Abstract
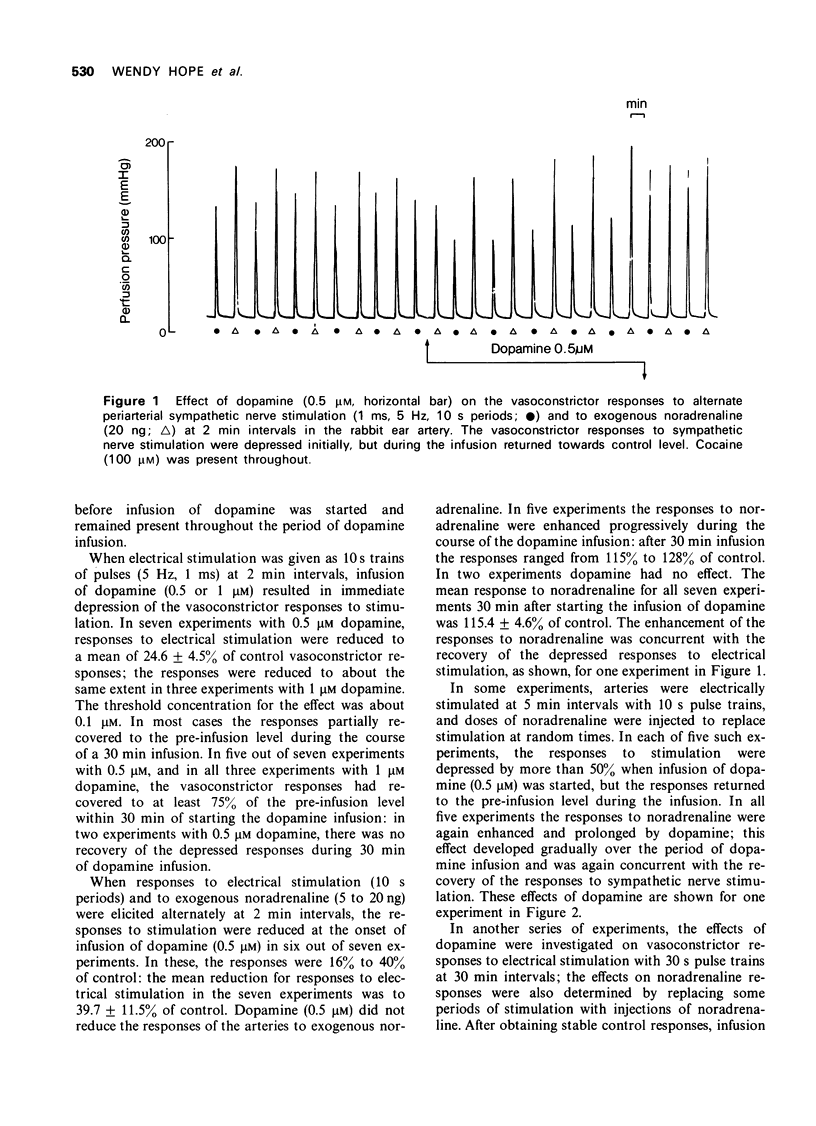
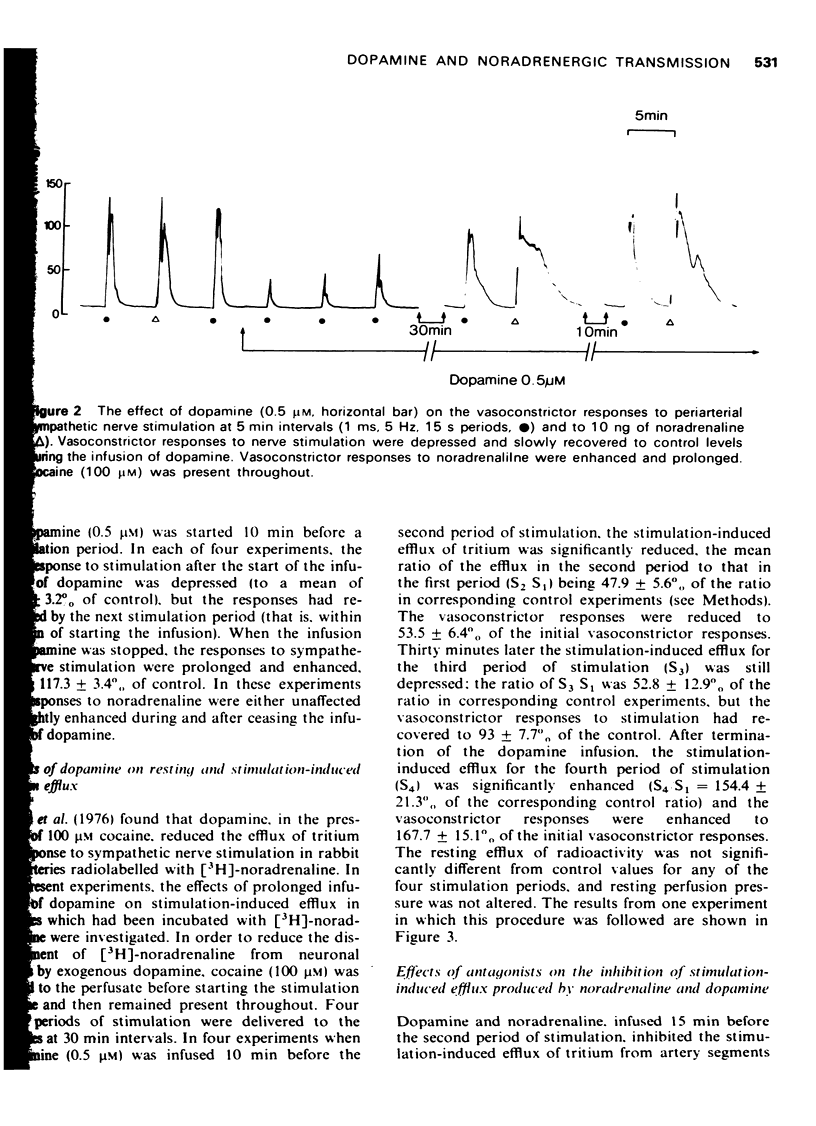
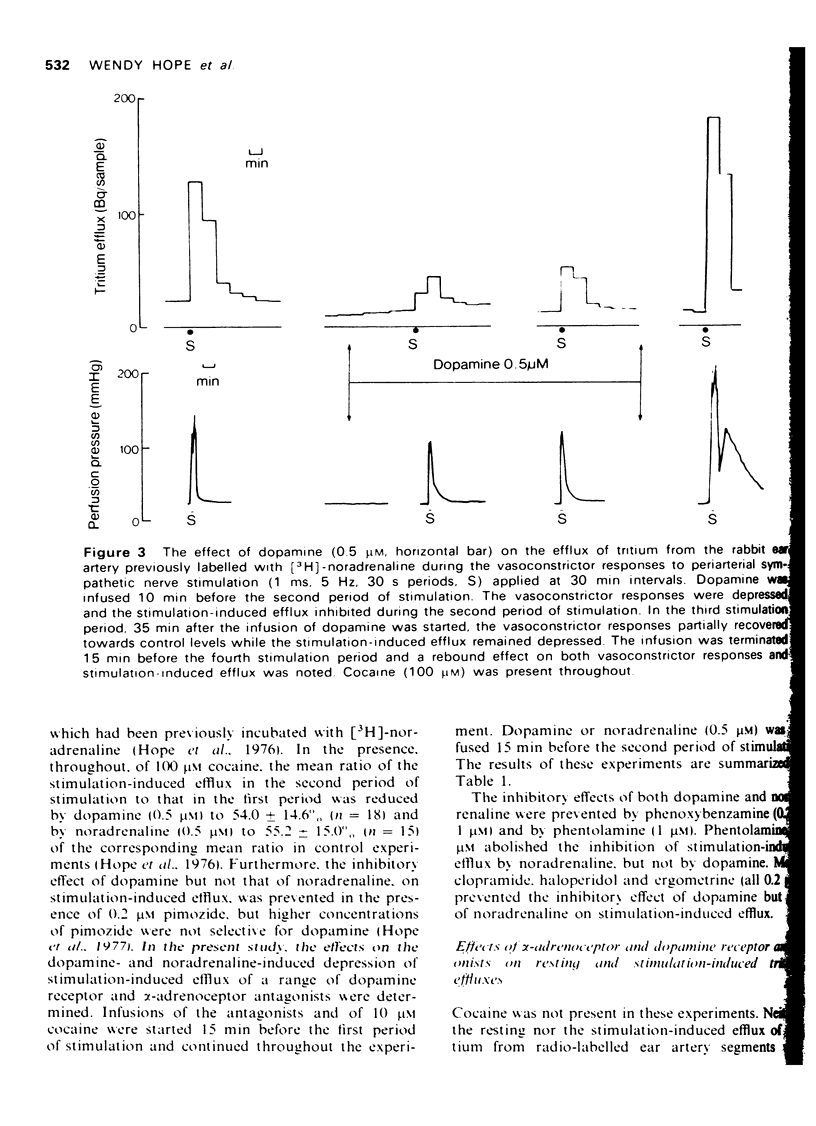
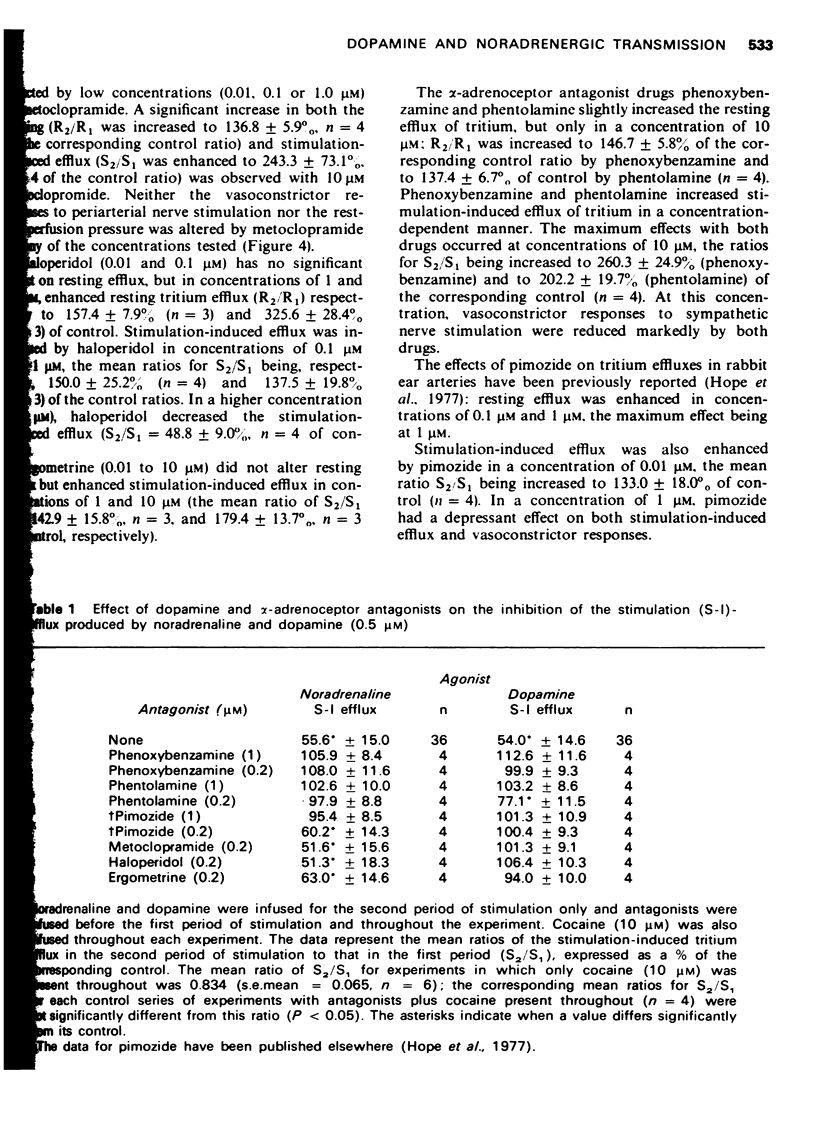
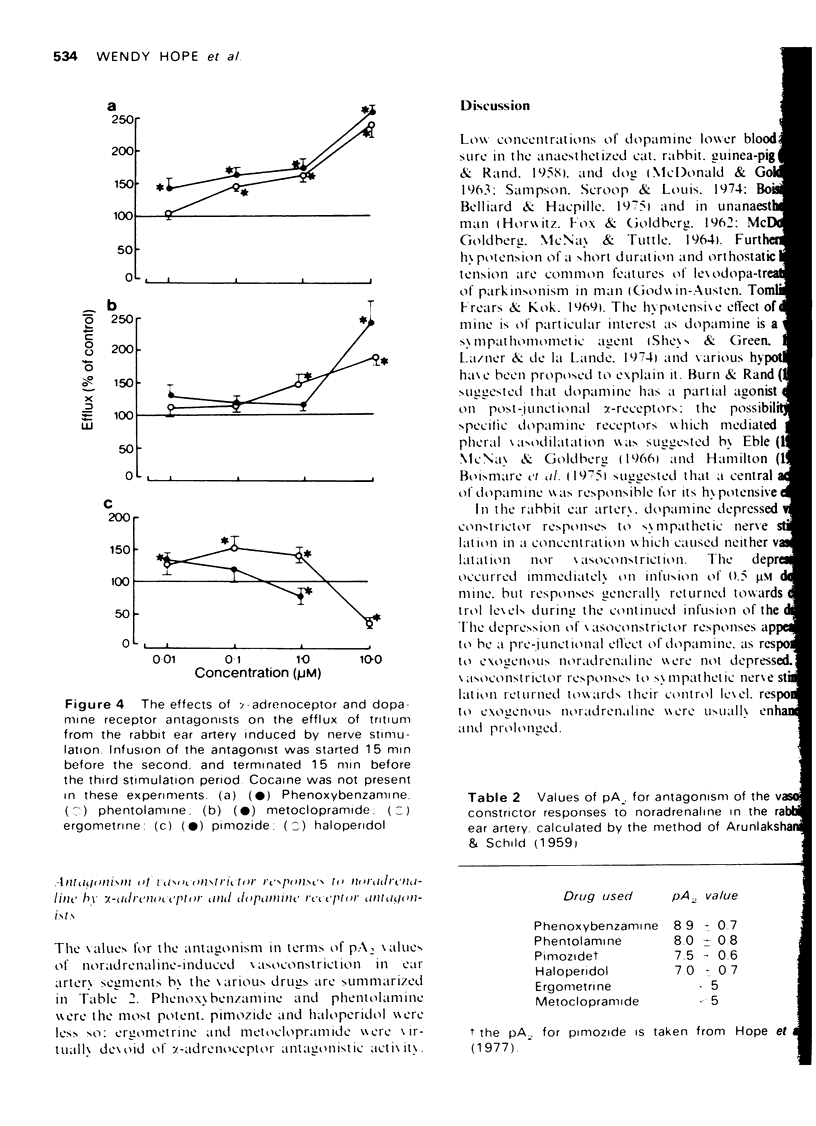
1. The effects of dopamine on vasoconstrictor responses to field stimulation of sympathetic nerves and to exogenous noradrenaline were studied in the isolated ear artery of the rabbit. Responses to noradrenaline were unchanged at the start of the dopamine infusions but were enhanced as the infusions continued and also after cessation of the infustion. 2 Dopamine (0.5 muM) reduced the stimulation-induced efflux of tritium from segments of ear artery labelled with [3H]-noradrenaline. The reduction persisted during 65 min of dopamine infusion, after which time the vasoconstrictor responses had generally recovered to 93% of control level. On ceasing the infusion, the stimulation-induced efflux and the vasoconstrictor responses were enhanced. 3 Metoclopramide, haloperidol and ergometrine, each in a concentration of 0.2 muM, prevented the inhibitory effect of 0.5 muM dopamine on the stimulation-induced tritium release, but not the inhibitory effect of 0.5 muM noradrenaline. Phenoxybenzamine (0.2 and 1 muM) and phentolamine (1 muM) prevented the inhibitory effects of both noradrenaline and dopamine on the stimulation-induced efflux, and phentolamine (0.2 muM) prevented the inhibition of the stimulation-induced release by noradrenaline but only partially prevented the inhibitory effect of dopamine on the stimulation-induced efflux. 