Abstract
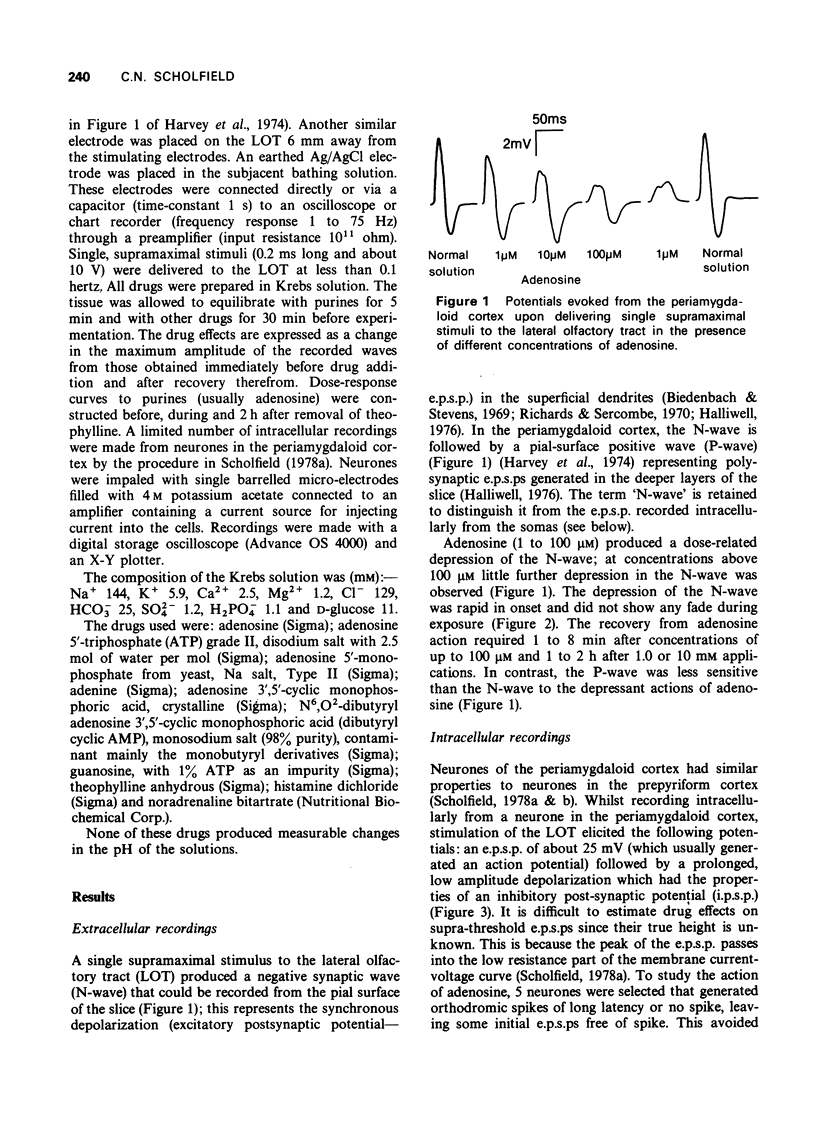
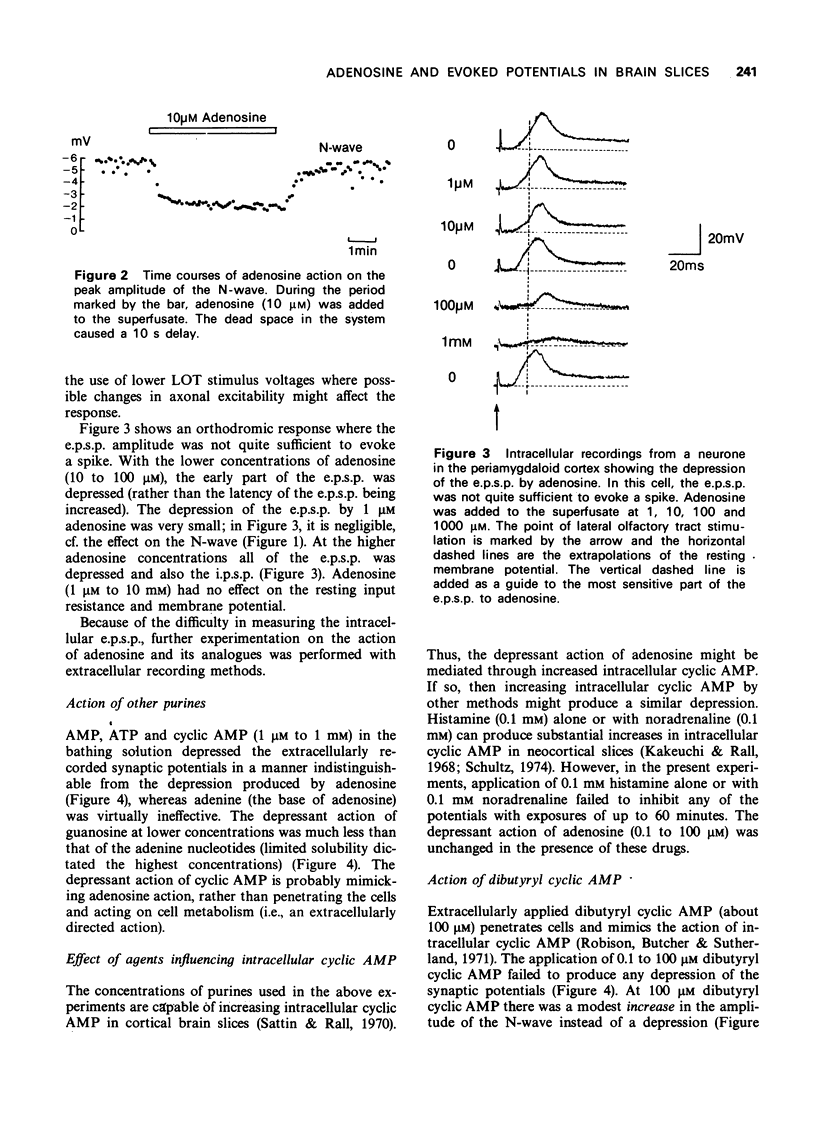
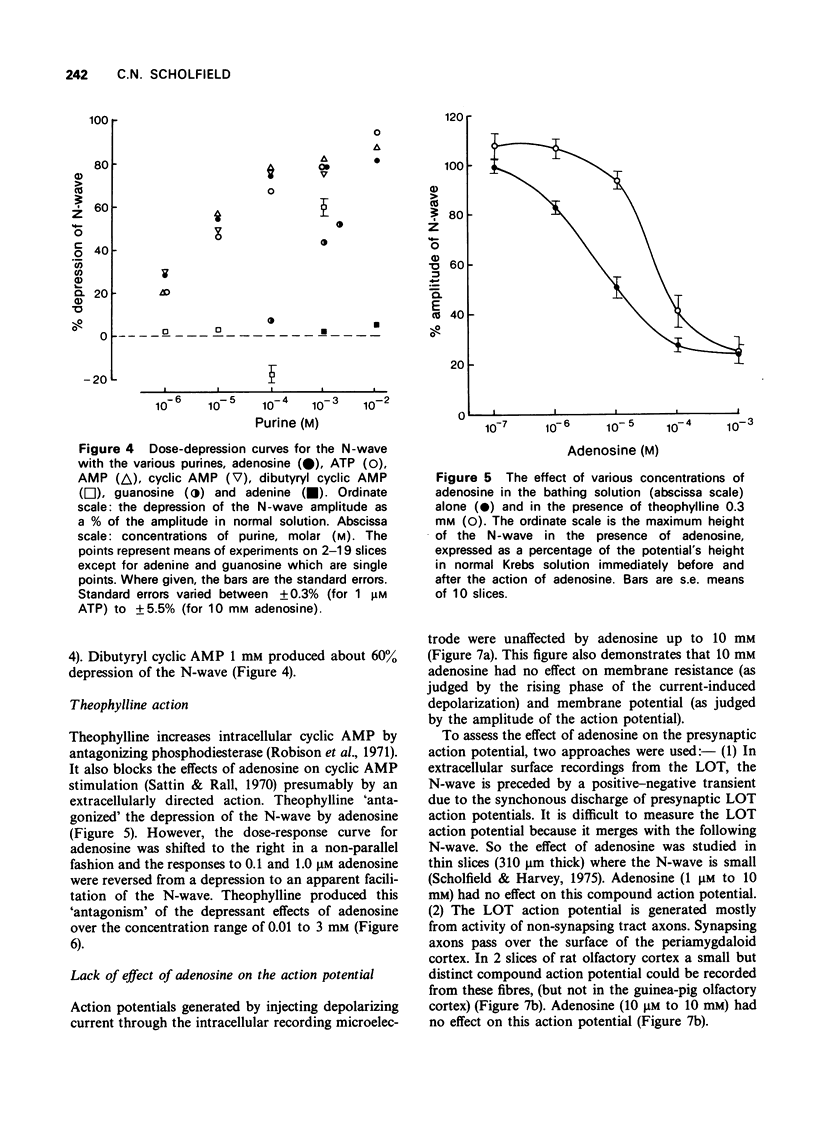
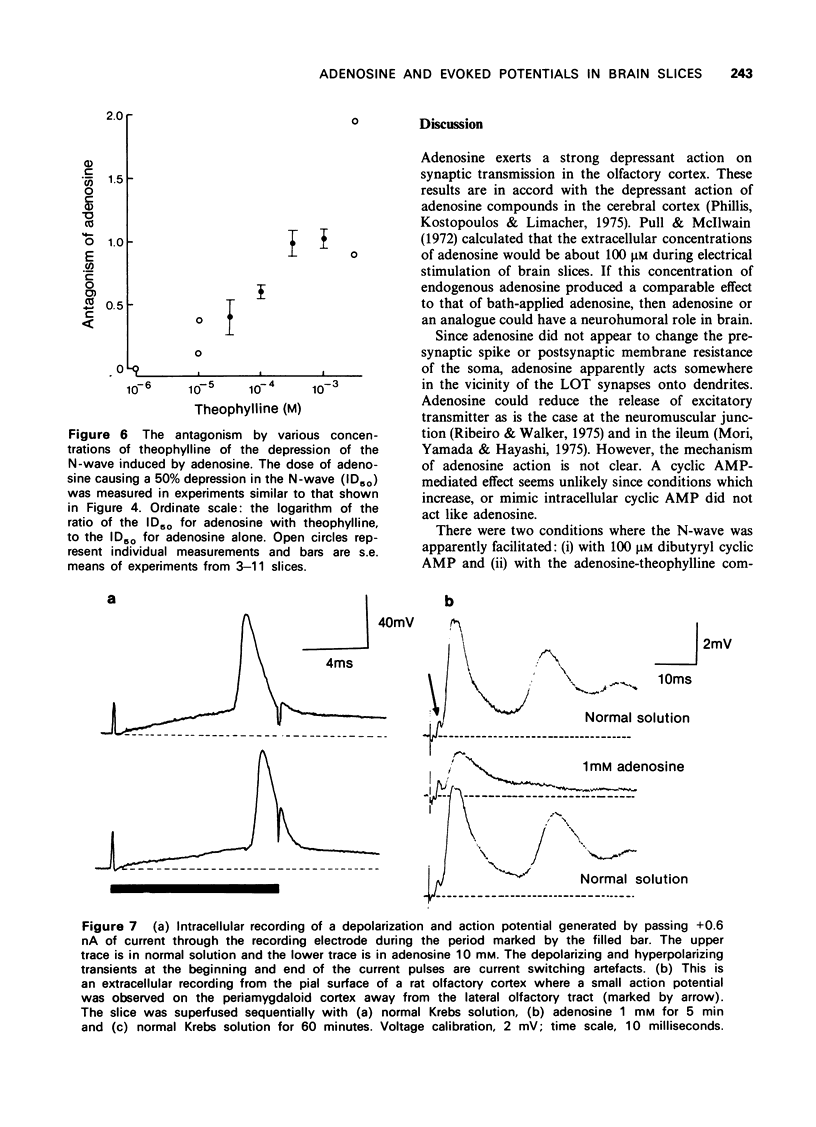
1 A study has been made of the action of adenosine on surface slices of guinea-pig olfactory cortex in vitro. 2 With extracellular recordings from the pial surface and stimulation of the presynaptic input, the lateral olfactory tract (LOT) generated a monosynaptic negative wave representing dendritic excitatory potentials. This negative wave was depressed by bath application of 1 micron adenosine with increasing effect up to 1 mM. Adenosine 5'--triphosphate (ATP), adenosine 5'-monophosphate (AMP) and cyclic adenosine 3',5'-monophosphate (cyclic AMP) had similar depressant actions. Adenine and guanosine were very weak depressants. 3 Theophylline concentrations in the range 10 micron to 3 mM progressively antagonized the action of adenosine. 4 Dibuyryl cyclic AMP (100 micron) and agents which increase intracellular cyclic AMP were not depressants, suggesting that the action of adenosine was not cyclic AMP-mediated. 5 Intracellular recordings confirmed the depressant effect of adenosine on excitatory potentials generated by LOT stimulation and also showed that postsynatpic action potentials and the membrane of the soma were unaffected by adenosine. 