Abstract
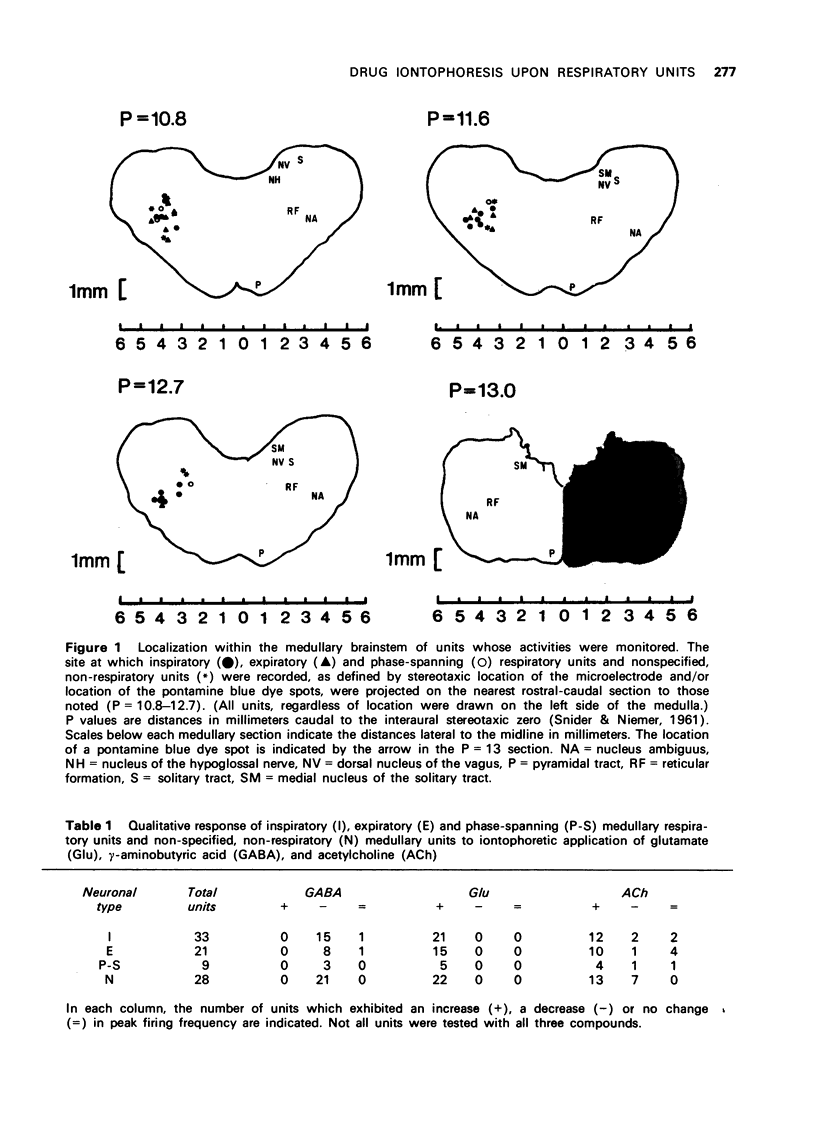
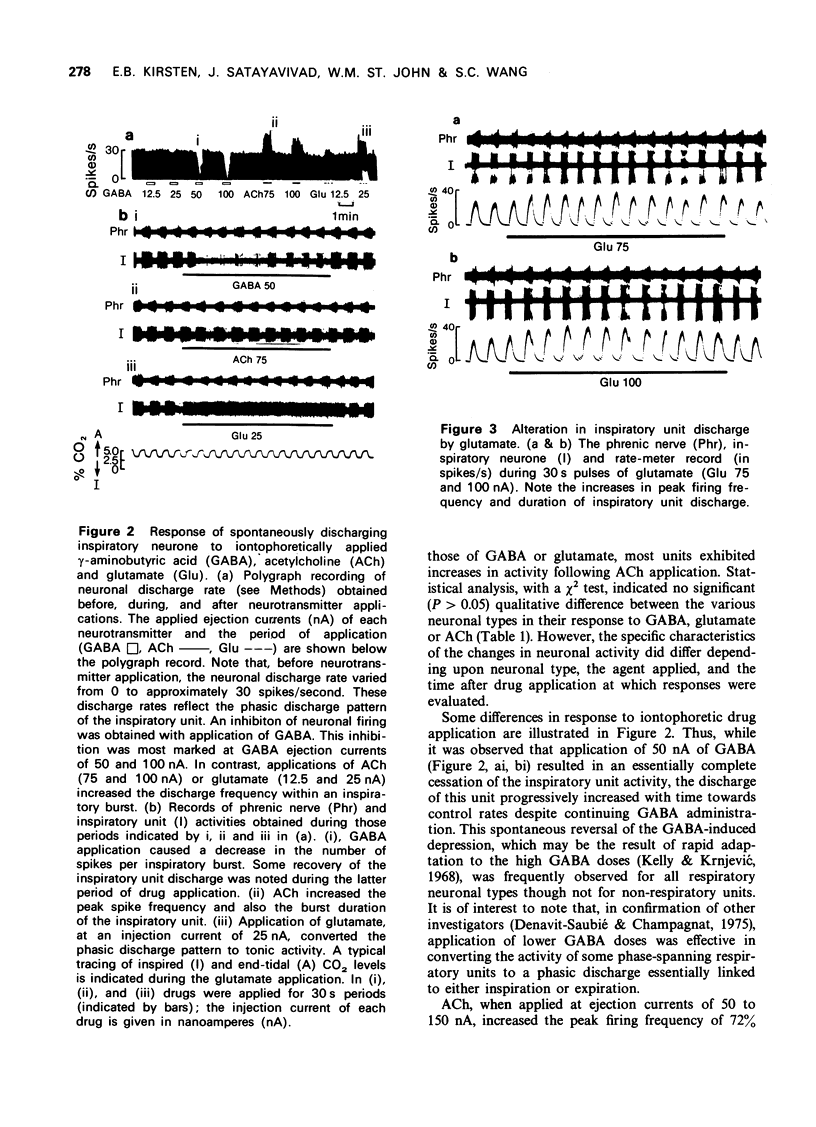
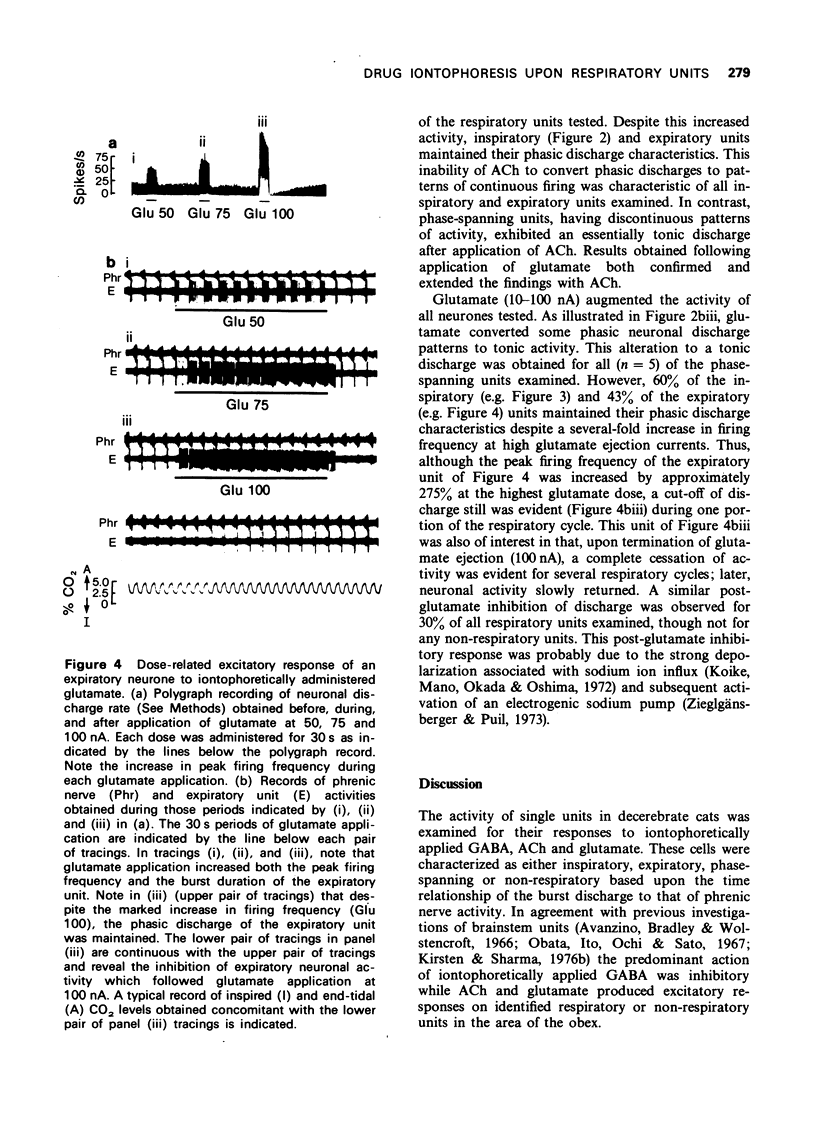
1 Cats with midcollicular decerebration were vagotomized, paralyzed and artificially ventilated. Phrenic nerve activity was recorded as an index of central respiratory rhythm. Medullary respiratory neurones and non-respiratory cells located in approximation to the ventral respiratory nucleus were tested for their responsiveness to iontophoretically applied gamma-aminobutyric acid (GABA), acetylcholine (ACh) and glutamate. 2 GABA tended to inhibit, whereas ACh and glutamate excited activity both of respiratory and non-respiratory units. Some phase-spanning respiratory unit activities were converted to phasic discharge patterns linked to either inspiration or expiration concomitant with application of low GABA doses. Appropriate applications of GABA also resulted in a complete cessation of the respiratory or non-respiratory neuronal activities. 3 While application of ACh or glutamate induced continuous firing in phasic, phase-spanning respiratory neurones, the periodic discharge patterns of inspiratory or expiratory units was not altered by ACh or, in many instances, by glutamate. Only at high doses of glutamate was the phasic discharge of some inspiratory or expiratory units converted to tonic activity. 4 These observations suggest that strong inhibitory processes serve to maintain the phasic firing pattern of respiratory units. These data also support the concept that active-inhibitory phase-switching mechanisms serve to define respiratory rhythmicity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avanzino G. L., Bradley P. B., Wolstencroft J. H. Pharmacological properties of neurons of the paramedian reticular nucleus. Experientia. 1966 Jun 15;22(6):410–410. doi: 10.1007/BF01901170. [DOI] [PubMed] [Google Scholar]