4 A possible role for dopamine in the modulation of noradrenergic transmission is suggested.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G. S., Rand M. J., Story D. F. Techniques for studying adrenergic transmitter release in an isolated perfused artery. Cardiovasc Res. 1973 May;7(3):423–428. doi: 10.1093/cvr/7.3.423. [DOI] [PubMed] [Google Scholar]
- Andén N. E., Butcher S. G., Corrodi H., Fuxe K., Ungerstedt U. Receptor activity and turnover of dopamine and noradrenaline after neuroleptics. Eur J Pharmacol. 1970;11(3):303–314. doi: 10.1016/0014-2999(70)90006-3. [DOI] [PubMed] [Google Scholar]
- BURN J. H., RAND M. J. The depressor action of dopamine and adrenaline. Br J Pharmacol Chemother. 1958 Dec;13(4):471–479. doi: 10.1111/j.1476-5381.1958.tb00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant B. J., McCulloch M. W., Rand M. J., Story D. F. Release of 3-H-(--)-noradrenaline from guinea-pig hypothalamic slices: effects of adrenoceptor agonists and antagonists. Br J Pharmacol. 1975 Mar;53(3):454P–454P. [PMC free article] [PubMed] [Google Scholar]
- Costa E., Green A. R., Koslow S. H., LeFevre H. F., Revuelta A. V., Wang C. Dopamine and norepinephrine in noradrenergic axons: a study in vivo of their precursor product relationship by mass fragmentography and radiochemistry. Pharmacol Rev. 1972 Jun;24(2):167–190. [PubMed] [Google Scholar]
