Abstract
In plants, sugars are the main respiratory substrates and important signaling molecules in the regulation of carbon metabolism. Sugar signaling studies suggested that sugar sensing involves several key components, among them hexokinase (HXK). Although the sensing mechanism of HXK is unknown, several experiments support the hypothesis that hexose phosphorylation is a determining factor. Glucose (Glc) analogs transported into cells but not phosphorylated are frequently used to test this hypothesis, among them 3-O-methyl-Glc (3-OMG). The aim of the present work was to investigate the effects and fate of 3-OMG in heterotrophic plant cells. Measurements of respiration rates, protein and metabolite contents, and protease activities and amounts showed that 3-OMG is not a respiratory substrate and does not contribute to biosynthesis. Proteolysis and lipolysis are induced in 3-OMG-fed maize (Zea mays L. cv DEA) roots in the same way as in sugar-starved organs. However, contrary to the generally accepted idea, phosphorous and carbon nuclear magnetic resonance experiments and enzymatic assays prove that 3-OMG is phosphorylated to 3-OMG-6-phosphate, which accumulates in the cells. Insofar as plant HXK is involved in sugar sensing, these findings are discussed on the basis of the kinetic properties because the catalytic efficiency of HXK isolated from maize root tips is five orders of magnitude lower for 3-OMG than for Glc and Man.
In plants, the supply of carbohydrates to growing organs can vary greatly with plant development and external conditions. Changing the sugar supply modulates cell metabolism and gene expression (Koch, 1996; Yu, 1999). An unlimited sugar supply triggers the enhancement of carbohydrate-utilizing functions and represses utilization of alternative carbon sources in sinks on one hand, and on the other hand, photosynthesis in source leaves. Conversely, a limited sugar supply has opposite effects. Thus, in sugar-starved plants, proteins and lipids are degraded to supply carbon skeletons to respiration and biosynthetic processes (Peoples and Dalling, 1988; Yu, 1999; Brouquisse et al., 2001). In this case, protein degradation leads to an increase in Asn (Genix et al., 1990; King et al., 1990; Brouquisse et al., 1992), and is linked to the induction of exo- and endopeptidases (Tassi et al., 1992; James et al., 1993, 1996; Chevalier et al., 1995; Moriyasu and Ohsumi, 1996). In maize (Zea mays L. cv DEA) root tips, sugar starvation induces a vacuolar Ser endopeptidase, named root starvation-induced protease (RSIP), which accounts for about 80% of the endopeptidase activity measured in vitro (James et al., 1996; Brouquisse et al., 2001). The protease induction can be reversed by supplying sugars (James et al., 1993; Chevalier et al., 1995; Brouquisse et al., 2001). Thus, the expression of some proteins is clearly related to the amount of sugars available to the cells.
The mechanisms used by cells to sense sugars have been extensively studied in yeast, animal, and plants, and there is increasing evidence that sugar-sensing mechanisms are partly conserved in eukaryotes (Jang and Sheen, 1997; Halford et al., 1999; Johnston, 1999; Rolland et al., 2002). In plants, sugar sensing might involve multiple receptors and different signal transduction pathways probably interact. Studies using mutants or non-metabolizable sugar analogs, or metabolic intermediates, suggested that sugar transporters at the plasmalemma and hexokinase (HXK) were potential locations of signal input into the sugar signaling system (Lalonde et al., 1999; Sheen et al., 1999; Smeekens, 2000). Among the Glc analogs used to differentiate transport from other steps, 6-deoxy-Glc (6-DOG) has been used because it is readily transported into cells but cannot be phosphorylated by HXK. Similarly, 3-O-methyl-Glc (3-O-methyl-d-glucopyranose, 3-OMG) is known to be transported into the cell and said not to be phosphorylated by HXK (Jang and Sheen, 1997; Lalonde et al., 1999; Smeekens, 2000; Rolland et al., 2002). Therefore, it has been widely used to investigate hexose transport (Reinhold and Eshhar, 1968; Gogarten and Bentrup, 1983; Sauer and Stadler, 1993; Wiese et al., 2000) or sugar signaling (Graham et al., 1994; Jang and Sheen, 1994; Godt et al., 1995; Martin et al., 1997; Fujiki et al., 2000; Ichimura et al., 2000; Ho et al., 2001; Oesterhelt and Gross, 2002). In most cases, 3-OMG did not trigger a sugar signal (Graham et al., 1994; Jang and Sheen, 1994; Ho et al., 2001; Oesterhelt and Gross, 2002), supporting the conclusion that HXK phosphorylation of Glc is required for sugar perception in plant cells. In some cases, a sugar signal was triggered by 6-DOG (Godt et al., 1995; Roitsch et al., 1995) or 3-OMG (Martin et al., 1997), suggesting a sensing mechanism and a signaling pathway independent of HXK. However, 3-OMG can be phosphorylated in vivo in rat (Rattus norvegicus) heart (Gatley et al., 1984), yeast (Saccharomyces cerevisiae; Gancedo and Gancedo, 1984), and probably in the mycoplasma Acholeplasma laidlawii (Tarshis et al., 1976). Moreover, beef heart and yeast HXK catalyze the phosphorylation of 3-OMG in vitro, although at maximal rates three orders of magnitude lower than those observed for Glc (Malaisse-Lagae et al., 1986).
These somewhat conflicting data prompted us to reinvestigate the effects and fate of 3-OMG in heterotrophic plant cells, asking the following questions: Is 3-OMG phosphorylated by HXK, is it a respiratory substrate, and to what extent can it be used to study sugar signaling? To answer these questions, we characterized the biochemical and physiological responses of excised maize root tips and isolated plant cells when 3-OMG was substituted for Glc in the culture medium. For root tips, the time courses of metabolite concentrations (sugars, ester-phosphates, adenine nucleotides [AdNs], and amino acids) were monitored in vivo using 13C and 31P NMR along with respiration rate measurements. The effects of 3-OMG on protein contents, proteolytic activities, and RSIP amounts were observed after a 48-h incubation period, after which time the changes in enzymatic activities and metabolic contents are clearly marked and yet fully reversible (Brouquisse et al., 1991). We also looked for 3-OMG phosphorylation in the maize root tips and in suspension-cultivated cells of Arabidopsis and tomato (Lycopersicon esculentum Mill.). Last, we studied 3-OMG phosphorylation by HXK purified from maize roots. Our results support two major conclusions: First, 3-OMG is phosphorylated to 3-OMG-6-phosphate (3-OMG-6P) by HXK in heterotrophic plant cells; and second, proteolysis is induced in 3-OMG-fed root tips in the same way as in sugar-starved or 6-DOG-fed root tips, which means that 3-OMG is not sensed as a sugar by the cells. This dual effect does not contradict the hypothesis of HXK as a sensor if we take into account the kinetic properties of HXK; the catalytic efficiency of HXK is five orders of magnitude lower for 3-OMG than for Glc and Man.
