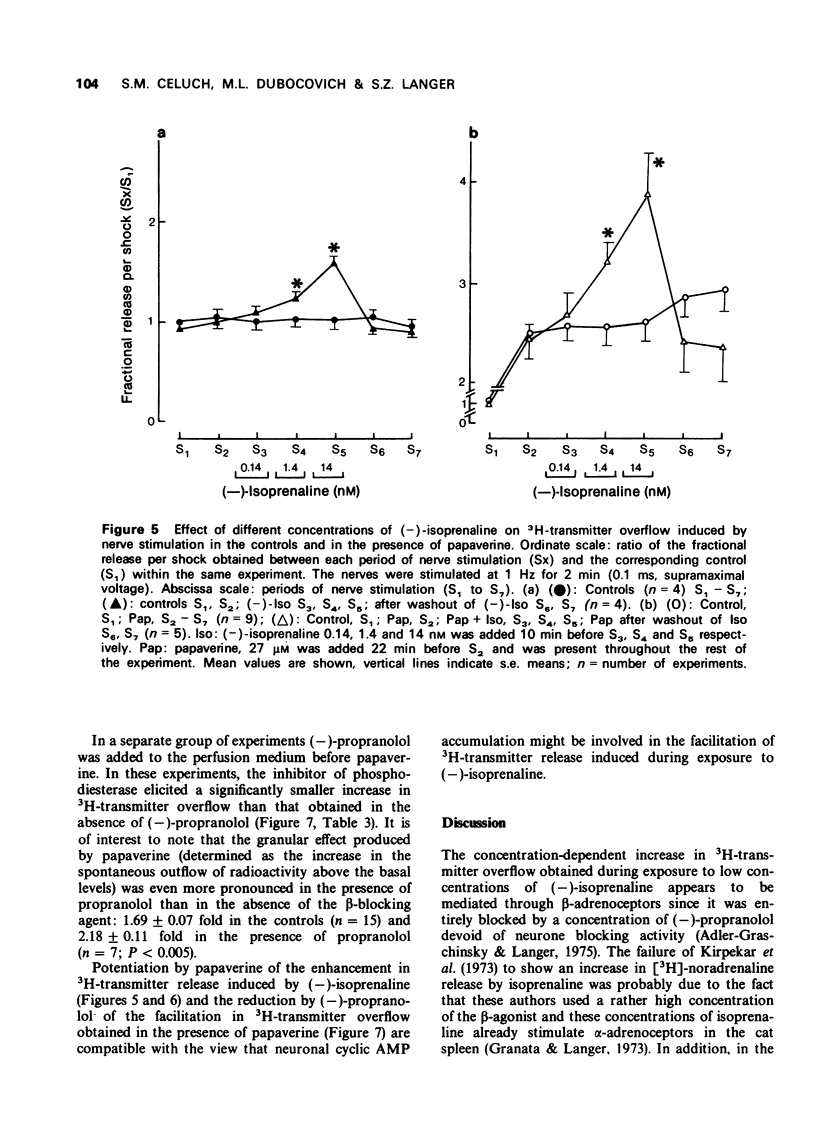
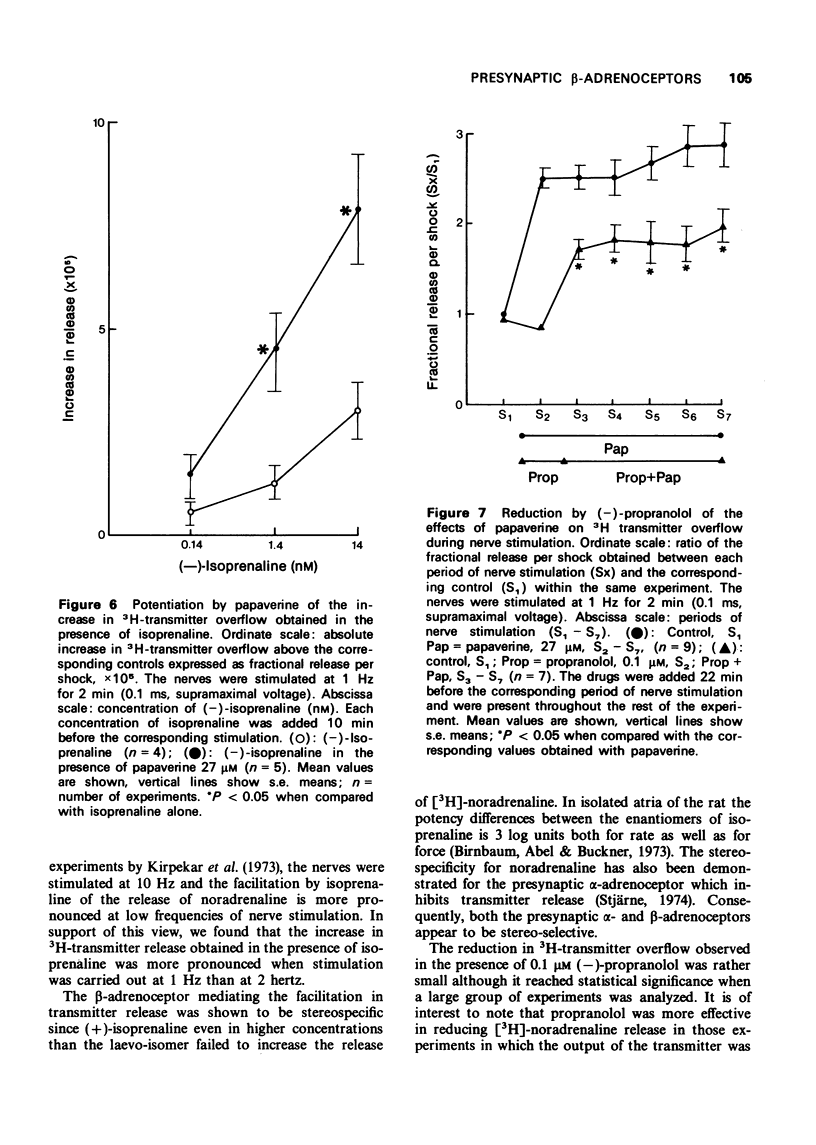
5 The increase in 3H-transmitter overflow induced by nerve stimulation during exposure to the phosphodiesterase inhibitor, papaverine (27 μM) was more pronounced than that obtained in the presence of 3-isobutyl-1-methyl xanthine (IBMX) 0.5 mM. The facilitation in transmitter release induced by papaverine was not correlated with the granular effect produced by this drug.
6 In the presence of papaverine, the concentration-effect curve for (-)-isoprenaline on transmitter release was shifted to the left and its maximum was increased. In addition, propranolol significantly reduced the enhancement in noradrenaline release obtained by exposure to papaverine under conditions in which the granular effect produced by the phosphodiesterase inhibitor was even greater than in the absence of the β-blocker.
7 It is concluded that activation of presynaptic β-adrenoceptors in the perfused cat spleen leads to an enhancement in transmitter release which appears to be linked to an increase in cyclic adenosine 3′,5′-monophosphate levels in noradrenergic nerve endings.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ablad B., Almgren O., Carlsson A., Henning M., Jonasson J., Ljung B. Reduced adrenal amine synthesis in spontaneously hypertensive rats after long-term treatment with propranolol. Br J Pharmacol. 1977 Oct;61(2):318–320. doi: 10.1111/j.1476-5381.1977.tb08422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ablad B., Ek L., Johansson B., Waldeck B. Inhibitory effect of propranolol on the vasoconstrictor response to sympathetic nerve stimulation. J Pharm Pharmacol. 1970 Aug;22(8):627–628. doi: 10.1111/j.2042-7158.1970.tb10585.x. [DOI] [PubMed] [Google Scholar]
- Adler-Graschinsky E., Langer S. Z. Possible role of a beta-adrenoceptor in the regulation of noradrenaline release by nerve stimulation through a positive feed-back mechanism. Br J Pharmacol. 1975 Jan;53(1):43–50. doi: 10.1111/j.1476-5381.1975.tb07328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. M., Nunn B. Adrenergic neuron blocking properties of (plus or minus)-propranolol and (plus)-propranolol. J Pharm Pharmacol. 1970 Nov;22(11):806–810. doi: 10.1111/j.2042-7158.1970.tb08443.x. [DOI] [PubMed] [Google Scholar]