6 Since presynaptic action potentials were also unaffected by adenosine, these experiments suggest that adenosine reduces excitatory transmission at LOT synapses and fortifies the idea that adenosine has a 'neurohumoral' action.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biedenbach M. A., Stevens C. F. Electrical activity in cat olfactory cortex produced by synchronous orthodromic volleys. J Neurophysiol. 1969 Mar;32(2):193–203. doi: 10.1152/jn.1969.32.2.193. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Harvey J. A., Scholfield C. N., Brown D. A. Evoked surface-positive potentials in isolated mammalian olfactory cortex. Brain Res. 1974 Aug 16;76(2):235–245. doi: 10.1016/0006-8993(74)90457-0. [DOI] [PubMed] [Google Scholar]
- Harvey J. A., Scholfield C. N., GRAHM L. T., Jr, Aprison M. H. Putative transmitters in denervated olfactory complex. J Neurochem. 1975 Mar;24(3):445–449. doi: 10.1111/j.1471-4159.1975.tb07660.x. [DOI] [PubMed] [Google Scholar]
- Kakiuchi S., Rall T. W. Studies on adenosine 3',5'-phosphate in rabbit cerebral cortex. Mol Pharmacol. 1968 Jul;4(4):379–388. [PubMed] [Google Scholar]
- Miyamota M. D., Breckenridge B. M. A cyclic adenosine monophosphate link in the catecholamine enhancement of transmitter release at the neuromuscular junction. J Gen Physiol. 1974 May;63(5):609–624. doi: 10.1085/jgp.63.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis J. W., Kostopoulos G. K., Limacher J. J. A potent depressant action of adenine derivatives on cerebral cortical neurones. Eur J Pharmacol. 1975 Jan;30(1):125–129. doi: 10.1016/0014-2999(75)90214-9. [DOI] [PubMed] [Google Scholar]
- Pull I., McIlwain H. Adenine derivatives as neurohumoral agents in the brain. The quantities liberated on excitation of superfused cerebral tissues. Biochem J. 1972 Dec;130(4):975–981. doi: 10.1042/bj1300975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro J. A., Walker J. The effects of adenosine triphosphate and adenosine diphosphate on transmission at the rat and frog neuromuscular junctions. Br J Pharmacol. 1975 Jun;54(2):213–218. doi: 10.1111/j.1476-5381.1975.tb06931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards C. D., Sercombe R. Calcium, magnesium and the electrical activity of guinea-pig olfactory coex in vitro. J Physiol. 