RESULTS
Effects of 3-OMG on Protein Content, Proteolytic Activities, and RSIP Expression
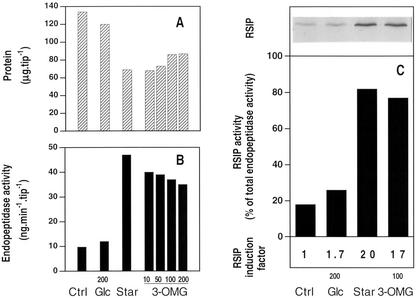
Figure 1, A and B, report the changes in total protein content and endopeptidase activity (measured against azocasein) that occurred in maize root tips incubated for 48 h in the presence of either 200 mm Glc or increasing concentrations of 3-OMG, or in the absence of any sugar. In comparison with the control (freshly excised root tips incubated for 4 h in 200 mm Glc), sugar starvation triggered a 50% decrease in protein content and a 5-fold increase in endopeptidase activity, whereas small changes occurred in Glc-fed root tips. In the presence of 3-OMG, protein content and endopeptidase activity changed nearly as much as in sugar-starved root tips. However, high concentrations of 3-OMG in the incubation medium (100 and 200 mm) led to slightly higher protein content and lower endopeptidase activity than lower concentrations (10 and 50 mm). Because of the similar effect of 100 and 200 mm 3-OMG on proteolysis and protease induction, 100 mm was routinely used. The expression of the RSIP and its contribution to endopeptidase activity were estimated through western-blot and immunoprecipitation experiments, using polyclonal antibodies raised against RSIP (Fig. 1C). RSIP was barely present in control root tips or root tips incubated with 200 mm Glc, where it represented 18% and 28%, respectively, of the total endopeptidase activity in vitro. In root tips, either starved or incubated with 100 mm 3-OMG, the increase in total endopeptidase activity was related to an increase in the amount of RSIP, the activity of which represents respectively 82% and 76% of the endopeptidase activity. From these data, the induction factors for RSIP were calculated to be 1.7, 20, and 17, respectively, in Glc-fed, -starved, and 3-OMG-fed root tips. Incubation of root tips for 48 h with 100 mm 6-DOG triggered a decrease in protein, and an increase in endopeptidase activity and RSIP expression, similar to that observed for starved root tips (data not shown). Therefore, at this stage, the biochemical response of the plant cells to 3-OMG is similar to the responses observed for starved or 6-DOG-fed cells. Our results are consistent with the concept that 3-OMG does not repress the sugar starvation response because, although transported into eukaryotic cells, it is not metabolized. To check this point, we investigated the effects of 3-OMG on respiratory metabolism in maize root tips.
Figure 1.
Effects of 3-OMG on protein content (A), proteolytic activity (B), and RSIP expression (C) in maize root tips. Incubation of freshly excised root tips during 4 h in 200 mm Glc, defined as control (Ctrl). Otherwise, excised root tips were incubated for 48 h without carbon substrate (Star), with 200 mm Glc (Glc), or in the presence of 10, 50, 100, or 200 mm 3-OMG, and analyzed for proteins (A), endopeptidase activity (B), and western-blot RSIP detection (C) as described in “Materials and Methods.” C, Total proteins from one root tip equivalent (between 134 and 65 μg protein according to A) were loaded in each lane for western-blot RSIP analysis. The percentage of endopeptidase activity due to RSIP in control (Ctrl), 200 mm Glc-fed (Glc), Glc-starved (Star), and 100 mm 3-OMG-fed root tips was measured after immunoprecipitation experiments with anti-RSIP antibodies. Data are the mean of five (protein), three (endopeptidase activities), and two (immunoprecipitation) independent experiments. sds are less than 15%.
Effects of 3-OMG on Growth and Respiratory Metabolism
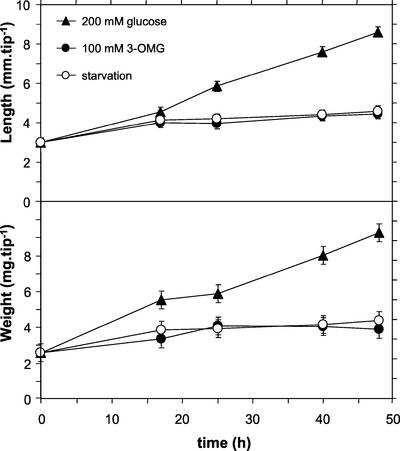
The growth of excised maize root tips incubated with 3-OMG was very limited compared with that of Glc-fed tips and similar to the growth observed in sugar starvation (Fig. 2). The slight increase of mass and the related increase in length, observed during the first 15 to 20 h of incubation, are attributed to the consumption of endogenous sugars and to water uptake resulting from the increase in cell osmolarity (Brouquisse et al., 1991).
Figure 2.
Growth of excised maize root tips in different nutritive media. Three sets of maize root tips were incubated either with or without the hexose indicated by symbols. Each point was obtained from a sample of 20 root tips. Data are the mean ± sd of two independent experiments.
After a 48-h incubation period, the respiration rate of 3-OMG-fed root tips dropped by 75% and was close to that of carbon-starved root tips (Table I). Thus, 3-OMG was not able to sustain cell metabolism, and 3-OMG-fed root tips became rapidly carbon starved. AdN contents of maize root tips incubated for 48 h in media of different composition were measured (Table I). The total AdN content decreased in 3-OMG-treated root tips as in starved root tips, but the ATP to ADP ratio and AEC remained high (around 5 and 0.90, respectively), as in the control. The addition of 10 mm Pi in the incubation medium did not change either the total AdN content or the AEC value significantly. Together, these data show that 3-OMG triggers the same effects as starvation on common indicators of growth and respiratory metabolism.
Table I.
