Abstract
Tocopherols are essential components of the human diet and are synthesized exclusively by photosynthetic organisms. These lipophilic antioxidants consist of a chromanol ring and a 15-carbon tail derived from homogentisate (HGA) and phytyl diphosphate, respectively. Condensation of HGA and phytyl diphosphate, the committed step in tocopherol biosynthesis, is catalyzed by HGA phytyltransferase (HPT). To investigate whether HPT activity is limiting for tocopherol synthesis in plants, the gene encoding Arabidopsis HPT, HPT1, was constitutively overexpressed in Arabidopsis. In leaves, HPT1 overexpression resulted in a 10-fold increase in HPT specific activity and a 4.4-fold increase in total tocopherol content relative to wild type. In seeds, HPT1 overexpression resulted in a 4-fold increase in HPT specific activity and a total seed tocopherol content that was 40% higher than wild type, primarily because of an increase in γ-tocopherol content. This enlarged pool of γ-tocopherol was almost entirely converted to α-tocopherol by crossing HPT1 overexpressing plants with lines constitutively overexpressing γ-tocopherol methyltransferase. Seed of the resulting double overexpressing lines had a 12-fold increase in vitamin E activity relative to wild type. These results indicate that HPT activity is limiting in various Arabidopsis tissues and that total tocopherol levels and vitamin E activity can be elevated in leaves and seeds by combined overexpression of the HPT1 and γ-tocopherol methyltransferase genes.
Tocopherols, collectively known as vitamin E, are a class of lipid-soluble antioxidants synthesized exclusively by photosynthetic organisms. Tocopherols are essential components of the human diet because they perform numerous critical functions including quenching and scavenging various reactive oxygen species and free radicals and protecting polyunsaturated fatty acids from lipid peroxidation (Fukuzawa and Gebicky, 1983; Neely et al., 1988; Fryer, 1993; Bramley et al., 2000). Because of these and other activities, dietary tocopherols are thought to play an important role in improving immune function and in limiting the incidence and progression of several degenerative human diseases including certain types of cancer, cataracts, neurological disorders, and cardiovascular disease (Brigelius-Flohe and Traber, 1999; Bramley et al., 2000; Pryor, 2000).
In plants, indirect evidence suggests that tocopherols perform antioxidant and radical quenching functions similar to those in animals (Fryer, 1992) and that tocopherols may have additional roles related to photosynthesis (Munne-Bosch and Alegre, 2002). Plants alter their tocopherol levels during development (Molina-Torres and Martinez, 1991; Tramontano et al., 1992) and in response to a variety of stresses, including high-light, low-temperature, drought, and salt stress (Gossett et al., 1994; Streb et al., 1998; Leipner et al., 1999; Havaux et al., 2000; Munne-Bosch and Alegre, 2000). In addition, during leaf senescence, a process accompanied by chlorophyll degradation and oxidative damage in photosynthetic membranes, there is an increased accumulation of tocopherols (Rise et al., 1989). These combined studies suggest that the synthesis of tocopherols is highly regulated during plant growth and development.
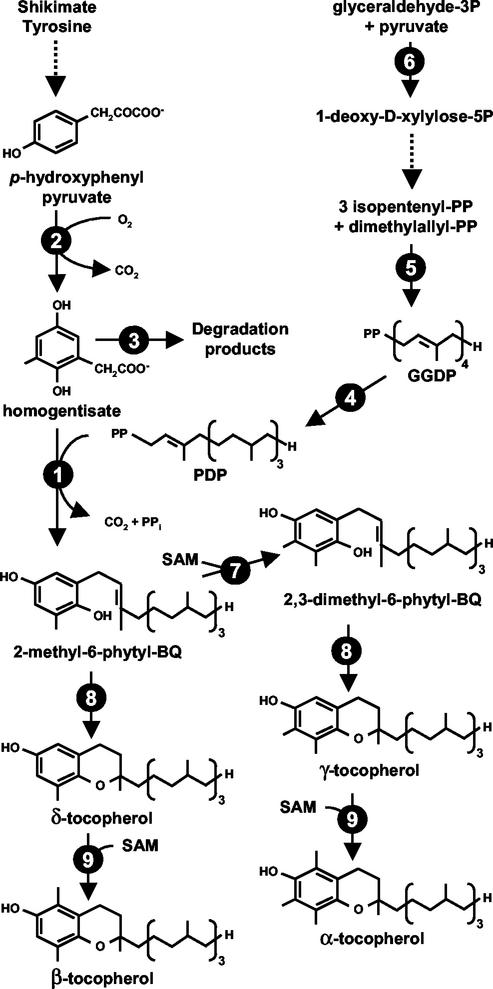
Tocopherols are amphipatic molecules, consisting of a polar chromanol head group and a lipophilic isoprenoid tail derived from homogentisate (HGA) and phytyl diphosphate (PDP), respectively. The tocopherol biosynthetic pathway is depicted in Figure 1. In plants, the aromatic precursor of tocopherols, HGA, is synthesized from p-hydroxyphenyl pyruvate (HPP) by a cytosolic HPPD (Norris et al., 1995; Garcia et al., 1997, 1999). The biosynthetic source of HPP in tissues is unclear because HPP can originate from the shikimate pathway via prephenate or by transamination of Tyr (Threlfall and Whistance, 1971; Fiedler et al., 1982; Garcia et al., 1999; Lopukhina et al., 2001). PDP is formed by the sequential action of the plastidic GGPS1 and GGDR (Kuntz et al., 1992; Addlesee et al., 1996; Keller et al., 1998; Addlesee and Hunter, 1999; Okada et al., 2000). As with other plastid-synthesized isoprenoids, PDP is derived from the DXP pathway (Eisenreich et al., 1998; Lichtenthaler, 1998).
Figure 1.
The tocopherol biosynthetic pathway in plants. Dashed arrows represent multiple steps. Enzymes are indicated by circled numbers: 1, HGA phytyltransferase (HPT); 2, p-hydroxyphenyl pyruvate dioxygenase (HPPD); 3, HGA dioxygenase; 4, geranylgeranyl diphosphate reductase (GGDR); 5, geranylgeranyl diphosphate synthase (GGPS); 6, 1-deoxy-d-xylulose-5-phosphate synthase (DXPS); 7, 2-methyl-6-phytyl-1,4-benzoquinol methyltransferase (MPBQ); 8, tocopherol cyclase (TC); and 9, γ-tocopherol methyltransferase (γ-TMT).
In photosynthetic organisms, condensation of HGA and PDP, the committed step in tocopherol biosynthesis, is catalyzed by HPT (Soll et al., 1980, 1984; Soll, 1987; Collakova and DellaPenna, 2001; Savidge et al., 2002). The product of this reaction, MPBQ, is the first prenylquinol intermediate in the pathway and can be methylated to 2,3-dimethyl-6-phytyl-1,4-benzoquinol (DMPBQ) by MPBQ methyltransferase (MPBQ MT; Soll and Schultz, 1979, 1980; Hutson and Threlfall, 1980; Marshall et al., 1985; Soll, 1987; Shintani et al., 2002). Both MPBQ and DMPBQ are substrates for TC to yield the first tocopherols of the pathway, γ-tocopherol and δ-tocopherol, respectively (Stocker et al., 1996; Arango and Heise, 1998; Porfirova et al., 2002). Both γ- and δ-tocopherol can be methylated by γ-TMT to yield α- and β-tocopherol, respectively (D'Harlingue and Camara, 1985; Shintani and DellaPenna, 1998).
Because of the importance of vitamin E in human and plant physiology, the tocopherol biosynthetic pathway has become a focus for plant metabolic engineering. To successfully manipulate the tocopherol content and/or composition of various plant tissues, enzymes with high flux coefficients must be identified in the pathway. This requires the cloning of individual tocopherol biosynthetic enzymes and a detailed understanding of the molecular and biochemical regulation of individual steps of the pathway. In recent years, several genes encoding enzymes directly or indirectly involved in tocopherol biosynthesis have been cloned and overexpressed in plants to test whether they are limiting for tocopherol synthesis in various tissues (Garcia et al., 1997, 1999; Norris et al., 1998; Shintani and DellaPenna, 1998; Collakova and DellaPenna, 2001; Estevez et al., 2001; Schledz et al., 2001; Savidge et al., 2002; Tsegaye et al., 2002).