- Dougan D. F., Mearrick P. T., Wade D. N. Metoclopramide as a dopamine antagonist in the heart and gut of the mollusc Tapes watlingi. Clin Exp Pharmacol Physiol. 1974 Nov-Dec;1(6):473–478. doi: 10.1111/j.1440-1681.1974.tb00568.x. [DOI] [PubMed] [Google Scholar]
- Drew G. M. Effects of alpha-adrenoceptor agonists and antagonists on pre- and postsynaptically located alpha-adrenoceptors. Eur J Pharmacol. 1976 Apr;36(2):313–320. doi: 10.1016/0014-2999(76)90084-4. [DOI] [PubMed] [Google Scholar]
- Drew G. M. Pharmacological characterisation of the presynaptic alpha-adrenoceptor in the rat vas deferens. Eur J Pharmacol. 1977 Mar 21;42(2):123–130. doi: 10.1016/0014-2999(77)90351-x. [DOI] [PubMed] [Google Scholar]
- Dubocovich M. L., Langer S. Z. Negative feed-back regulation of noradrenaline release by nerve stimulation in the perfused cat's spleen: differences in potency of phenoxybenzamine in blocking the pre- and post-synaptic adrenergic receptors. J Physiol. 1974 Mar;237(3):505–519. doi: 10.1113/jphysiol.1974.sp010495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EBLE J. N. A PROPOSED MECHANISM FOR THE DEPRESSOR EFFECT OF DOPAMINE IN THE ANESTHETIZED DOG. J Pharmacol Exp Ther. 1964 Jul;145:64–70. [PubMed] [Google Scholar]
- Endo T., Starke K., Bangerter A., Taube H. D. Presynaptic receptor systems on the noradrenergic neurones of the rabbit pulmonary artery. Naunyn Schmiedebergs Arch Pharmacol. 1977 Feb;296(3):229–247. doi: 10.1007/BF00498689. [DOI] [PubMed] [Google Scholar]