Effect of 3-OMG on respiration, adenine nucleotide contents, ATP/ADP ratio, and AEC in maize root tips
| Condition | Respiration | ATP | ADP | AMP | Σ AdN | ATP/ADP | AEC |
|---|---|---|---|---|---|---|---|
| nmol O2 min−1 tip−1 | pmol tip−1 | ||||||
| Control | 3.45 ± 0.30 | 2,130 ± 382 | 360 ± 66 | 49 ± 26 | 2,540 ± 465 | 5.92 | 0.91 |
| Starvation | 0.70 ± 0.10 | 1,213 ± 273 | 225 ± 34 | 33 ± 17 | 1,471 ± 324 | 5.40 | 0.90 |
| 3-OMG (100 mm) | 0.85 ± 0.15 | 1,239 ± 141 | 216 ± 15 | 30 ± 8 | 1,485 ± 164 | 5.74 | 0.91 |
| 3-OMG (100 mm) + Pi (10 mm) | 0.78 ± 0.15 | 1,364 ± 367 | 270 ± 66 | 43 ± 30 | 1,677 ± 463 | 5.05 | 0.89 |
Except for control, defined as a 4-h incubation in 200 mm Glc, root tips were incubated for 48 h without carbon substrate or with 100 mm 3-OMG. When not mentioned, Pi concentration was 0.25 mm. AdNs were extracted and analyzed, and the values of Σ AdN and adenylate energy charge (AEC) were calculated as described in “Materials and Methods.” These data represent the mean of at least two (3-OMG and 3-OMG + Pi), four (starvation), and six (control) independent experiments.
Effects of 3-OMG on the Metabolite Contents
13C and 31P in Vivo NMR Study
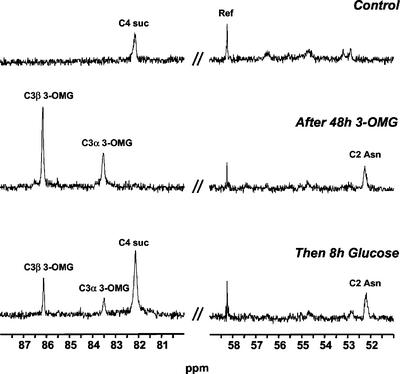
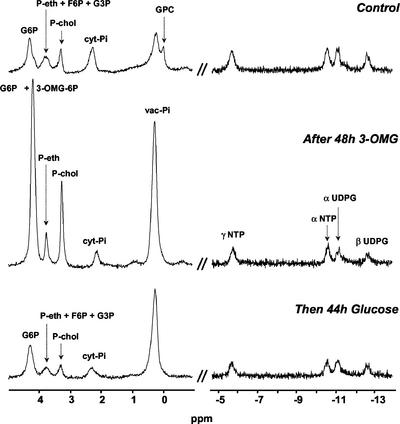
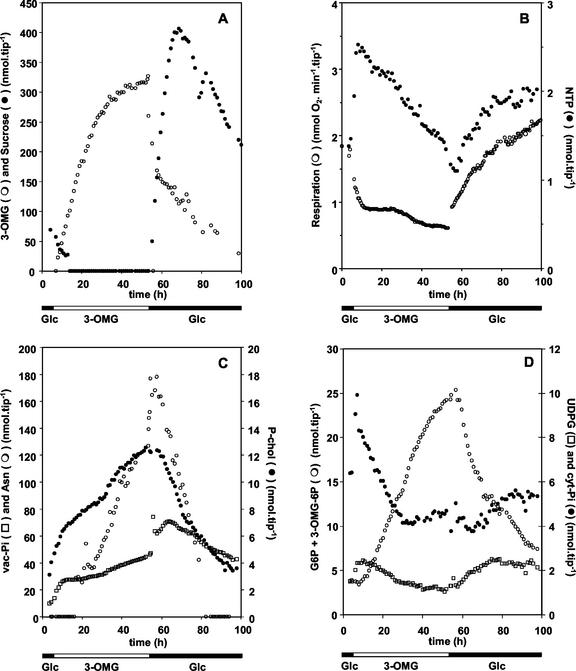
13C and 31P NMR spectroscopy were used to monitor the time course changes in major pools of metabolites in excised root tips. Figures 3 and 4 display typical 13C and 31P spectra obtained with root tips successively fed with 200 mm Glc, 100 mm 3-OMG, and 200 mm Glc again. 13C NMR (Fig. 3) was used to measure the amounts of Suc, 3-OMG, and Asn, a transient marker of protein degradation (Brouquisse et al., 1992). 31P NMR (Fig. 4) was used to analyze the most abundant phosphorylated compounds, such as Glc-6-phosphate (G6P); phosphorylcholine (P-chol), which is a marker of membrane degradation (Roby et al., 1987); cytoplasmic phosphate (cyt-Pi) and vacuolar phosphate (vac-Pi); UDP-Glc (UDPG); and nucleoside triphosphates (NTPs). The growth of root tips was taken into account for the quantification of metabolites throughout the experiment.
Figure 3.
13C NMR spectra of excised maize root tips in different nutritive media: regions used for quantification of sugars and amino acids. Excised maize root tips were first perifused with a medium containing 200 mm Glc during 4 h (Control, top). Then, 100 mm 3-OMG was substituted for Glc for 48 h (middle). Finally, root tips were perifused with 200 mm Glc (bottom). Ref, 13CH2 resonance of ethanol contained in a capillary. Spectra were acquired at 100.61 MHz with a free induction decay (FID) resolution of 1.4 Hz, a 45° radiofrequency (RF) pulse (45 μs), and 3,000 transients repeated every 1 s.
Figure 4.
31P NMR spectra of excised maize root tips in different nutritive media: regions used for quantification of phosphoesters and nucleoside phosphates. Excised maize root tips were first perifused with a medium containing 200 mm Glc during 4 h (Control, top). Then, 100 mm 3-OMG was substituted for Glc during 48 h (middle). Finally, the sample was perifused with 200 mm Glc (bottom). GPC, glycerylphosphorylcholine. Spectra were acquired at 161.98 MHz with an FID resolution of 1.7 Hz, a 45° RF pulse (32.5 μs), and 1,000 transients repeated every 0.6 s.