Precursor feeding studies with safflower (Carthamus tinctorius) cell cultures suggested that levels of HGA, PDP, or both might be limiting for flux through the tocopherol biosynthetic pathway (Furuya et al., 1987). HPPD produces HGA from HPP and has been cloned and characterized from a variety of plants (Garcia et al., 1997, 1999; Norris et al., 1998). Overexpression of HPPD in Arabidopsis leaves and seeds resulted in a 10-fold increase in HPPD specific activity but only a 10% to 30% increase in total tocopherol levels (Tsegaye et al., 2002). This result may be attributable to enhanced degradation of HGA in transgenics or to a low flux coefficient of HPPD in the tocopherol pathway (Tsegaye et al., 2002). Plastidic phytol levels may also be limiting for tocopherol synthesis because feeding phytol to safflower cell cultures stimulated tocopherol levels to an even higher extent than HGA (Furuya et al., 1987). Other studies suggest that DXP pathway-derived isopentenyl diphosphate, which is used for the synthesis of phytol and PDP, may limit isoprenoid synthesis in Arabidopsis chloroplasts (Estevez et al., 2001). Constitutive overexpression of the first enzyme of the DXP pathway, DXP synthase (DXPS) in Arabidopsis leaves resulted in elevated levels of several plastidic isoprenoids including tocopherols, which were increased up to 2-fold relative to wild type (Estevez et al., 2001).
Arabidopsis leaves accumulate predominantly α-tocopherol, whereas the major tocopherol present in Arabidopsis seeds is γ-tocopherol (Shintani and DellaPenna, 1998). This difference was shown to be the result of low-seed γ-TMT activity because overexpression of γ-TMT in Arabidopsis seeds led to the conversion of more than 95% of the γ-tocopherol to α-tocopherol. In these experiments, total tocopherol levels remained unchanged in the transgenic seed. These results indicated that γ-TMT was a key enzyme controlling seed tocopherol composition but had no effect on flux through the pathway (Shintani and DellaPenna, 1998).
HPT is one of the most recent tocopherol biosynthetic enzyme to be cloned and characterized (Collakova and DellaPenna, 2001; Schledz et al., 2001; Savidge et al., 2002). HPT catalyzes the committed step of tocopherol biosynthesis (Fig. 1), making it a likely candidate for an enzyme with a high flux coefficient. To test whether HPT activity is limiting for tocopherol synthesis in different tissues, HPT was overexpressed in Arabidopsis leaves and seeds, and the resulting transgenic lines were characterized at both the molecular and biochemical levels. In addition, simultaneous overexpression of HPT and γ-TMT in Arabidopsis leaves and seeds was performed to determine whether the transgenic phenotypes conferred by these two genes were additive.
RESULTS
Biochemical and Molecular Characterization of Wild-Type and 35S::HPT1 Plants
Using a genomics-based approach, we have recently cloned HPT1, the gene encoding HPT in Arabidopsis, which catalyzes the condensation of HGA and PDP in tocopherol synthesis (Collakova and DellaPenna, 2001). Because both HGA and PDP are substrates for other prenyltransferases (Threlfall and Whistance, 1971; Schulze-Siebert et al., 1987; Oster et al., 1997) and HPT activity is low in plant chloroplasts, we hypothesized that HPT could be a highly regulated enzyme with a high flux coefficient (Collakova and DellaPenna, 2001). To assess whether HPT activity is limiting for tocopherol synthesis in different tissues, the enzyme was expressed under the control of the cauliflower mosaic virus 35S rRNA (CaMV 35S) promoter in Arabidopsis. Sixty-six independent primary transformant lines (35S::HPT1) were generated and analyzed (data not shown). 35S::HPT1 lines showing antibiotic resistance segregation ratios consistent with a single insertion locus and exhibiting leaf tocopherol levels higher than wild type were taken to homozygosity.
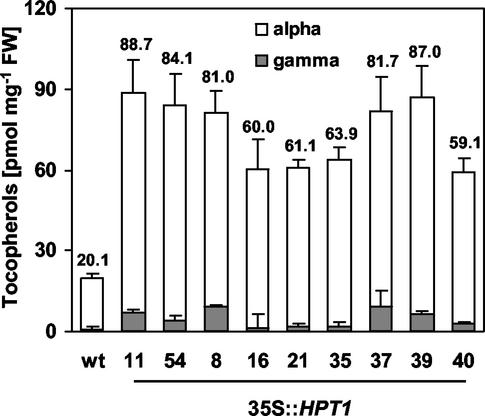
To address the question of whether HPT activity was limiting for tocopherol synthesis in photosynthetic tissue, tocopherol composition and levels were analyzed in leaves of wild-type and homozygous 35S::HPT1 lines. Plants overexpressing HPT1 contained 3- to 4.4-fold higher total tocopherol levels than wild type that were highly significant (P < 0.01; Fig. 2). Both wild-type and 35S::HPT1 leaves contained predominantly α-tocopherol, although γ-tocopherol levels were also elevated in 35S::HPT1. Wild-type leaves accumulated α-tocopherol at 19.1 ± 1.5 pmol mg−1, whereas α-tocopherol levels in leaves of 35S::HPT1 ranged between 55 and 80 pmol mg−1. Leaf γ-tocopherol content was 0.6 ± 0.1 pmol mg−1 in wild type and 1 to 9 pmol mg−1 in HPT1 overexpressers. The levels of β- and δ-tocopherols in both wild type and 35S::HPT1 were below detection. MPBQ and DMPBQ, prenylquinol intermediates in tocopherol biosynthesis, were not detectable in wild-type and 35S::HPT1 leaves (data not shown). These results suggest that MPBQ MT and TC are not limiting activities for tocopherol synthesis in leaf tissue. Two independent transgenic lines exhibiting relatively high α- and γ-tocopherol levels, 35S::HPT1-11 and -54 (Fig. 2), were selected for subsequent analyses.
Figure 2.
Tocopherol composition and levels in leaves of 4-week-old wild-type and homozygous 35S::HPT1 Arabidopsis plants. Thirty milligrams of leaf tissue was extracted, and individual tocopherols were separated and quantified by reverse-phase HPLC. Each line is represented as an average ± sd of tocopherol levels in three plants analyzed in duplicate. Total tocopherol levels in picomoles per milligram of leaf are indicated above the error bar of each line. Total tocopherol levels of 35S::HPT1 lines were significantly higher than wild-type levels (P < 0.01). β- and δ-tocopherols were not detected.
To confirm that the increased tocopherol levels in transgenic lines were attributable to elevated HPT activity resulting from overexpression of HPT1, HPT mRNA levels and specific activity were measured in wild-type and transgenic lines. Real-time PCR showed that HPT mRNA levels were extremely low in wild type and 20- to 100-fold higher in 35S::HPT1 lines (data not shown). Using isolated chloroplasts, prenyltransferase assays demonstrated that HPT specific activity in 2-, 4-, and 6-week-old wild-type plants was also very low (0.11–0.34 pmol h−1 mg−1 protein) and that transgenic lines had 4- to 10-fold higher HPT specific activity than the corresponding wild-type plants (Table I). In general, the highest HPT specific activity in both wild type and 35S::HPT1 was observed in chloroplasts isolated from young, 2-week-old plants. The specific activity of both wild-type and HPT1 overexpressing lines decreased approximately by 2-fold by 6 weeks of age. However, the elevated specific activity in transgenics relative to wild type was maintained throughout plant development (Table I). These results are consistent with previous findings that HPT activity is highest in young plants and diminishes with age (Hutson and Threlfall, 1980).
Table I.
HPT specific activity in chloroplasts isolated from 2-, 4-, and 6-week-old wild-type and 35S::HPT1-11 and -54 leaves.
| Plant Age | Specific Activity
|
||
|---|---|---|---|
| Wild Type | 35S::HPT1-11 | 35S::HPT1-54 | |
| weeks | pmol h−1 mg−1 protein (-fold change) | ||
| 2 | 0.34 ± 0.06* | 1.69 ± 0.39 (5.1) | 1.36 ± 0.11 (4.1) |
| 4 | 0.11 ± 0.03* | 1.14 ± 0.48 (10.3) | 0.84 ± 0.27 (7.6) |
| 6 | 0.15 ± 0.10 | 0.71 ± 0.41 (4.7) | 0.75 ± 0.36 (5.0) |
HPT specific activity was determined using radiolabeled HGA and unlabeled PDP and expressed as an average ± sd of two to three independent experiments performed in triplicate. Values in parentheses represent -fold increases in the specific activity of transgenics compared with wild-type plants of the corresponding age. HPT specific activity in leaves of both 35S::HPT1 overexpressers was significantly higher than wild type (P < 0.05). Though there was a general trend of HPT specific activity decreasing in older relative to younger plants in each line, this was only statistically significant between 2- and 4-week-old wild-type plants (*, P < 0.01).