- Berger A. J., Mitchell R. A. Lateralized phrenic nerve responses to stimulating respiratory afferents in the cat. Am J Physiol. 1976 May;230(5):1314–1320. doi: 10.1152/ajplegacy.1976.230.5.1314. [DOI] [PubMed] [Google Scholar]
- Bertrand F., Hugelin A., Vibert J. F. A stereologic model of pneumotaxic oscillator based on spatial and temporal distributions of neuronal bursts. J Neurophysiol. 1974 Jan;37(1):91–107. doi: 10.1152/jn.1974.37.1.91. [DOI] [PubMed] [Google Scholar]
- Bianchi A. L. Localisation et étude des neurones respiratoires bulbaires. Mise en jeu antidromique par stimulation spinale ou vagale. J Physiol (Paris) 1971 Jan-Feb;63(1):5–40. [PubMed] [Google Scholar]
- Boakes R. J., Bramwell G. J., Briggs I., Candy J. M., Tempesta E. Localization with Pontamine Sky Blue of neurones in the brainstem responding to microiontophoretically applied compounds. Neuropharmacology. 1974 Jun;13(6):475–479. doi: 10.1016/0028-3908(74)90136-1. [DOI] [PubMed] [Google Scholar]
- Bradley G. W., von Euler C., Marttila I., Roos B. A model of the central and reflex inhibition of inspiration in the cat. Biol Cybern. 1975 Aug 8;19(2):105–116. doi: 10.1007/BF00364107. [DOI] [PubMed] [Google Scholar]
- COHEN M. I., WANG S. C. Respiratory neuronal activity in pons of cat. J Neurophysiol. 1959 Jan;22(1):33–50. doi: 10.1152/jn.1959.22.1.33. [DOI] [PubMed] [Google Scholar]
- Clark F. J., von Euler C. On the regulation of depth and rate of breathing. J Physiol. 1972 Apr;222(2):267–295. doi: 10.1113/jphysiol.1972.sp009797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. I. Discharge patterns of brain-stem respiratory neurons in relation to carbon dioxide tension. J Neurophysiol. 1968 Mar;31(2):142–165. doi: 10.1152/jn.1968.31.2.142. [DOI] [PubMed] [Google Scholar]