- Enero M. A., Langer S. Z. Inhibition by dopamine of 3H-noradrenaline release elicited by nerve stimulation in the isolated cat's nictitating membrane. Naunyn Schmiedebergs Arch Pharmacol. 1975;289(2):179–203. doi: 10.1007/BF00501305. [DOI] [PubMed] [Google Scholar]
- Farmer J. B. Impairment of sympathetic nerve responses by dopa, dopamine and their alpha-methyl analogues. J Pharm Pharmacol. 1965 Oct;17(10):640–646. doi: 10.1111/j.2042-7158.1965.tb07576.x. [DOI] [PubMed] [Google Scholar]
- Geffen L. B., Livett B. G., Rush R. A. Immunological localization of chromogranins in sheep sympathetic neurones, and their release by nerve impulses. J Physiol. 1969 Oct;204(2):58P–59P. [PubMed] [Google Scholar]
- Godwin-Austen R. B., Tomlinson E. B., Frears C. C., Kok H. W. Effects of L-dopa in Parkinson's disease. Lancet. 1969 Jul 26;2(7613):165–168. doi: 10.1016/s0140-6736(69)91417-2. [DOI] [PubMed] [Google Scholar]
- HORWITZ D., FOX SM 3. D., GOLDBERG L. I. Effects of Dopamine in man. Circ Res. 1962 Feb;10:237–243. doi: 10.1161/01.res.10.2.237. [DOI] [PubMed] [Google Scholar]
- Hamilton T. C. Effects of dopamine on the conductance of perfused vascular beds of the chloralosed cat. Br J Pharmacol. 1972 Mar;44(3):442–450. doi: 10.1111/j.1476-5381.1972.tb07282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope W., Law M., McCulloch M. W., Rand M. J., Story D. F. Effects of some catecholamines on noradrenergic transmission in the rabbit ear artery. Clin Exp Pharmacol Physiol. 1976 Jan-Feb;3(1):15–28. [PubMed] [Google Scholar]