- Cubeddu L., 5th, Barnes E., Weiner N. Release of norepinephrine and dopamine-beta-hydroxylase by nerve stimulation. IV. An evaluation of a role for cyclic adenosine monophosphate. J Pharmacol Exp Ther. 1975 Apr;193(1):105–127. [PubMed] [Google Scholar]
- Cubeddu L., Barnes E., Weiner N. Release of norepinephrine and dopamine-beta-hydroxylase by nerve stimulation. II. Effects of papaverine. J Pharmacol Exp Ther. 1974 Dec;191(3):444–457. [PubMed] [Google Scholar]
- Del Río J., López De Ceballos M. The adrenergic neuron blocking activity of propranolol and alprenolol. Arch Farmacol Toxicol. 1975 Aug;1(2):125–136. [PubMed] [Google Scholar]
- Dretchen K. L., Standaert F. G., Skirboll L. R., Morgenroth V. H., 3rd Evidence for a prejunctional role of cyclic nucleotides in neuromuscular transmission. Nature. 1976 Nov 4;264(5581):79–81. doi: 10.1038/264079a0. [DOI] [PubMed] [Google Scholar]
- Eliash S., Weinstock M. Factors influencing the adrenergic neurone blocking action of propranolol. Br J Pharmacol. 1972 Aug;45(4):630–634. doi: 10.1111/j.1476-5381.1972.tb08120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliash S., Weinstock M. Role of adrenergic neurone blockade in the hypotensive action of propranolol. Br J Pharmacol. 1971 Oct;43(2):287–294. [PMC free article] [PubMed] [Google Scholar]
- Endo T., Starke K., Bangerter A., Taube H. D. Presynaptic receptor systems on the noradrenergic neurones of the rabbit pulmonary artery. Naunyn Schmiedebergs Arch Pharmacol. 1977 Feb;296(3):229–247. doi: 10.1007/BF00498689. [DOI] [PubMed] [Google Scholar]
- Granata A. R., Langer S. Z. Effects of cocaine or denervation on responses of isolated strips of cat spleen to (-)-noradrenaline and (-)-isoprenaline. Br J Pharmacol. 1973 Aug;48(4):667–675. doi: 10.1111/j.1476-5381.1973.tb08255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedqvist P., Moawad A. Presynaptic alpha- and beta-adrenoceptor medicated control of noradrenaline release in human oviduct. Acta Physiol Scand. 1975 Dec;95(4):494–496. doi: 10.1111/j.1748-1716.1975.tb10079.x. [DOI] [PubMed] [Google Scholar]
- Hughes I. E., Kneen B. The effect of propranolol on sympathetic nerve stimulation in isolated vasa deferentia. J Pharm Pharmacol. 1976 Mar;28(3):200–205. doi: 10.1111/j.2042-7158.1976.tb04131.x. [DOI] [PubMed] [Google Scholar]