In addition to HPT, the enzymes shown in Figure 1, HPPD, HGAD, GGPS1, GGDR, and γ-TMT, are also directly or indirectly involved in tocopherol synthesis. Given the elevated levels of tocopherols in 35S::HPT1 lines, it is possible that the expression of other enzymes involved in tocopherol synthesis might also be indirectly affected in the transgenics. To test this hypothesis, the mRNA levels of all available genes of the pathway in wild-type and 35S::HPT1 leaves were measured. With the exception of the 20- to 100-fold increase in HPT mRNA levels in 35S::HPT1, no significant differences in the expression of these other tocopherol-related genes were observed between wild-type and 35S::HPT1 Arabidopsis plants (data not shown).
Effects of HPT1 Overexpression on Tocopherol Levels in Arabidopsis Seed
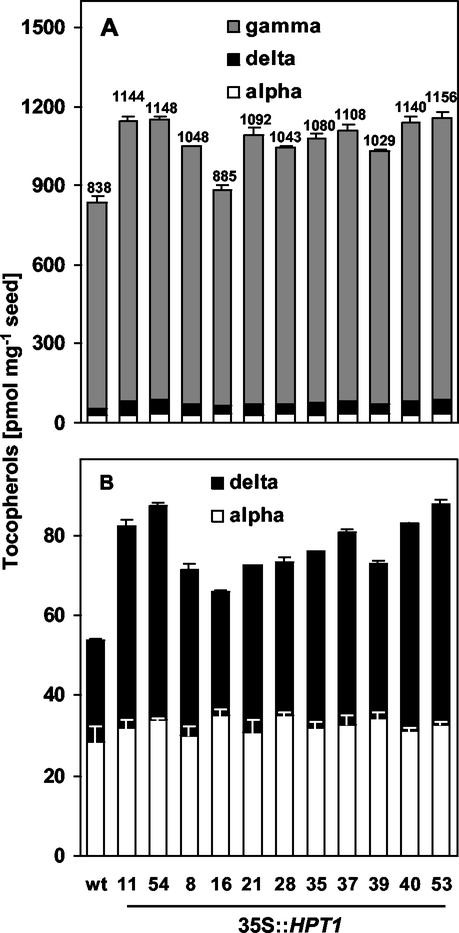
Molecular and biochemical analyses of the tocopherol biosynthetic pathway in leaves of wild-type and 35S::HPT1 transgenic plants demonstrated that HPT expression and activity are limiting for tocopherol synthesis in Arabidopsis leaf tissue. To test whether HPT is also limiting in seeds, total and individual tocopherol levels were determined in seeds of wild-type and homozygous 35S::HPT1 plants. Total tocopherol levels in wild-type Arabidopsis seeds were 838 ± 18 pmol mg−1 seed. In eight of the nine homozygous 35S::HPT1 lines shown in Figure 3, 35S::HPT1-16 being the exception, highly significant increases (P < 0.001) in total seed tocopherol content were observed. The best HPT1 overexpressing lines (11 and 54) contained up to 37% higher seed tocopherol levels than wild type, corresponding to an additional 310 pmol tocopherols mg−1 seed (Fig. 3). The majority of this increase was attributable to elevation of γ-tocopherol levels, the predominant tocopherol in wild-type Arabidopsis seeds, although in some transgenic lines, δ-tocopherol levels also doubled. α-Tocopherol levels did not change significantly (Fig. 3).
Figure 3.
Tocopherol composition and levels in seed of wild-type and homozygous 35S::HPT1 Arabidopsis plants. Seed was extracted and analyzed for tocopherols by normal phase HPLC. Tocopherol levels are expressed as an average ± sd of three analyses per each line in a representative experiment. Total tocopherol levels in picomoles per milligram of seed are indicated above the error bar of each line. Arabidopsis seeds accumulate predominantly γ-tocopherol, which increased up to 40% in 35S::HPT1 compared with wild type. The levels of α-tocopherol did not change significantly, whereas δ-tocopherol increased up to 2-fold in some transgenic lines. β-Tocopherol was not detected. With the exception of 35S::HPT1-16, statistical significance for total tocopherol levels of all transgenic lines relative to wild type was P < 0.001.
We have previously demonstrated that 35S::HPT1-11 and -54 have significantly higher HPT specific activity relative to wild type (Table I). HPT specific activity in seeds was also determined to assess whether the observed differences in seed tocopherol levels between wild-type and 35S::HPT1 lines were correlated with increased HPT activity. Both transgenic lines (-11 and -54) exhibited nearly a 4-fold increase in HPT specific activity relative to wild-type seeds (Table II). When compared with leaf HPT specific activities, the HPT specific activity in seeds was similar to that of chloroplasts from 6-week-old plants (Tables I and II). These results collectively suggest that as in photosynthetic tissues, HPT activity is limiting for tocopherol synthesis in Arabidopsis seed.
Table II.
HPT specific activity in seed of wild-type and 35S::HPT1 overexpressers (lines 11 and 54)
| Line | HPT Specific Activity |
|---|---|
| pmol h−1 mg−1 protein | |
| Wild type | 0.17 ± 0.05 |
| 35S::HPT1-11 | 0.66 ± 0.10 |
| 35S::HPT1-54 | 0.67 ± 0.08 |
Protein extracts from dry Arabidopsis seeds were used to determine HPT specific activity as described. Values are an average ± sd of three independent experiments performed in triplicate. HPT specific activity in seed of both 35S::HPT1 overexpressers was significantly higher than wild type (P < 0.01).
Tocopherol Analysis of Arabidopsis Leaves and Seeds Overexpressing Both HPT1 and γ-TMT Transgenes
Constitutive HPT1 overexpression in Arabidopsis led to a maximal 4.4-fold and 40% increase in total leaf and seed tocopherol levels, respectively. The increase in leaves was primarily attributable to an elevation in α-tocopherol levels and to a lesser extent γ-tocopherol. In Arabidopsis seed, γ-tocopherol was the major contributor to the 40% increase (Fig. 3). This is a result of a limitation in seed γ-TMT activity, which catalyzes methylation of γ- and δ-tocopherols to α- and β-tocopherols, respectively, and this metabolic block can be overcome by overexpressing γ-TMT during seed development (Shintani and DellaPenna, 1998).
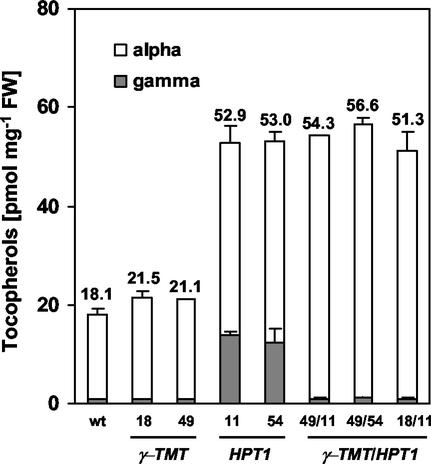
To test whether the traits conferred individually by γ-TMT and HPT overexpression are additive, individual 35S::HPT1 and 35S::γ-TMT lines were crossed, double homozygotes were selected, and their leaf and seed tocopherol content and composition were determined. The levels of individual tocopherols in leaves of wild-type, double overexpressers, and the corresponding single-transgene homozygous parent lines are shown in Figure 4. The total leaf tocopherol levels of 35S::HPT1-11 and -54 in Figure 4 are somewhat lower than in Figure 2. Leaf tocopherol content is extremely sensitive to environmental stimuli and plant developmental stage (Munne-Bosch and Alegre, 2002), and these differences are consistent with the normal range of interexperiment variation observed in tocopherol content (data not shown).
Figure 4.