- Hope W., McCulloch M. W., Story D. F., Rand M. J. Effects of pimozide on noradrenergic transmission in rabbit isolated ear arteries. Eur J Pharmacol. 1977 Nov 15;46(2):101–111. doi: 10.1016/0014-2999(77)90245-x. [DOI] [PubMed] [Google Scholar]
- Ilhan M., Long J. P. Inhibition of the sympathetic nervous system by dopamine. Arch Int Pharmacodyn Ther. 1975 Jul;216(1):4–10. [PubMed] [Google Scholar]
- Kier L. B., Truitt E. B., Jr The preferred conformation of dopamine from molecular orbital theory. J Pharmacol Exp Ther. 1970 Jul;174(1):94–98. [PubMed] [Google Scholar]
- Kopin I. J., Breese G. R., Krauss K. R., Weise V. K. Selective release of newly synthesized norepinephrine from the cat spleen during sympathetic nerve stimulation. J Pharmacol Exp Ther. 1968 Jun;161(2):271–278. [PubMed] [Google Scholar]
- Langer S. Z. Presynaptic regulation of catecholamine release. Biochem Pharmacol. 1974 Jul 1;23(13):1793–1800. doi: 10.1016/0006-2952(74)90187-7. [DOI] [PubMed] [Google Scholar]
- Langer S. Z. Sixth gaddum memorial lecture, National Institute for Medical Research, Mill Hill, January 1977. Presynaptic receptors and their role in the regulation of transmitter release. Br J Pharmacol. 1977 Aug;60(4):481–497. doi: 10.1111/j.1476-5381.1977.tb07526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S. Z. The metabolism of (3H)noradrenaline released by electrical stimulation from the isolated nictitating membrane of the cat and from the vas deferens of the rat. J Physiol. 