1970 Dec;211(3):571–584. doi: 10.1113/jphysiol.1970.sp009294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattin A., Rall T. W. The effect of adenosine and adenine nucleotides on the cyclic adenosine 3', 5'-phosphate content of guinea pig cerebral cortex slices. Mol Pharmacol. 1970 Jan;6(1):13–23. [PubMed] [Google Scholar]
- Scholfield C. N. A depolarizing inhibitory potential in neurones of the olfactory cortex in vitro. J Physiol. 1978 Feb;275:547–557. doi: 10.1113/jphysiol.1978.sp012207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholfield C. N. Electrical properties of neurones in the olfactory cortex slice in vitro. J Physiol. 1978 Feb;275:535–546. doi: 10.1113/jphysiol.1978.sp012206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholfield C. N., Harvey J. A. Local anesthetics and barbiturates: effects on evoked potentials in isolated mammalian cortex. J Pharmacol Exp Ther. 1975 Dec;195(3):522–531. [PubMed] [Google Scholar]
- Schultz J. Adenosine 3',5'-monophosphate in guinea pig cerebral cortical slices: effect of benzodiazepines. J Neurochem. 1974 May;22(5):685–690. [PubMed] [Google Scholar]
- Yamamoto C., McIlwain H. Electrical activities in thin sections from the mammalian brain maintained in chemically-defined media in vitro. J Neurochem. 1966 Dec;13(12):1333–1343. doi: 10.1111/j.1471-4159.1966.tb04296.x. [DOI] [PubMed] [Google Scholar]