Tocopherol composition and levels in leaves of 5-week-old wild-type, 35S::γ-TMT, 35S::HPT1, and double 35S::γ-TMT/ 35S::HPT1 overexpressers. Leaf tissue (approximately 70 mg) was extracted, and individual tocopherols were separated and quantified by normal phase HPLC. Each line is represented as an average ± sd from three plants. Total tocopherol levels of 35S::HPT1 and 35S::γ-TMT/35S::HPT1 plants were significantly higher than wild-type levels (P < 0.005). The excess γ-tocopherol in double transgenics was methylated to α-tocopherol.
There was no significant difference in tocopherol composition or levels between wild-type and 35S::γ-TMT leaves. However, the leaf γ-tocopherol present in 35S::HPT1-11 and -54 was converted to α-tocopherol in 35S::HPT1/35S::γ-TMT double homozygotes (Fig. 4). In seed of double overexpressers, nearly the entire pool of γ- and δ-tocopherols was similarly methylated to α- and β-tocopherols, respectively (Fig. 5, A and B). These data indicate that the traits conferred by each single transgene are additive.
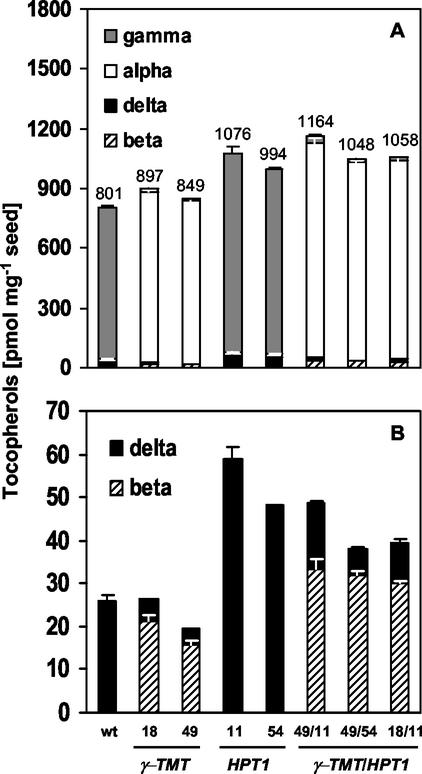
Figure 5.
Tocopherol composition and levels in seed of wild-type, 35S::γ-TMT, 35S::HPT1, and 35S::γ-TMT/35S::HPT1 overexpressers. A, α-, β-, γ-, and δ- tocopherols; B, β- and δ-tocopherols. Tocopherol analysis of mature dry seed was performed as described in “Materials and Methods.” Each line is presented as an average of three independent measurements of three replicates. Total tocopherol levels of 35S::HPT1 and 35S::γ-TMT/35S::HPT1 lines were significantly higher than wild type (P < 0.05). The majority of γ- and δ-tocopherols was converted to α- and β-tocopherols in 35S::γ-TMT/35S::HPT1 overexpressers.
Effects of Increased Tocopherol Levels on Chlorophyll, Carotenoid, and Plastoquinone-9 (PQ-9) Content in 35S::HPT1
In plastids, the HGA and isopentenyl diphosphate (as geranylgeranyl diphosphate [GGDP] or PDP) used in tocopherol synthesis are also used in the synthesis of chlorophylls, carotenoids, and PQ-9 (Threlfall and Whistance, 1971; Schulze-Siebert et al., 1987; Oster et al., 1997). Several studies suggest that the plastidic GGDP pool originating from the DXP pathway may be limiting for isoprenoid synthesis (Fray et al., 1995; Shewmaker et al., 1999; Estevez et al., 2001). Increased flux through the tocopherol pathway may therefore have an effect on the levels of other compounds formed from GGDP or HGA. To test this hypothesis, levels of HGA-derived PQ-9 and two major GGDP-derived compounds, chlorophylls and carotenoids, were measured in leaves and seeds of wild type and 35S::HPT1-11 and -54. No statistically significant differences in total chlorophyll, carotenoids, or PQ-9 levels were observed between wild-type and transgenic plants (Table III). In leaf, the average chlorophyll and carotenoid levels were approximately 1 and 0.44 nmol mg−1 tissue, respectively. In wild type and 35S::HPT1, PQ-9 levels were approximately 82 pmol mg−1 leaf tissue. In seed, chlorophylls and PQ-9 were not detected, whereas carotenoid levels were approximately 31 pmol mg−1 seed (Table III).
Table III.
Total chlorophyll, carotenoids, and PQ-9 levels in wild-type and 35S::HPT1 Arabidopsis leaves and seeds
| Line | Leaf Content
|
Seed Content
|
|||
|---|---|---|---|---|---|
| Chlorophylls | Carotenoids | PQ-9 | Chlorophylls and PQ-9 | Carotenoids | |
| nmol mg−1 | pmol mg−1 | pmol mg−1 | |||
| Wild type | 1.00 ± 0.06 | 0.43 ± 0.04 | 82 ± 8 | nd | 30.3 ± 0.2 |
| 35S::HPT1-11 | 1.01 ± 0.10 | 0.45 ± 0.04 | 80 ± 5 | nd | 28.3 ± 0.6 |
| 35S::HPT1-54 | 0.98 ± 0.08 | 0.43 ± 0.05 | 83 ± 4 | nd | 35.9 ± 0.2 |
In leaves, chlorophyll and carotenoid levels were determined in 90% (v/v) methanol as described previously (Lichtenthaler, 1987). Twenty milligrams of seed was extracted, and pigments were analyzed by reverse-phase HPLC. Lutein made up >95% of total seed carotenoids (data not shown), and its levels were taken as total carotenoid levels. Chlorophylls and PQ-9 were not detected (nd) in dry Arabidopsis seed. Chlorophyll and carotenoid levels in 35S::HPT1 leaves and seeds were comparable with wild-type levels. Pigment levels are an average ± sd of three measurements.
DISCUSSION
HPT is a likely candidate for an enzyme with a high flux coefficient in tocopherol biosynthesis. HPT uses HGA and PDP to catalyze formation of the first committed prenylquinol intermediate in the tocopherol biosynthetic pathway, MPBQ. Because HGA and PDP are also used in the synthesis of plastoquinones, phylloquinones, and chlorophylls (Threlfall and Whistance, 1971; Schulze-Siebert et al., 1987; Oster et al., 1997) and because HPT acts at the branch-point for these plastidic prenyllipid pathways, it could be a key enzyme controlling flux through the tocopherol pathway. To test this hypothesis, the Arabidopsis HPT1 cDNA was constitutively expressed under the control of the CaMV 35S promoter. Significant increases in HPT specific activity and total tocopherol levels were observed in both leaf and seed of 35S::HPT1 plants compared with wild type.
In leaves, HPT1 overexpression resulted in a 4.4-fold increase in total tocopherols, the highest percentage increase in engineering leaf tocopherol content yet reported in Arabidopsis. Three other enzymes affecting tocopherol biosynthesis, DXPS, HPPD, and γ-TMT, have been overexpressed in Arabidopsis leaves with varying degrees of success in altering leaf tocopherol levels. Estevez et al. (2001) reported significantly elevated levels of several plastidic isoprenoids, including a 2-fold increase in tocopherols, when DXPS was constitutively expressed in Arabidopsis leaves. Only a 30% increase in total leaf tocopherol content was observed in transgenic Arabidopsis lines overexpressing HPPD (Tsegaye et al., 2002). Constitutive overexpression of γ-TMT in Arabidopsis had no effect on tocopherol composition or total tocopherol levels in leaves (Shintani and DellaPenna, 1998). With the exception of γ-TMT, it appears that HPT, DXPS, and HPPD activities all limit tocopherol accumulation to some degree in wild-type Arabidopsis leaves.