- Kirpekar S. M., Furchgott R. F., Wakade A. R., Prat J. C. Inhibition by sympathomimetic amines of the release of norepinephrine evoked by nerve stimulation in the cat spleen. J Pharmacol Exp Ther. 1973 Dec;187(3):529–538. [PubMed] [Google Scholar]
- Langer S. Z., Enero M. A. The potentiation of responses to adrenergic nerve stimulation in the presence of cocaine: its relationship to the metabolic fate of released norepinephrine. J Pharmacol Exp Ther. 1974 Dec;191(3):431–443. [PubMed] [Google Scholar]
- Langer S. Z. Presynaptic regulation of catecholamine release. Biochem Pharmacol. 1974 Jul 1;23(13):1793–1800. doi: 10.1016/0006-2952(74)90187-7. [DOI] [PubMed] [Google Scholar]
- Langer S. Z. Sixth gaddum memorial lecture, National Institute for Medical Research, Mill Hill, January 1977. Presynaptic receptors and their role in the regulation of transmitter release. Br J Pharmacol. 1977 Aug;60(4):481–497. doi: 10.1111/j.1476-5381.1977.tb07526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P. J., Haeusler G. Reduction in sympathetic nervous activity as a mechanism for hypotensive effect of propranolol. Nature. 1975 Jul 31;256(5516):440–440. doi: 10.1038/256440a0. [DOI] [PubMed] [Google Scholar]
- Ljung B., Ablad B., Dahlöf C., Henning M., Hultberg E. Impaired vasoconstrictor nerve function in spontaneously hypertensive rats after long-term treatment with propranolol and metroprolol. Blood Vessels. 1975;12(5):311–315. doi: 10.1159/000158067. [DOI] [PubMed] [Google Scholar]
- Mylecharane E. J., Raper C. Further studies on the adrenergic neuron blocking activity of some -adrenoceptor antagonists and guanethidine. J Pharm Pharmacol. 1973 Mar;25(3):213–220. doi: 10.1111/j.2042-7158.1973.tb10627.x. [DOI] [PubMed] [Google Scholar]
- Mylecharane E. J., Raper C. Prejunctional actions of some beta-adrenoreceptor antagonists in the vas deferens preparation of the guinea-pig. Br J Pharmacol. 1970 May;39(1):128–138. doi: 10.1111/j.1476-5381.1970.tb09562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- Roth R. H., Morgenroth V. H., 3rd, Salzman P. M. Tyrosine hydroxylase: allosteric activation induced by stimulation of central noradrenergic neurons. Naunyn Schmiedebergs Arch Pharmacol. 1975;289(4):327–343. doi: 10.1007/BF00508408. [DOI] [PubMed] [Google Scholar]