Transition from 200 mm Glc to 100 mm 3-OMG induced a rapid accumulation of 3-OMG in the root tips. After 20 h of incubation, its intracellular content reached 330 nmol tip−1 (about 120 mm) and then stabilized (Fig. 5A). As previously observed in sugar-starved root tips (Saglio and Pradet, 1980; Brouquisse et al., 1991), endogenous Suc and Fru were totally consumed within 15 h. Unexpectedly, Glc was also totally consumed within 15 h after the transition to 3-OMG (data not shown) instead of stabilizing at 30% of its initial value (Brouquisse et al., 1991). Such a decrease in Glc content may be explained by the stimulation of Glc efflux in response to 3-OMG uptake (Gogarten and Bentrup, 1983). Respiration rate dropped quickly by 50% after the transition to 3-OMG, but decreased at a lower rate beyond 10 h (Fig. 5B). After a transient increase, the concentrations of NTP (Fig. 5B) and UDPG (Fig. 5D) continuously decreased to reach about 60% of their initial values after 48 h of 3-OMG treatment. Cyt-Pi concentration evolved with kinetics similar to NTP (Fig. 5D), whereas the Cyt-Pi resonance chemical shift indicated cytoplasmic acidification (data not shown). In the same time, the concentrations of P-chol, vac-Pi, and Asn, three common markers of intracellular autophagy (Roby et al., 1987; Genix et al., 1990), increased during the incubation with 3-OMG (Fig. 5C). To summarize, in 3-OMG-fed root tips, the decrease in respiration rate and in NTP and UDPG concentrations, and the steady accumulation of P-chol, vac-Pi, and Asn (Fig. 5), added to the protein decrease and the induction of proteolytic activities (Fig. 1), characterize carbon starvation (Journet et al., 1986; Brouquisse et al., 1991; Yu, 1999).
Figure 5.
Time courses of respiration and metabolite contents in excised maize root tips. Metabolite concentrations, deduced from spectral intensities as described in “Materials and Methods,” are plotted as a function of the incubation time. The composition of the external medium is indicated at the bottom. Data are from one representative experiment of three. A, Time courses of intracellular hexose concentrations. B, Time courses of respiration rate and NTP concentration. C, Time courses of concentrations of starvation markers: Asn, P-chol, and vac-Pi. D, Time courses of concentrations of cyt-Pi, UDPG, and C6 hexose phosphates.
Starvation was still reversible after 48 h of incubation in 100 mm 3-OMG. Replacing 100 mm 3-OMG with 200 mm Glc in the external medium induced a bimodal efflux of 3-OMG together with a boost in Suc content and respiration rate (Fig. 5, A and B). Suc was de novo synthesized at a high rate (Fig. 3, bottom) and transiently accumulated up to 400 nmol tip−1 (115 mm) before returning to standard levels. In the same time, NTP, cyt-Pi, and UDPG pools (Fig. 5, B and D) gradually returned to levels close to the control. The decrease in Asn and P-chol contents (Fig. 5C) matches the cessation of protein and lipid remobilization.
Accumulation of an Unusual Phosphomonoester
In control root tips, the chemical shift of the G6P resonance is 4.3 ppm (Fig. 4; Saint-Ges et al., 1991; Roscher et al., 1998; Brouquisse et al., 2001). Upon replacement of Glc by 3-OMG in the external medium, the 4.3-ppm resonance first decreased by 15% (Fig. 5D). Then, the intensity of a resonance progressively centered at 4.2 ppm increased dramatically (Figs. 4 and 5D). This resonance, which arises from at least one phosphomonoester, might have been attributed to G6P on a chemical shift basis because this parameter is very sensitive to pH (Robitaille et al., 1991). However, the intensity of this 4.2-ppm resonance evolved quasi-linearly as a function of time (Fig. 5D), revealing a quite unusual rate of accumulation for G6P. When Glc was substituted for 3-OMG in the external medium, not only did the accumulation of this molecule cease, but the intensity of the 4.2-ppm resonance decreased quickly down to a point where a resonance centered at 4.3 ppm could be easily distinguished (Fig. 4).
Such an increase in the G6P resonance was unexpected for several reasons. First, it is known that G6P content decreases in sugar-starved cells (Roby et al., 1987; Brouquisse et al., 2001). Second, variations in UDPG and G6P concentrations are usually correlated. The phosphoglucomutase and UDPG pyrophosphorylase enzymes generally function near equilibrium and stabilize the G6P to UDPG ratio (ap Rees, 1988; Roscher et al., 1998). This is clearly not the case in 3-OMG-fed root tips (Fig. 5D). Third, demethylation of 3-OMG, which could provide Glc to HXK to generate G6P, is very limited in vivo (Csaky and Wilson, 1956; see 14C observations below). These considerations led us to hypothesize that, during 3-OMG treatment, the accumulated phosphomonoester was not G6P but possibly a phosphorylated form of 3-OMG.
Identification of 3-OMG-6P in Tissue and Cell Extracts
31P NMR Analysis of Maize Root Tip Acid Extracts
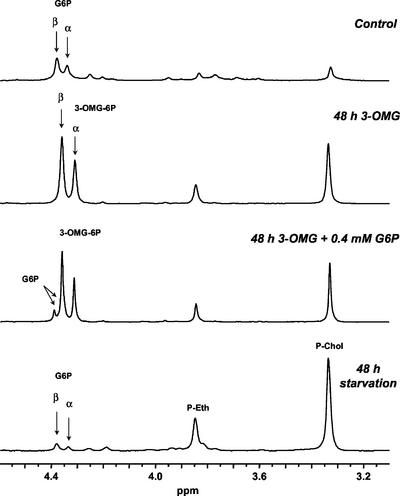
To test the hypothesis of 3-OMG phosphorylation, we proceeded toward the formal identification of 3-OMG-phosphate (3-OMG-P) in acid and ethanolic extracts of excised maize root tips and suspension-cultured cells incubated with 3-OMG for 48 h by analyzing 31P and 13C NMR spectra of these extracts. Figure 6 presents the spectral region of the phosphomonoester resonances in a 31P NMR spectra of plant material. In the spectrum of the control extract, G6P is characterized by the two resonances of the α- and β-anomers, at 4.38 and 4.34 ppm, respectively. With sugar-starved root tips, the starvation symptoms clearly appear through an increase in the P-chol and P-ethanolamine resonance intensities and a decrease in the G6P one. In the spectrum of the 3-OMG-fed root tips, an increase is also observed for the P-chol and P-ethanolamine resonances, but contrary to sugar starvation, there is a significant increase of the intensities of two unassigned resonances at 4.36 and 4.30 ppm. These intensities are in the proportion of 60% to 40%, as usually measured for the β- and α-resonances of G6P, respectively. A small amount of G6P was added directly to the extract (0.4 mm final) and a new spectrum was recorded. The appearance of a resolved β-G6P resonance demonstrated that the intense resonances of the 3-OMG spectrum do not arise from G6P (the α-G6P resonance 0.4 ppm upfield is hidden). The chemical shifts of these unassigned resonances sustain the hypothesis of a phosphorylation of 3-OMG in C6 because the 31P resonance of C6-phosphohexoses always lie in the 5- to 4-ppm interval at neutral pH (Fan, 1996).