In seeds, HPT1 overexpression resulted in accumulation of 37% more total tocopherols than wild type, which corresponds to an additional 310 pmol mg−1 seed versus 70 pmol mg−1 tissue for the 4.4-fold increase in leaf tocopherol levels. The increase in the absolute tocopherol levels in leaf tissue appears less significant than in seed until one considers the relative water content of the two tissues. Because Arabidopsis leaves contain 92% water, total tocopherol levels increased from 250 to 1,100 pmol mg−1 dry weight in wild-type and 35S::HPT1 leaves, respectively. In contrast, because mature Arabidopsis seed contain 11% water, total tocopherol content increased from 940 to 1,290 pmol mg−1 dry weight in wild-type and 35S::HPT1 leaves, respectively. Thus, overexpressing HPT1 elevated the HPT specific activity of seeds and leaves to similar levels, leading to similar final levels of total tocopherols in the two tissues but a proportionately higher impact on tocopherol accumulation in leaves. These data suggest that HPT has a relatively high flux coefficient in both leaves and seed, although indirect evidence suggests HPT activity is still limiting for tocopherol synthesis in 35S::HPT1 seeds. Savidge et al. (2002) reported that HPT1 overexpression driven by the strong, seed-specific napin promoter resulted in a 75% increase in seed tocopherol levels relative to wild type, which would correspond to approximately 1,650 pmol tocopherols mg−1 dry weight. Although HPT activity was not determined in these studies (Savidge et al., 2002), the higher tocopherol levels obtained are consistent with reports that the CaMV 35S promoter is less effective in developing seed than the napin promoter (Eccleston and Ohlrogge, 1998). Despite these differences, both studies demonstrate that HPT activity is limiting for tocopherol synthesis in wild-type Arabidopsis seed and is a key enzyme regulating tocopherol accumulation in plant tissues.
By alleviating the HPT limitation in the tocopherol pathway, it is possible that other enzyme activities downstream of HPT (TC, MPBQ MT, or γ-TMT) or their substrates might become limiting in 35S::HPT1 plants. A limitation in TC activity would result in the accumulation of DMPBQ, whereas a limitation in MPBQ MT activity would lead to the increased levels of β- and δ-tocopherols. If both enzymes were limiting, MPBQ in addition to DMPBQ and β- and δ-tocopherols would accumulate. None of these compounds were detected in leaves of wild-type or 35S::HPT1 plants. In 35S::HPT1 seed, δ-tocopherol levels were doubled, but still accounted for less than 5% of the total tocopherol pool. These data indicate that neither TC nor MPBQ MT are limiting in 35S::HPT1 seeds and leaves. Recent radiotracer studies using HGA and PDP also suggested that TC and MPBQ MT are not limiting for α-tocopherol synthesis in chromoplasts of yellow pepper (Arango and Heise, 1998).
In wild-type Arabidopsis seeds, but not in leaves, γ-TMT activity has previously been shown to be limiting for α-tocopherol synthesis (Shintani and DellaPenna, 1998). In leaves of 35S::HPT1, γ-tocopherol levels were up to 15-fold higher than wild type, whereas a 37% increase in γ-tocopherol levels was observed in 35S::HPT1 seeds. To test whether γ-TMT or S-adenosyl-l-Met was limiting in 35S::HPT1, the previously characterized 35S::γ-TMT lines (Shintani and DellaPenna, 1998) were crossed to 35S::HPT1 lines, and the resulting double homozygous transgenic progeny were selected and analyzed for tocopherol content and composition. Overexpression of γ-TMT in the 35S::HPT1 background resulted in almost complete methylation of the excess γ-tocopherol to α-tocopherol in both leaves and seeds. The persistence of small quantities of γ-tocopherol in 35S::γ-TMT and the double transgenics may reflect a distinct functional role for γ-tocopherol or may indicate that a fraction of the γ-tocopherol pool is not accessible for further methylation. These data collectively indicate that the biochemical phenotypes conferred individually by HPT and γ-TMT overexpression are additive in both seeds and leaves and that S-adenosyl-l-Met is not limiting for α-tocopherol synthesis in 35S::HPT1 lines.
Altering flux through a metabolic pathway can have unanticipated effects on compounds synthesized by biochemically related pathways, especially when some of the substrates are shared among these pathways (Fray et al., 1995; Shewmaker et al., 1999; Estevez et al., 2001). Overexpression of DXPS in Arabidopsis leaves increased the levels of several isoprenoid-derived compounds including carotenoids, chlorophylls, and tocopherols, suggesting that availability of chloroplastic GGDP, a common precursor in their biosynthesis, may be limiting in wild-type plants (Estevez et al., 2001). In canola (Brassica napus) seed, overexpression of phytoene synthase led to a 50-fold increase in carotenoids and a 50% decrease in tocopherol levels relative to wild type (Shewmaker et al., 1999). Constitutive overexpression of the GGDP-using carotenoid biosynthetic enzyme phytoene synthase in tomato caused chlorosis and dwarfism, most likely by redirecting the limited GGDP pool from the gibberellin and chlorophyll biosynthetic pathways to carotenoid synthesis (Fray et al., 1995). In light of these reports, the effects of HPT1 overexpression on the levels of other HGA- and GGDP-derived compounds in Arabidopsis tissues were also assessed.
Although we did not directly measure gibberellin levels in leaves of 35S::HPT1 plants, gibberellin metabolism appeared to be unaffected because no obvious differences in growth, flowering time, dormancy, or physical appearance were observed relative to wild type (data not shown). PQ-9, chlorophyll, and carotenoid levels in leaf and seed were also unaffected by HPT1 overexpression in Arabidopsis. One molecule of GGDP is used in the synthesis of each chlorophyll molecule, whereas carotenoids contain two molecules of GGDP or “GGDP equivalents.” The total chlorophyll and carotenoid content in leaves corresponds to 1,900 pmol mg−1 tissue of GGDP equivalents; the 70 pmol mg−1 increase in incorporation of GGDP equivalents into leaf tocopherols in 35S::HPT1-11 is negligible by comparison. In mature wild-type Arabidopsis seeds, this composition is reversed, with tocopherols predominating (up to 1,000 pmol mg−1 seed) and carotenoids being a minor component (approximately 30 pmol mg−1 seed). The 310 pmol mg−1 seed increase in GGDP equivalents incorporated into tocopherols in 35S::HPT1-11 seed is highly significant relative to the level of carotenoids, but apparently did not affect GGDP availability for carotenoid synthesis. This increased demand for GGDP in seeds may be compensated in part by increased flux through the DXP pathway. A similar phenomenon was observed in canola seed engineered for elevated carotenoid synthesis (Shewmaker et al., 1999). There seems to be a relatively high flexibility for flux through the seed DXP pathway leading to GGDP, likely by feedback regulation.
Outlook for Metabolic Engineering of Vitamin E Levels in Crops
Tocopherols are essential components of the human diet. Because of the relatively low levels of α-tocopherol, the most effective form of vitamin E, in most commonly consumed vegetables and oils (Eitenmiller, 1997; Bramley et al., 2000; Ching and Mohamed, 2001), α-tocopherol is limited in the average American diet (Grusak and DellaPenna, 1999; Horwitt, 2001). It is recommended that 35 μmol (15 mg) of α-tocopherol be consumed daily (Food and Nutrition Board, Institute of Medicine, 2000). Although Arabidopsis is not of direct agricultural importance, it has become a model for metabolic engineering of the tocopherol pathway and can be used to extrapolate the impact of engineering on dietary vitamin E intakes. In Table IV, we estimate the vitamin E activity of wild-type and various transgenic lines reported in this study in terms of the α-tocopherol equivalents (α-TE) per “serving size” of 100 g of leaves and seeds (for definition of α-TE see Table IV). In leaves of 35S::HPT1/35S::γ-TMT double overexpressers, which accumulate predominantly α-tocopherol, vitamin E activity was 3.2-fold higher than wild type. Because γ-tocopherol is the major tocopherol in mature wild-type Arabidopsis seed, the vitamin E activity is relatively low (3.9 mg of α-TE) despite the high levels of total tocopherols. In seed of 35S::HPT1/35S::γ-TMT lines, α-tocopherol is the major tocopherol, and the vitamin E activity increased 12-fold relative to wild type (Table IV). Similar increases in seed vitamin E activity are anticipated for genetically engineered crops (Grusak and DellaPenna, 1999).
Table IV.
Vitamin E activity in wild-type, single, and double overexpressers
| Line | Vitamin E Activity
|
|
|---|---|---|
| Leaves | Seeds | |
| mg α-TE 100 g−1 tissue | ||
| Wild type | 0.75 | 3.9 |
| 35S::HPT1 | 1.81 | 5.2 |
| 35S::γ-TMT | 0.87 | 35.6 |
| 35S::HPT1/35S::γ-TMT | 2.39 | 47.4 |
Vitamin E activity was calculated for each line using the absolute levels of individual tocopherols from Figures 4 and 5 and the relative biological activities of R,R,R-tocopherols. One milligram of α-, β-, γ-, and δ-tocopherol corresponds to 1, 0.5, 0.1, and 0.03 mg of α-TE, respectively (Food and Nutrition Board, Institute of Medicine, 2000).