- Serck-Hanssen G. Effects of theophylline and propranolol on acetylcholine-induced release of adrenal medullary catecholamines. Biochem Pharmacol. 1974 Aug 15;23(16):2225–2234. doi: 10.1016/0006-2952(74)90552-8. [DOI] [PubMed] [Google Scholar]
- Skirboll L. R., Baizer L., Dretchen K. L. Evidence for a cyclic nucleotide-mediated calcium flux in motor nerve terminals. Nature. 1977 Jul 28;268(5618):352–355. doi: 10.1038/268352a0. [DOI] [PubMed] [Google Scholar]
- Starke K., Schümann H. J. Interactions of angiotensin, phenoxybenzamine and propranolol on noradrenaline release during sympathetic nerve stimulation. Eur J Pharmacol. 1972 Apr;18(1):27–30. doi: 10.1016/0014-2999(72)90127-6. [DOI] [PubMed] [Google Scholar]
- Stj-5aarne L., Brundin J. Dual adreoceptor-mediated control of noradrenaline secretion from human vasoconstrictor nerves: facilitation by BETA-receptors and inhibitor by alpha-receptors. Acta Physiol Scand. 1975 May;94(1):139–141. doi: 10.1111/j.1748-1716.1975.tb05872.x. [DOI] [PubMed] [Google Scholar]
- Stjärne L. Selectivity for catecholamines of presynaptic alpha-receptors involved in feedback control of sympathetic neurotransmitter secretion in guinea-pig vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1975;288(2-3):296–303. doi: 10.1007/BF00500534. [DOI] [PubMed] [Google Scholar]
- Stjärne L. Stereoselectivity of presynaptic alpha-adrenoceptors involved in feedback control of sympathetic neurotransmitter secretion. Acta Physiol Scand. 1974 Jan;90(1):286–288. doi: 10.1111/j.1748-1716.1974.tb05590.x. [DOI] [PubMed] [Google Scholar]
- Weller M. Evidence for the presynaptic location of adenylate cyclase and the cyclic AMP-stimulated protein kinase which is bound to synaptic membranes. Biochim Biophys Acta. 1977 Sep 19;469(3):350–354. doi: 10.1016/0005-2736(77)90171-7. [DOI] [PubMed] [Google Scholar]
- Wooten G. F., Thoa N. B., Kopin I. J., Axelrod J. Enhanced release of dopamine -hydroxylase and norepinephrine from sympathetic nerves by dibutyryl cyclic adenosine 3', 5'-monophosphate and theophylline. Mol Pharmacol. 1973 Mar;9(2):178–183. [PubMed] [Google Scholar]
- Yamaguchi N., de Champlain J., Nadeau R. A. Regulation of norepinephrine release from cardiac sympathetic fibers in the dog by presynaptic alpha- and beta-receptors. Circ Res. 1977 Jul;41(1):108–117. doi: 10.1161/01.res.41.1.108. [DOI] [PubMed] [Google Scholar]