Figure 6.
31P NMR spectra of acid extracts of maize root tips. Samples of 2,000 excised root tips were filtered and frozen in liquid N2 after 4-h incubation with 200 mm Glc (Control, top spectrum), or 48-h incubation with 100 mm 3-OMG (second spectrum from top), or 48-h incubation without substrate (bottom spectrum). The third spectrum from top was recorded after an addition of 0.4 mm G6P into the extract of 3-OMG-treated root tips. Acid extracts were prepared as described in “Materials and Methods” and pH was adjusted at 7.5. Spectra were acquired at 161.98 MHz with an FID resolution of 0.6 Hz, a 60° RF pulse (15 μs), and 2,048 transients repeated every 3.5 s. The exponential apodization was 0.5 Hz.
G6P Enzymatic Assay of Maize Root Tip Ethanolic Extracts
To confirm this hypothesis, G6P contents were measured in ethanolic extracts using an enzymatic assay. The G6P concentrations were 3.2 ± 0.6, 2.4 ± 0.4, and 1.3 ± 0.4 nmol tip−1 in control, 48-h-starved, and 48-h 3-OMG-fed (100 mm) root tips, respectively (mean ± sd of five independent experiments). Thus, G6P content decreased from control to starvation, as expected (Roby et al., 1987; Brouquisse et al., 2001), but decreased even more in root tips incubated with 3-OMG. This proved definitely that the phosphomonoester accumulated in 3-OMG-fed root tips was not G6P.
13C NMR Analysis of Maize Root Tip Ethanolic Extracts
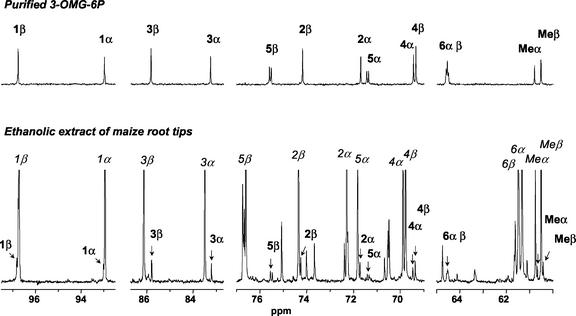
The 13C spectra of phosphomonoesters being characteristic of their molecular structure, ethanolic extracts of maize root tips were analyzed by 13C NMR spectroscopy. Because the attribution of the resonances is based on the spectra of pure compounds, 3-OMG-P was produced in vitro from 3-OMG using yeast HXK (Gancedo and Gancedo, 1984) and purified. It was thoroughly characterized with 1H and 13C NMR and shown to be 3-OMG-6P (M. Gromova, S. Cortès, A. Heyraud, R. Brouquisse, and C. Roby, unpublished data). The 13C spectra of 3-OMG (Bock and Pedersen, 1983) and 3-OMG-6P (Fig. 7, top) were identified among the different spectra arising from the more abundant components in the extract of 3-OMG-fed maize root tips (Fig. 7, bottom). Quantification of the amounts of 3-OMG and 3-OMG-6P from the 13C NMR spectra of the extracts indicated that 8% ± 1% of the substrate had been phosphorylated in planta after 48 h.
Figure 7.
13C NMR identification of 3-OMG-6P. Synthesis of 3-OMG-6P from 3-OMG and ATP using yeast HXK, and isolation using exclusion-diffusion chromatography as described in “Materials and Methods.” Top, 13C NMR spectrum of purified 3-OMG-6P. Bottom, 13C NMR spectrum of an ethanolic extract of 1,000 maize root tips incubated with 100 mm 3-OMG for 48 h. Arrows and bold labels indicate the resonances of 3-OMG-6P, whereas italic labels identify 3-OMG resonances. Spectra were acquired at 100.61 MHz with an FID resolution of 0.7 Hz, a 60° RF pulse (10 μs), and 2,048 transients repeated every 1.75 s. The exponential apodization was 0.5 Hz.
NMR Analysis of Arabidopsis and Tomato Cell Acid Extracts
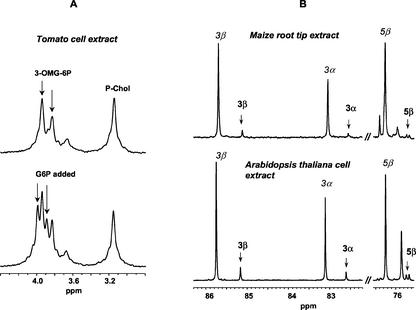
To generalize this finding to other plants, we also looked for 3-OMG phosphorylation in two dicotyledon species, Arabidopsis and tomato, by analyzing acid extracts of heterotrophic cells incubated for 24 h with 50 mm 3-OMG. In analogy to Figure 6, Figure 8A shows that the 31P NMR resonances of the phosphomonoester accumulated in tomato cells have the same chemical shift as 3-OMG-6P. Figure 8B proves that 3-OMG-6P is present in the acid extract of Arabidopsis. Determination of the amounts of 3-OMG and 3-OMG-6P from the 13C NMR spectrum indicated that 12% ± 1% of the substrate had been phosphorylated in Arabidopsis cells.
Figure 8.