1970 Jul;208(3):515–546. doi: 10.1113/jphysiol.1970.sp009135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazner M. A., De la Lande I. S. Comparative potencies of dopamine and noradrenaline on the rabbit ear artery. J Pharm Pharmacol. 1974 Jan;26(1):62–65. doi: 10.1111/j.2042-7158.1974.tb12822.x. [DOI] [PubMed] [Google Scholar]
- MCDONALD R. H., Jr, GOLDBERG L. I., MCNAY J. L., TUTTLE E. P., Jr EFFECT OF DOPAMINE IN MAN: AUGMENTATION OF SODIUM EXCRETION, GLOMERULAR FILTRATION RATE, AND RENAL PLASMA FLOW. J Clin Invest. 1964 Jun;43:1116–1124. doi: 10.1172/JCI104996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch M. W., Rand M. J., Story D. F. Proceedings: Evidence for a dopaminergic mechanism for modulation of adrenergic transmission in the rabbit ear artery. Br J Pharmacol. 1973 Sep;49(1):141P–142P. doi: 10.1111/j.1476-5381.1973.tb08279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay J. L., Goldberg L. I. Comparison of the effects of dopamine, isoproterenol, norepinephrine and bradykinin on canine renal and femoral blood flow. J Pharmacol Exp Ther. 1966 Jan;151(1):23–31. [PubMed] [Google Scholar]
- Sampson R. G., Scroop G. C., Louis W. J. Cardiovascular effects of dopamine in the anaesthetized dog. Clin Exp Pharmacol Physiol. 1974 Jan-Feb;1(1):3–12. doi: 10.1111/j.1440-1681.1974.tb00521.x. [DOI] [PubMed] [Google Scholar]
- Sheys E. M., Green R. D. A quantitative study of alpha adrenergic receptors in the spleen and aorta of the rabbit. J Pharmacol Exp Ther. 1972 Feb;180(2):317–325. [PubMed] [Google Scholar]