- Feldman J. L. A network model for control of inspiratory cutoff by the pneumotaxic center with supportive experimental data in cats. Biol Cybern. 1976 Jan 10;21(3):131–138. doi: 10.1007/BF00337420. [DOI] [PubMed] [Google Scholar]
- Geller H. M., Woodward D. J. An improved constant current source for micro-iontophoretic drug application studies. Electroencephalogr Clin Neurophysiol. 1972 Oct;33(4):430–432. doi: 10.1016/0013-4694(72)90125-3. [DOI] [PubMed] [Google Scholar]
- Kelly J. S., Krnjević K. Effects of gamma-aminobutyric acid and glycine on cortical neurons. Nature. 1968 Sep 28;219(5161):1380–1381. doi: 10.1038/2191380a0. [DOI] [PubMed] [Google Scholar]
- Kirsten E. B., Sharma J. N. Characteristicas and response differences to iontophoretically applied norepinephrine, D-amphetamine and acetylcholine on neurons in the medial and lateral vestibular nuclei of the cat. Brain Res. 1976 Aug 6;112(1):77–90. doi: 10.1016/0006-8993(76)90335-8. [DOI] [PubMed] [Google Scholar]
- Kirsten E. B., Sharma J. N. Microiontophoresis of acetylcholine, histamine and their antagonists on neurones in the medial and lateral vestibular nuclei of the cat. Neuropharmacology. 1976 Dec;15(12):743–753. doi: 10.1016/0028-3908(76)90003-4. [DOI] [PubMed] [Google Scholar]
- Koike H., Mano N., Okada Y., Oshima T. Activities of the sodium pump in cat pyramidal tract cell studied with intracellular injection of sodium ions. Exp Brain Res. 1972 Apr 27;14(5):449–462. doi: 10.1007/BF00236587. [DOI] [PubMed] [Google Scholar]