NMR identification of 3-OMG-6P in extracts of different species. A, 31P NMR spectra of tomato cell extracts. Cell extracts were prepared from 4-d-old cells incubated during 24 h with 100 mm 3-OMG. The figure shows the phosphomonoester resonance interval of the 31P NMR spectra of one acid extract before (top) and after (bottom) addition of 2 mm G6P in the sample. It contained 80 mm trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid (CDTA) and pH was adjusted to 6.9 to improve resolution in this spectral interval. These spectra are the sum of 1,024 transients obtained with the acquisition parameters reported in Figure 6. B, 13C NMR spectra of maize root and Arabidopsis cell extracts. Comparison of two intervals of the 13C NMR spectra of acid extracts of maize root tips (top) and Arabidopsis cells (bottom) proving the presence of 3-OMG-6P in these cells incubated for 24 h with 100 mm 3-OMG. The selected 3-OMG-6P resonances (bold labels) are characteristic of the 13C NMR spectrum of this molecule (Fig. 7). The intense 3-OMG resonances (italic labels) reveal the accumulation of the precursor in the cells. No CDTA was added to this sample, and pH was 7.0. Spectra were obtained by adding 6,144 transients recorded with the acquisition parameters reported in Figure 7.
Activity and Kinetic Parameters of Maize HXK
Purification of maize root HXK was undertaken to assess consistently HXK involvement in the phosphorylation of 3-OMG. High neutral phosphatase (measured at pH 7.5) and G6P phosphatase activities were found in clarified homogenates of freshly excised maize root tips (Table II). Moreover, as compared with the control, neutral phosphatase and G6P phosphatase activities doubled in 48-h-starved and 48-h 3-OMG-fed root tips, whereas HXK activity decreased by a factor of 1.7 (Table II). Considering the slow kinetics of 3-OMG phosphorylation in vivo (Fig. 5D), contaminating phosphatase activities are likely to impair the in vitro measurements of 3-OMG phosphorylation kinetics. Thus, the activity and kinetic parameters of maize HXK against 3-OMG, Glc, and Man were investigated with a HXK preparation devoid of phosphatase activity.
Table II.
Activities of HXK, neutral phosphatase, and G6P phosphatase in clear homogenates of maize root tips and in partially purified HXK fraction
| Condition | HXK | Neutral Phosphatase | G6P Phosphatase |
|---|---|---|---|
| nmol min−1 tip−1 | |||
| Control | 4.8 ± 0.8 | 4.45 ± 0.15 | 0.9 ± 0.5 |
| Starvation | 2.9 ± 0.7 | 9.1 ± 0.1 | 1.9 ± 0.6 |
| 3-OMG (100 mm) | 2.7 ± 0.7 | 10.1 ± 0.8 | 1.8 ± 0.5 |
| μmol min−1 mg protein−1 | |||
| Purified HXK | 2.5 ± 0.3 | 0.024 ± 0.001 | n.d. |
Excised root tips were incubated either during 4 h with 200 mm Glc (control) or during 48 h in a medium either devoid of carbon substrate (starvation) or containing 100 mm 3-OMG. Data are the mean of three independent experiments for clarified homogenates and for purified HXK. n.d., Not determined.
The HXK-specific activity was 100-fold higher than that of phosphatase activity in the HXK-enriched fraction obtained after a five-step purification procedure (Table II). Remaining phosphatase was inhibited (>95%) with the use of phosphatase inhibitors (Pi, phosphatase inhibitor cocktail II). The Km and Vmax values of purified HXK for Glc, Man, and ATP are given in Table III. ADP inhibited HXK activity measured against 2 mm ATP in a partially noncompetitive mode (data not shown). The Km and Vmax/Km values found for Glc and ATP, as well as inhibition by ADP, were similar to those previously found for the organelle-bound maize root HXK (Galina et al., 1995; da-Silva et al., 2001). As compared with Glc and Man, the affinity of maize root HXK for 3-OMG and the Vmax are two and three orders of magnitude lower, respectively. Phosphorylation of 3-OMG by purified maize HXK was inhibited by HXK inhibitors such as ZnCl2, ADP, and mannoheptulose (Table III). Thus, maize root HXK does phosphorylate 3-OMG in vitro, and probably in vivo (Fig. 5D), but with a catalytic efficiency (Vmax/Km) five orders of magnitude lower than for Glc and Man.
Table III.
Maize root HXK kinetic parameters for the phosphorylation of hexoses
| Glca | Man | 3-OMGa | ATP | |
|---|---|---|---|---|
| Km (μmol L−1) | 76 ± 9 | 105 ± 40 | 21,000 ± 7,000 | 42 ± 8 |
| Vmax (μmol min−1 mg protein−1) | 2.52 ± 0.31 | 1.26 ± 0.06 | 0.007 ± 0.002 | 2.16 ± 0.32 |
| 103 × Vmax/Km(min−1 mg protein−1 L) | 33 | 12 | 3.10−4 | 51 |
The Km and Vmax values were calculated using the Lineweaver-Burk reciprocal plot method. Data are the mean of three measurements from two independent HXK preparations.
HXK activity was inhibited to the same extent with either Glc (2 mm) or 3-OMG (50 mm), when measured in the presence of either 10 mm ZnCl2 (55% inhibition) or 0.5 mm ADP (15% inhibition). With 10 mm mannoheptulose, Glc and 3-OMG phosphorylations were inhibited by 31% and 78%, respectively.
Overall metabolism of 3-OMG
Maize root tips were incubated for 48 h in the presence of 100 mm [methyl-14C]3-OMG. During this period, the production of [14C]CO2 was measured and root tip samples were harvested after 24 and 48 h for [14C] metabolite analysis. As reported in Table IV, after 24 and 48 h of incubation, respectively, 88% and 84% of the total radioactivity were recovered in neutral fractions. Anions represented 8% to 11% of the total radioactivity. After treatment of the anionic fractions with alkaline phosphatase and fractionation on an anionic exchange column, more than 98% of the radioactivity was retrieved in the neutral fraction obtained. This result and that presented in Figure 5D indicate that the radioactivity of the anionic fraction was associated with esterphosphates, most being 3-OMG-6P. This matches a previous observation showing that after 6 d, 8% of the radioactivity incorporated as [U-14C]3-OMG comigrated with sugar phosphates in Chenopodium rubrum suspension cells (Gogarten and Bentrup, 1989). In the present study, less than 5% of the radioactivity was either respired or retrieved in cationic and insoluble fractions (1.06%, 0.73%, and 2.17%, respectively, of the total incorporated 3-OMG after 48 h). It was calculated from the data in Tables I and IV that 3-OMG is respired about 600 times slower than Glc, which agrees with the in vitro kinetic data (Table III). Moreover, by comparing the amount of [14C]CO2 released by the root tips during the 40- to 48-h incubation period (data not shown), and the respiration rate measured during the same time (Fig. 5B), we calculated that [methyl-14C]3-OMG contributed less than 0.1% to the respired substrates. Taken together, these results show that 3-OMG is poorly metabolized in excised maize root tips. First, demethylation of 3-OMG occurs very slowly in vivo (less than 4 pmol min−1 tip−1). Second, 3-OMG does not contribute much to respiration and biosynthetic processes.