- Starke K., Endo T., Taube H. D. Pre- and postsynaptic components in effect of drugs with alpha adrenoceptor affinity. Nature. 1975 Apr 3;254(5499):440–441. doi: 10.1038/254440a0. [DOI] [PubMed] [Google Scholar]
- Starke K., Endo T., Taube H. D. Relative pre- and postsynaptic potencies of alpha-adrenoceptor agonists in the rabbit pulmonary artery. Naunyn Schmiedebergs Arch Pharmacol. 1975;291(1):55–78. doi: 10.1007/BF00510821. [DOI] [PubMed] [Google Scholar]
- Starke K., Montel H. Alpha-receptor-mediated modulation of transmitter release from central noradrenergic neurones. Naunyn Schmiedebergs Arch Pharmacol. 1973;279(1):53–60. doi: 10.1007/BF00502067. [DOI] [PubMed] [Google Scholar]
- Starke K. Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]
- Stjärne L., Brundin J. Affinity of Noradrenaline and dopamine for neural alpha-receptors mediating negative feedback control of noradrenaline secretion in human vasoconstrictor nerves. Acta Physiol Scand. 1975 Sep;95(1):89–94. doi: 10.1111/j.1748-1716.1975.tb10029.x. [DOI] [PubMed] [Google Scholar]
- Stjärne L. Selectivity for catecholamines of presynaptic alpha-receptors involved in feedback control of sympathetic neurotransmitter secretion in guinea-pig vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1975;288(2-3):296–303. doi: 10.1007/BF00500534. [DOI] [PubMed] [Google Scholar]
- Whitsett T. L., Halushka P. V., Goldberg L. I. Attenuation of postganglionic sympathetic nerve activity by L-dopa. Circ Res. 1970 Oct;27(4):561–570. doi: 10.1161/01.res.27.4.561. [DOI] [PubMed] [Google Scholar]