MATERIALS AND METHODS
Chemicals
Tocopherol standards and tocol were purchased from Matreya (Pleasant Gap, PA). PDP was a gift from Dr. Stephanie Sen (Purdue University, West Lafayette, IN). [U-14C]HPP was prepared from l-[U-14C]Tyr (specific activity 464 mCi mmol−1; Amersham Biosciences Inc., Piscataway, NJ) according to Schulz et al. (1993). Minor modifications were introduced to obtain more concentrated [U-14C]HPP. In brief, l-[U-14C]Tyr was dried under a stream of nitrogen and dissolved in the original volume of phosphate buffer (0.5 m, pH 6.5). Catalase (Roche Diagnostics, Indianapolis) and l-amino acid oxidase type IV (Sigma-Aldrich, St. Louis) were both added to the final concentration of 0.4 mg mL−1. After 2 h of incubation at room temperature, [U- 14C]HPP was purified on an ion-exchange column (Dowex, Sigma-Aldrich) equilibrated with 0.1 n HCl and used immediately for prenyltransferase assays.
Generation of HPT1 Overexpressing Lines
A cDNA encoding HPT was excised from pSKHPT1 (Collakova and DellaPenna, 2001) by digestion with EcoRI and KpnI and subcloned into EcoRI and KpnI digested pART7 (Gleave, 1992). The resulting construct was mobilized by NotI digestion and ligation into the pART27-based vector pMLBART (Gleave, 1992), which contains the bar gene for selection of transformed plants. This construct was introduced into Agrobacterium tumefaciens and used to transform wild-type Arabidopsis plants (ecotype Columbia) by the floral dip method (Clough and Bent, 1998) to obtain 35S::HPT1 T0 sense plants.
Sixty-six independent transformants were selected by glufosinate (120 mg L−1) resistance and analyzed for tocopherol content. Seeds of lines exhibiting elevated leaf tocopherol levels were harvested and subjected to segregation analysis. All T235S::HPT1 transgenic plants segregating 3:1 for glufosinate resistance were carried through the next generation, and plants homozygous for glufosinate resistance were selected for further experiments. Plants overexpressing γ-TMT (Shintani and DellaPenna, 1998) under the control of 35S CaMV promoter were crossed with 35S::HPT1, and double homozygotes were selected based on their dual resistance to kanamycin and BASTA. Transgenic lines, 35S::HPT1-11 and -54, and 35S::γ-TMT-18 and -49 were used for these crosses.
Prenyllipid Analyses of Transgenic Plants
For leaf prenyllipid analyses, plants were grown in a 16-h photoperiod (70–100 μE) at 22°C/19°C day/night cycle for 4 to 5 weeks. For tocopherol analysis, lipids from 30 to 35 mg of leaf tissue or 10 to 13 mg of seeds were extracted in the presence of butylated hydroxytoluene (2 mg mL−1) to prevent tocopherol degradation (Bligh and Dyer, 1959). Tocol was used as an internal standard. For leaf analysis, tocopherols were separated on a reverse-phase HPLC (C18, ODS2, 4.6 × 250 mm; Column Engineering, Ontario, Canada; VP HPLC system, Shimadzu, Kyoto) using an isocratic solvent system of 5% (v/v) isopropanol in methanol at 2 mL min−1. For leaf and seed analyses, where separation of β- and γ-tocopherols was required, a normal phase HPLC system (LiChrosorb 5 Si60A 4.6- × 250-mm silica column, Column Engineering; HP 1100 series HPLC system, Agilent Technologies, Palo Alto, CA) and a 10-min isocratic method using 17% (v/v) di-isopropyl ether in hexane at 42°C at 2 mL min−1 were used. Tocopherols were detected by fluorescence at 290-nm excitation and 325-nm emission. Chlorophylls, carotenoids, and PQ-9 were analyzed as described (Collakova and DellaPenna, 2001). For PQ-9 analysis, 50 mg of leaves or 15 mg of seeds was used for extractions. Detection limit for PQ-9 was 4 pmol in seed.
Real-Time PCR
To obtain sufficient amounts of tissue, plants (wild type and 35S::HPT1-11 and -54) were grown at a 10-h photoperiod at 75 to 100 μE for 6 weeks. Tissue from six representative plants was harvested 2 to 3 h after the start of the light cycle and immediately frozen in liquid nitrogen. Total RNA was isolated and any contaminating genomic DNA was removed by treatment with RQ1-RNase free DNase (Promega, Madison, WI). Ten micrograms of total RNA was reverse transcribed to generate cDNA in two 50-μL reactions for each sample using a Taq-Man kit according to manufacturer's recommendations (Applied Biosystems, Foster City, CA). An aliquot of cDNA corresponding to 100 to 200 ng of total RNA was used in each Taq-Man real-time PCR assay (Applied Biosystems). Elongation factor EF1α was used to normalize RNA concentrations. Standard curves were constructed for each gene and were used to calculate the corresponding mRNA concentrations. Sequences of the primers and probes used in the real-time PCR assays and their optimal final concentrations are available upon request.
Prenyltransferase Assays
HPT assays were performed using crude chloroplast preparations or seed protein extracts from wild-type plants and 35S::HPT1-11 and -54 lines grown as described for real-time PCR assays. Chloroplasts were prepared from 25 to 30 g of leaf tissue. Tissue was disrupted using a blender in a buffer containing 0.6 m sorbitol, 0.5 m HEPES, pH 8.4, 4 mm EDTA, 4 mm EGTA, 10 mm Na2CO3, 0.2% (w/v) bovine serum albumin, 1 μm benzamidine, and 5 μm 4-aminocaproic acid. Chloroplasts were filtered through two layers of Miracloth and centrifuged at 4,000g for 5 min at 4°C. Crude chloroplasts were washed once in a buffer containing 50 mm HEPES, pH 7.6, 4 mm MgCl2, 1 μm benzamidine, and 5 μm 4-aminocaproic acid and once more in the same buffer lacking proteinase inhibitors.
Each prenyltransferase reaction (0.1 mL) contained 0 or 100 μm PDP, approximately 5 μm [U-14C]HPP (1 μCi), 20 μg of HPPD protein, and chloroplasts corresponding to about 0.45 to 0.65 mg of protein (0.10–0.15 mg of chlorophyll) in HPT reaction buffer (50 mm HEPES, pH 7.6, 4 mm MgCl2, 1 mm KF, 0.2% [v/v] Tween 80, and 50 mm potassium ascorbate). Reactions were incubated at room temperature for 2 h with shaking and quinones were extracted with two volumes of acetone:light petroleum ether (1:1, v/v). After centrifugation, the ether phase was loaded on a 4-mL silica column (pore size, 60 μm; Sigma-Aldrich), and quinones were eluted with 4 volumes of 20% (v/v) diethyl ether in petroleum ether. After drying, 5 mL of scintillation liquid was added to each sample and radioactivity was determined by scintillation counting. Background activities obtained from control reactions containing 14C-labeled HGA without exogenous PDP were subtracted from the activities when both substrates were added, which was always at least 3.5-fold higher than the background activities to obtain HPT specific activity. Chloroplasts were extracted with 90% (v/v) methanol and centrifuged to determine chlorophyll concentration (Lichtenthaler, 1987). Proteins present in the remaining pellet were solubilized by NaOH in the presence of SDS and determined by the method of Lowry (Stoscheck, 1990).
In seeds, protein extracts were prepared as follows: Dry mature seeds were ground in 50 mm HEPES (pH 7.6) containing 4 mm MgCl2 and centrifuged. The resulting pellet of water-insoluble proteins was resuspended in HPT reaction buffer to a protein concentration of 20 to 30 mg mL−1. HPT assays containing 2 to 3 mg of seed protein were performed in a volume of 0.2 mL as described for chloroplasts. Seed HPT specific activities were about 3-fold higher than the corresponding background activities.
ACKNOWLEDGMENTS
We thank Dr. Bart Janssen for providing pMLBART, Dr. Scott Sattler for optimizing real-time PCR probes and primers, and Maria Magallanes-Lundback for seed carotenoid analyses. We are very grateful to the members of the DellaPenna laboratory for reviewing this manuscript and for their helpful comments.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.015222.