- Mitchell R. A., Berger A. J. Neural regulation of respiration. Am Rev Respir Dis. 1975 Feb;111(2):206–224. doi: 10.1164/arrd.1975.111.2.206. [DOI] [PubMed] [Google Scholar]
- Mitchell R. A., Herbert D. A. The effect of carbon dioxide on the membrane potential of medullary respiratory neurons. Brain Res. 1974 Jul 26;75(2):345–349. doi: 10.1016/0006-8993(74)90759-8. [DOI] [PubMed] [Google Scholar]
- Obata K., Ito M., Ochi R., Sato N. Pharmacological properties of the postsynaptic inhibition by Purkinje cell axons and the action of gamma-aminobutyric acid on deiters NEURONES. Exp Brain Res. 1967;4(1):43–57. doi: 10.1007/BF00235216. [DOI] [PubMed] [Google Scholar]
- Richter D. W., Heyde F., Gabriel M. Intracellular recordings from different types of medullary respiratory neurons of the cat. J Neurophysiol. 1975 Sep;38(5):1162–1171. doi: 10.1152/jn.1975.38.5.1162. [DOI] [PubMed] [Google Scholar]
- SALMOIRAGHI G. C., STEINER F. A. Acetylcholine sensitivity of cat's medullary neurons. J Neurophysiol. 1963 Jul;26:581–597. doi: 10.1152/jn.1963.26.4.581. [DOI] [PubMed] [Google Scholar]
- Vibert J. F., Bertrand F., Denavit-Saubié M., Hugelin A. Three dimensional representation of bulbo-pontine respiratory networks architecture from unit density maps. Brain Res. 1976 Sep 17;114(2):227–244. doi: 10.1016/0006-8993(76)90668-5. [DOI] [PubMed] [Google Scholar]
- Zieglgänsberger W., Puil E. A. Actions of glutamic acid on spinal neurones. Exp Brain Res. 1973 Mar 29;17(1):35–49. doi: 10.1007/BF00234562. [DOI] [PubMed] [Google Scholar]
- von Euler C., Trippenbach T. Excitability changes of the inspiratory "off-switch" mechanism tested by electrical stimulation in nucleus parabrachialis in the cat. Acta Physiol Scand. 1976 Jun;97(2):175–188. doi: 10.1111/j.1748-1716.1976.tb10250.x. [DOI] [PubMed] [Google Scholar]