Table IV.
Metabolism of [methyl-14C]3-OMG in maize root tips during an incubation of 24 and 48 h
| Fraction | Metabolized [Methyl-14C]3-OMG
|
|||
|---|---|---|---|---|
| 24 h | 48 h | |||
| (nmol tip−1) | % | (nmol tip−1) | % | |
| Respirationa | 0.85 | 0.34 | 2.85 | 1.06 |
| Neutrals | 221.00 | 88.20 | 227.00 | 84.64 |
| Anionsb | 20.30 | 8.09 | 29.90 | 11.40 |
| Cations | 2.75 | 1.10 | 1.95 | 0.73 |
| Insolublesc | 5.70 | 2.27 | 5.85 | 2.17 |
| Total | 250.60 | 100 | 267.50 | 100 |
Root tips were incubated with 100 mm [methyl[14C]3-OMG. After 24 and 48 h, 100 root tips were harvested and the different fractions were prepared and analyzed as described in “Materials and Methods.” Data are mean values of two independent experiments.
Respiration data refer to the amounts of 3-OMG respired by root tips between 0 and 24 h and between 0 and 48 h of incubation. Values were calculated from the amounts of [14C]CO2 released by the root tips and trapped in KOH solution.
After treatment of the anionic fraction with alkaline phosphatase and separation on anionic exchange resin, more than 98% of the radioactivity was retrieved in the neutral fraction, indicating that anions were almost totally ester-phosphates.
Insolubles refer to starch, cell wall, and water- and ethanol-insoluble compounds.
DISCUSSION
3-OMG Phosphorylation in Plant Cells
The present study shows that 3-OMG is phosphorylated steadily in monocotyledon and dicotyledon plant cells (Figs. 4, 6, and 8). It also demonstrates that in planta 3-OMG is phosphorylated at C6 (Figs. 7 and 8B), and that in vitro it is phosphorylated by maize root HXK, as are Glc and Man, but at a very slow rate (Table III). Soluble and membrane-bound HXK activities have been characterized in whole maize roots (Galina et al., 1995; Galina and da-Silva, 2000; da-Silva et al., 2001). On the basis of its kinetic parameters and ADP sensitivity, it appears that we purified the membrane-bound HXK form, which has been associated with hexose sensing (da-Silva et al., 2001). It would be interesting to know if the soluble form of HXK also phosphorylates 3-OMG.
In animal and yeast systems fed with 14C-labeled 3-OMG, previous studies found that a phosphorylated molecule in the anionic phase extract was radioactively labeled, and it was concluded that this molecule was 3-OMG-P (Gancedo and Gancedo, 1984; Gatley et al., 1984). Similar measurements, performed with HXK isolated from these organisms, led to the conclusion that 3-OMG was phosphorylated by HXK (Malaisse-Lagae et al., 1986). Even though these previous reports did not fully characterize the phosphorylated molecule, from now on it can be assumed that HXK phosphorylates 3-OMG in eukaryotes.
3-OMG Metabolism
The present work also shows that the product of the reaction, 3-OMG-6P, accumulates in the cytoplasm (Figs. 4 and 5D), and is practically not used as a respiratory substrate. The drop in respiration rate (Fig. 5B), and the very low amount of [14C]CO2 produced by [14C]3-OMG-fed root tips (Table IV) proves this directly. As a consequence, endogenous proteins and lipids are mobilized to support respiration and minimal biosynthetic processes (Fig. 5C), in analogy with sugar-starved plant cells where the amounts of proteins and lipids decrease strongly (Saglio and Pradet, 1980; Journet et al., 1986; Brouquisse et al., 1991). Also, as already observed (James at al., 1996; Brouquisse et al., 2001), protein degradation is related to an increase in total endopeptidase activity and RSIP expression (Fig. 1). Thus, 3-OMG is not recognized as a metabolizable sugar despite being phosphorylated. In comparison, metabolism of 3-OMG was more extensive in the alga Galdieria sulfuraria, where 14% of the radioactivity was lost in CO2 and 9% retrieved in insoluble compounds (Oesterhelt and Gross, 2002).
Cellular bioenergetics were modified during the 48-h 3-OMG treatment (decrease in respiration rate, NTP, and Cyt-Pi; Fig. 5, B and D) in a way similar to starvation (Table I). However, despite the intracellular accumulation of a large amount of phosphomonoesters (Figs. 4 and 5D), there was no imbalance in energy metabolism (Table I). Replacement of 3-OMG by Glc triggered a return to the reference metabolic state (Fig. 5). When root tips were fed again with Glc after 48 h of treatment with 3-OMG, their prompt respiratory and metabolic response (Fig. 5, A and B) was typical of a healthy organ. Thus, 3-OMG is not toxic to plant cells, in contrast to other Glc analogs such as Man, 2-DOG, and glucosamine, which induce Pi starvation, a drop in AdN content and in the AEC value, and an imbalance of metabolism (Herold and Lewis, 1977; Brouquisse et al., 2001). As a consequence, the metabolic observations reported herein present a reliable basis for discussing the use of 3-OMG in signaling studies.
Phosphatase Activities in 3-OMG-Fed and -Starved Root Tips
Along with phosphorylation of 3-OMG, a dephosphorylation of 3-OMG-6P was observed in living root tips (Figs. 4 and 5). The two in vivo activities, estimated from the rates of accumulation and consumption of the substrate and product, appear to be of the same order of magnitude. This observation reveals the existence of a non-negligible ester-phosphate phosphatase activity in maize root tips that has been estimated in clarified homogenates (Table II). The presence of phosphatase activities in root tips and their increase after 3-OMG feeding (Table II) can explain in vivo the slowing of accumulation of 3-OMG-6P in 3-OMG-fed root tips after 40 h, and the decrease in the 3-OMG-6P pool after Glc refeeding (Fig. 5D). In plants, phosphatase activities, mainly acid isoforms, have been shown to increase in response to Pi starvation, thus remobilizing the Pi moiety of phosphorylated compounds to maintain Pi turnover and cell metabolism (Plaxton, 1998; Raghotama, 1999). With 3-OMG, such an increase could also contribute to remobilizing the carbon moiety of phosphorylated compounds to feed respiration and biosynthetic processes in response to carbon starvation. Thus, the increase in phosphatase activities and the subsequent accumulation of vacuolar Pi (Fig. 5; Roby et al., 1987; Brouquisse et al., 2001) could be considered as a marker of carbon starvation, like protease induction and Asn accumulation (Brouquisse et al., 1992; James et al., 1993).