LITERATURE CITED
- Addlesee HA, Gibson LCD, Jensen PE, Hunter CN. Cloning, sequencing and functional assignment of the chlorophyll biosynthesis gene, chlP, of Synechocystis sp. PCC 6803. FEBS Lett. 1996;389:126–130. doi: 10.1016/0014-5793(96)00549-2. [DOI] [PubMed] [Google Scholar]
- Addlesee HA, Hunter CN. Physical mapping and functional assignment of the geranylgeranyl-bacteriochlorophyll reductase gene, bchP, of Rhodobacter sphaeroides. J Bacteriol. 1999;181:7248–7255. doi: 10.1128/jb.181.23.7248-7255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango Y, Heise K-P. Tocopherol synthesis from homogentisate in Capsicum anuum L. (yellow pepper) chromoplast membranes: evidence for tocopherol cyclase. Biochem J. 1998;336:531–533. doi: 10.1042/bj3360531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bramley PM, Elmadfa I, Kafatos A, Kelly FJ, Manios Y, Roxborough HE, Schuch W, Sheehy PJA, Wagner KH. Vitamin E. J Sci Food Agric. 2000;80:913–938. [Google Scholar]
- Brigelius-Flohe R, Traber M. Vitamin E: function and metabolism. FASEB J. 1999;13:1145–1155. [PubMed] [Google Scholar]
- Ching LS, Mohamed S. Alpha-tocopherol content in 62 edible tropical plants. J Agric Food Chem. 2001;49:3101–3105. doi: 10.1021/jf000891u. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Collakova E, DellaPenna D. Isolation and functional analysis of homogentisate phytyltransferase from Synechocystis sp. PCC 6803 and Arabidopsis. Plant Physiol. 2001;127:1113–1124. [PMC free article] [PubMed] [Google Scholar]
- D'Harlingue A, Camara B. Plastid enzymes of terpenoid biosynthesis: purification and characterization of a gamma-tocopherol methyltransferase. J Biol Chem. 1985;260:15200–15203. [PubMed] [Google Scholar]
- Eccleston V, Ohlrogge J. Expression of lauroyl-acyl carrier protein thioesterase in Brassica napus seeds induces pathways for both fatty acid oxidation and biosynthesis and implies a set point for triacylglycerol accumulation. Plant Cell. 1998;10:613–621. doi: 10.1105/tpc.10.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenreich W, Schwarz M, Cartayrade A, Arigoni D, Zenk MH, Bacher A. The deoxyxylulose phosphate pathway of terpenoid biosynthesis in plants and microorganisms. Chem Biol. 1998;5:R221–R233. doi: 10.1016/s1074-5521(98)90002-3. [DOI] [PubMed] [Google Scholar]
- Eitenmiller RR. Vitamin E content of fats and oils: nutritional implications. Food Technol. 1997;51:78–81. [Google Scholar]
- Estevez JM, Cantero A, Reindl A, Reichler S, Leon P. 1-Deoxy-d-xylulose-5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. J Biol Chem. 2001;276:22901–22909. doi: 10.1074/jbc.M100854200. [DOI] [PubMed] [Google Scholar]
- Fiedler E, Soll J, Schultz G. The formation of homogentisate in the biosynthesis of tocopherol and plastoquinone in spinach chloroplasts. Planta. 1982;155:511–515. doi: 10.1007/BF01607575. [DOI] [PubMed] [Google Scholar]
- Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium and Carotenoids. Washington DC: National Academy Press; 2000. Vitamin E; pp. 186–283. [Google Scholar]
- Fray RG, Wallace A, Fraser PD, Valero D, Hedden P, Bramley PM, Grierson D. Constitutive expression of a fruit phytoene synthase gene in transgenic tomatoes causes dwarfism by redirecting metabolites from the gibberellin pathway. Plant J. 1995;8:693–701. [Google Scholar]
- Fryer MJ. The antioxidant effects of thylakoid vitamin E (α-tocopherol) Plant Cell Environ. 1992;15:381–392. [Google Scholar]
- Fryer MJ. Evidence for the photoprotective effects of vitamin E. Photochem Photobiol. 1993;58:304–312. doi: 10.1111/j.1751-1097.1993.tb09566.x. [DOI] [PubMed] [Google Scholar]
- Fukuzawa K, Gebicky JM. Oxidation of α-tocopherol in micelles and liposomes by the hydroxyl, perhydroxyl, and superoxide free radicals. Arch Biochem Biophys. 1983;226:242–251. doi: 10.1016/0003-9861(83)90290-4. [DOI] [PubMed] [Google Scholar]
- Furuya T, Yoshikawa T, Kimura T, Kaneko H. Production of tocopherols by cell culture of safflower. Phytochemistry. 1987;26:2741–2747. [Google Scholar]
- Garcia I, Rodgers M, Lenne C, Rolland A, Sailland A, Matringe M. Subcellular localisation and purification of a p-hydroxyphenylpyruvate dioxygenase from cultured carrot cells and characterization of the corresponding cDNA. Biochem J. 1997;325:761–769. doi: 10.1042/bj3250761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia I, Rodgers M, Pepin R, Hssich T, Matringe M. Characterization and subcellular compartmentation of recombinant 4-hydroxyphenylpyruvate dioxygenase from Arabidopsis in transgenic tobacco. Plant Physiol. 1999;119:1507–1516. doi: 10.1104/pp.119.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleave AP. A versatile binary vector system with a T-DNA organisational structure conductive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol. 1992;20:1203–1207. doi: 10.1007/BF00028910. [DOI] [PubMed] [Google Scholar]
- Gossett DR, Millhollon EP, Lucas MC. Antioxidant response to NaCl stress in salt-tolerant and salt-sensitive cultivars of cotton. Crop Sci. 1994;34:706–714. [Google Scholar]
- Grusak MA, DellaPenna D. Improving the nutrient composition of plants to enhance human nutrition and health. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:133–161. doi: 10.1146/annurev.arplant.50.1.133. [DOI] [PubMed] [Google Scholar]
- Havaux M, Bonfils J, Lutz C, Niyogi K. Photodamage of the photosynthetic apparatus and its dependence on the leaf development stage in the npq1 Arabidopsis mutant deficient in the xanthophyll cycle enzyme violaxanthin de-epoxidase. Plant Physiol. 2000;124:273–284. doi: 10.1104/pp.124.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitt MK. Critique of the requirement for vitamin E. Am J Clin Nutr. 2001;73:1003–1005. doi: 10.1093/ajcn/73.6.1003. [DOI] [PubMed] [Google Scholar]
- Hutson KG, Threlfall DR. Synthesis of plastoquinone-9 and phytylplastoquinone from homogentisate in lettuce chloroplasts. Biochim Biophys Acta. 1980;632:630–648. doi: 10.1016/0304-4165(80)90339-6. [DOI] [PubMed] [Google Scholar]
- Keller Y, Bouvier F, D'Harlingue A, Camara B. Metabolic compartmentation of plastid prenyllipid biosynthesis: evidence for the involvement of a multifunctional geranylgeranyl reductase. Eur J Biochem. 1998;251:413–417. doi: 10.1046/j.1432-1327.1998.2510413.x. [DOI] [PubMed] [Google Scholar]
- Kuntz M, Romer S, Suire C, Hugueney P, Schantz JHWR, Camara B. Identification of a cDNA for the plastid-located geranylgeranyl pyrophosphate synthase from Capsicum annuum: correlative increase in enzyme activity and transcript level during fruit ripening. Plant J. 1992;2:25–34. doi: 10.1111/j.1365-313x.1992.00025.x. [DOI] [PubMed] [Google Scholar]
- Leipner J, Fracheboud Y, Stamp P. Effects of growing season on the photosynthetic apparatus and leaf antioxidative defenses in two maize genotypes of different chilling tolerance. Environ Exp Bot. 1999;42:129–139. [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. [Google Scholar]
- Lichtenthaler HK. The plants' 1-deoxy-d-xylulose-5-phosphate pathway for biosynthesis of isoprenoids. Fett Lipid. 1998;100:128–138. [Google Scholar]
- Lopukhina A, Dettenberg M, Weiler EW, Hollander-Czytko H. Cloning and characterization of a coronatine-regulated tyrosine aminotransferase from Arabidopsis. Plant Physiol. 2001;126:1678–1687. doi: 10.1104/pp.126.4.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PS, Morris SR, Threlfall DR. Biosynthesis of tocopherols: a re-examination of the biosynthesis and metabolism of 2-demethyl-6-phytyl-1,4-benzoquinol. Phytochemistry. 1985;24:1705–1711. [Google Scholar]
- Molina-Torres J, Martinez ML. Tocopherols and leaf age in Xanthium strumarium L. New Phytol. 1991;118:95–99. [Google Scholar]
- Munne-Bosch S, Alegre L. Changes in carotenoids, tocopherols and diterpenes during drought and recovery, and the biological significance of chlorophyll loss in Rosmarinus officinalis plants. Planta. 2000;210:925–931. doi: 10.1007/s004250050699. [DOI] [PubMed] [Google Scholar]
- Munne-Bosch S, Alegre L. The function of tocopherols and tocotrienols in plants. Crit Rev Plant Sci. 2002;21:31–57. [Google Scholar]
- Neely WC, Martin JM, Barker SA. Products and relative reaction rates of the oxidation of tocopherols with singlet molecular oxygen. Photochem Photobiol. 1988;48:423–428. doi: 10.1111/j.1751-1097.1988.tb02840.x. [DOI] [PubMed] [Google Scholar]
- Norris SR, Barrette TR, DellaPenna D. Genetic dissection of carotenoid synthesis in Arabidopsis defines plastoquinone as an essential component of phytoene desaturation. Plant Cell. 1995;7:2139–2149. doi: 10.1105/tpc.7.12.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris SR, Shen X, DellaPenna D. Complementation of the Arabidopsis pds1 mutation with the gene encoding p-hydroxyphenylpyruvate dioxygenase. Plant Physiol. 1998;117:1317–1323. doi: 10.1104/pp.117.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Saito T, Nakagawa T, Kawamukai M, Kamiya Y. Five geranylgeranyl diphosphate synthases expressed in different organs are localized into three subcellular compartments in Arabidopsis. Plant Physiol. 2000;122:1045–1056. doi: 10.1104/pp.122.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster U, Bauer CE, Rudiger W. Characterization of chlorophyll a and bacteriochlorophyll a synthases by heterologous expression in Escherichia coli. J Biol Chem. 1997;272:9671–9676. doi: 10.1074/jbc.272.15.9671. [DOI] [PubMed] [Google Scholar]
- Porfirova S, Bergmuller E, Tropf S, Lemke R, Dormann P. Isolation of an Arabidopsis mutant lacking vitamin E and identification of a cyclase essential for all tocopherol biosynthesis. Proc Natl Acad Sci USA. 2002;99:12495–12500. doi: 10.1073/pnas.182330899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor WA. Vitamin E and heart disease: basic science to clinical intervention trials. Free Radic Biol Med. 2000;28:141–164. doi: 10.1016/s0891-5849(99)00224-5. [DOI] [PubMed] [Google Scholar]
- Rise M, Cojocaru M, Gottlieb HE, Goldschmidt E. Accumulation of α-tocopherol in senescing organs as related to chlorophyll degradation. Plant Physiol. 1989;89:1028–1030. doi: 10.1104/pp.89.4.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savidge B, Weiss JD, Wong Y-HH, Lassner MW, Mitsky TA, Shewmaker CK, Post-Beittenmiller D, Valentin HE. Isolation and characterization of homogentisate phytyltransferase genes from Synechocystis sp. PCC 6803 and Arabidopsis. Plant Physiol. 2002;129:321–332. doi: 10.1104/pp.010747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schledz M, Seidler A, Beyer P, Neuhaus G. A novel phytyltransferase from Synechocystis sp. PCC 6803 involved in tocopherol biosynthesis. FEBS Lett. 2001;499:15–20. doi: 10.1016/s0014-5793(01)02508-x. [DOI] [PubMed] [Google Scholar]
- Schulz A, Ort O, Beyer P, Kleinig H. SC-0051, a 2-benzoyl-cyclohexane-1,3-dione bleaching herbicide, is a potent inhibitor of the enzyme p-hydroxyphenylpyruvate dioxygenase. FEBS Lett. 1993;318:162–166. doi: 10.1016/0014-5793(93)80013-k. [DOI] [PubMed] [Google Scholar]
- Schulze-Siebert D, Homeyer U, Soll J, Schultz G. Synthesis of plastoquinone-9, α-tocopherol and phylloquinone (vitamin K1) and its integration in chloroplast carbon metabolism of higher plants. In: Stumpf P, Mudd J, Nes W, editors. The metabolism, structure, and function of plant lipids. New York: Plenum Press; 1987. pp. 29–36. [Google Scholar]
- Shewmaker CK, Sheehy JA, Daley M, Colburn S, Ke DY. Seed-specific overexpression of phytoene synthase: increase in carotenoids and other metabolic effects. Plant J. 1999;20:401–412. doi: 10.1046/j.1365-313x.1999.00611.x. [DOI] [PubMed] [Google Scholar]
- Shintani DK, Cheng Z, DellaPenna D. The role of 2-methyl-6-phytylbenzoquinone methyltransferase in determining tocopherol composition in Synechocystis sp. PCC6803. FEBS Lett. 2002;511:1–5. doi: 10.1016/s0014-5793(01)03223-9. [DOI] [PubMed] [Google Scholar]
- Shintani DK, DellaPenna D. Elevating the vitamin E content of plants through metabolic engineering. Science. 1998;282:2098–2100. doi: 10.1126/science.282.5396.2098. [DOI] [PubMed] [Google Scholar]
- Soll J. α-Tocopherol and plastoquinone synthesis in chloroplast membranes. Methods Enzymol. 1987;148:383–392. [Google Scholar]
- Soll J, Kemmerling M, Schultz G. Tocopherol and plastoquinone synthesis in spinach chloroplasts subfractions. Arch Biochem Biophys. 1980;204:544–550. doi: 10.1016/0003-9861(80)90066-1. [DOI] [PubMed] [Google Scholar]
- Soll J, Schultz G. Comparison of geranylgeranyl and phytyl substituted methylquinols in the tocopherol synthesis of spinach chloroplasts. Biochem Biophys Res Comm. 1979;91:715–720. doi: 10.1016/0006-291x(79)91939-9. [DOI] [PubMed] [Google Scholar]
- Soll J, Schultz G. 2-Methyl-6-phytylquinol and 2,3-dimethyl-5-phytylquinol as precursors of tocopherol synthesis in spinach chloroplasts. Phytochemistry. 1980;19:215–218. [Google Scholar]
- Soll J, Schultz G, Joyard J, Douce R, Block MA. Localisation and synthesis of prenylquinones in isolated outer and inner envelope membranes from spinach chloroplasts. In: Siegenthaler P-A, Eichenberger W, editors. Structure, Function and Metabolism of Plant Lipids. Amsterdam: Elsevier Science Publishers B. V.; 1984. pp. 263–266. [Google Scholar]
- Stocker A, Fretz H, Frick H, Ruttimann A, Woggon W-D. The substrate specificity of tocopherol cyclase. Bioorg Med Chem. 1996;4:1129–1134. doi: 10.1016/0968-0896(96)00125-3. [DOI] [PubMed] [Google Scholar]
- Stoscheck CM. Quantitation of protein. In: Deutscher MP, editor. Guide to Protein Purification. San Diego: Academic Press; 1990. pp. 57–60. [Google Scholar]
- Streb P, Shang W, Feierabend J, Bligny R. Divergent strategies of photoprotection in high-mountain plants. Planta. 1998;207:313–324. [Google Scholar]
- Threlfall DR, Whistance GR. Biosynthesis of isoprenoid quinones and chromanols. In: Goodwin T, editor. Aspects of Terpenoid Chemistry and Biochemistry. Liverpool, UK: Academic Press; 1971. pp. 357–404. [Google Scholar]
- Tramontano WA, Ganci D, Pennino M, Dierenfeld ES. Age dependent α-tocopherol concentrations in leaves of soybean and pinto beans. Phytochemistry. 1992;31:3349–3351. [Google Scholar]
- Tsegaye Y, Shintani DK, DellaPenna D. Over-expression of the enzyme p-hydroxyphenylpyruvate dioxygenase in Arabidopsis and its relation to tocopherol biosynthesis. Plant Physiol Biochem. 2002;40:913–920. [Google Scholar]