Use of Glc Analogs
The question of sugar sensing is only indirectly addressed when studying the metabolic and physiological consequences of 3-OMG substitution for Glc. It is the biochemical response of the integrated system to the sugar signal that is observed over 48 h, and not the signal transduction process itself. However, when carefully screening the metabolic responses to Glc analogs, one can make some interpretations in terms of signaling from these systemic properties. In previous work, after making sure that the observed responses were not the result of a metabolic imbalance caused by Pi starvation, we have shown that Man could be considered to have the same signaling effect as Glc (Brouquisse et al., 2001). Here, we show that in 3-OMG-fed excised maize root tips, 3-OMG readily accumulates in large amounts while sugar starvation occurs (Figs. 1–5). This confirms that 3-OMG does not trigger any apparent sugar signal (Smeekens, 2000; Rolland et al., 2002). Considered together, our Man and 3-OMG data support an involvement of HXK in the perception of sugar supply and the regulation of starvation-induced proteolysis. The finding that 3-OMG is phosphorylated by HXK (Table IV), but does not trigger a sugar signal, in no way invalidates the hypothesis that HXK might be a hexose sensor in plants. It supports the idea that dynamic processes can have a significant function in sensing.
In yeast, it was concluded recently that HXK sugar sensing was correlated to the onset of catalysis by the eventual formation of a stable transition intermediate (Hohmann et al., 1999; Kraakman et al., 1999). On this basis, we looked at the kinetic properties of the maize HXK presumably involved in sugar sensing (da-Silva et al., 2001) to understand the impact of different hexoses in plant sugar signaling. The affinity and the Vmax value of maize root tip HXK are about 300 times higher for Glc than for 3-OMG, giving a catalytic efficiency five orders of magnitude higher for Glc than for 3-OMG (Table IV). The ratios are of the same order of magnitude when comparing Man with 3-OMG. We point out the correlation between the very low phosphorylation rate of 3-OMG and the starvation-like response. If HXK is the first element in a chain of signal transduction (Sheen et al., 1999; Smeekens, 2000), the large conformational change of this enzyme occurring upon binding of the substrates and/or product release could be the triggering signal itself, in plants (Jang and Sheen, 1997) as in yeast (Kraakman et al., 1999).
CONCLUSION
The present work shows that 3-OMG is phosphorylated by HXK in heterotrophic plant cells, but not used as a respiratory and growth substrate. Proteolysis and RSIP expression are induced in 3-OMG-fed as in carbon-starved tissues. Thus, despite being phosphorylated, 3-OMG is not sensed as a sugar and behaves as a transported, but not phosphorylated, Glc analog. Considering the low catalytic efficiency of HXK for 3-OMG, it remains valid to use this analog for further study of the potential role of HXK phosphorylation rate in sugar sensing.
MATERIALS AND METHODS
Preparation and Incubation of Excised Maize (Zea mays L. cv DEA) Root Tips
Maize (Pioneer France Maïs, Toulouse, France) seed germination, root tip preparation, and incubation conditions were described (Brouquisse et al., 2001). Incubation medium containing minerals (Saglio and Pradet, 1980), plus 10 mm MES-KOH at pH 6.0, is medium A. Root tip samples were either perifused in the NMR tube for in vivo measurements (2-mm root tips) or incubated in flasks or syringes for tissue extraction (3-mm root tips), in the presence or absence of 3-OMG (M-4879, Sigma, St. Quentin-Fallavier, France) or Glc. To monitor growth, samples of 20 root tips were harvested at different times, dried on filter paper, weighed on tinfoil, and root tip lengths were measured.
In vivo NMR experiments were performed as described (Brouquisse et al., 2001) except that 4,000 excised root tips were used for each experiment and the partial oxygen pressure was regulated at 80% in the reservoir of external medium. The renewal of the medium around the sample varied from 24 to eight times a minute between the start and the end of the experiment, because of the growth (the average root tip length was 2.3 mm at the start, 3.3 mm for the 3-OMG-fed sample after 48 h, and 7 mm at the end of the experiment).
Preparation of Suspension-Cultured Cells
Arabidopsis cells (ecotype Columbia), obtained from Pierre Carol (Unité Mixte de Recherche 5575, Grenoble, France) originated from the cell suspension culture described by Axelos et al. (1992). They were grown in the dark at 21°C in Gamborg medium containing 52 mm Suc. They were subcultured by diluting 50 mL of cell solution in 200 mL of fresh medium in 500-mL flasks continuously agitated on an orbital shaker (Laboshake, Gerhardt, Bonn) at 100 rpm. During the first 3 weeks, they were subcultured once a week and after that adaptation period in the dark they were subcultured every 5 d.
Tomato (Lycopersicon esculentum Mill. var cerasiformae cv Sweet 100) cells, obtained from Jean-Luc Montillet (DEVM, Commissariat à l'Energie Atomique, Cadarache, France), were grown at 23°C in Murashige and Skoog medium containing 166 mm Glc instead of Suc. They were kept in the dark and continuously agitated on a rotary shaker (HT, Infors AG, Bootmingen, Switzerland) at 150 rpm. Cells were subcultured every 5 d by diluting 10 mL of cell solution in 90 mL of fresh medium in 250-mL flasks. Initial cell density was 60 mg fresh weight mL−1.
Preparation of Extracts
Clarified Homogenates of Maize Root Tips
Clarified homogenates were prepared as described (Brouquisse et al., 2001). Before HXK and phosphatase activity measurements and immunoprecipitation experiments, 0.5 to 1 mL of supernatant was first desalted through an ECONO-PAC 10 DG column (Bio-Rad Laboratories, Hercules, CA) equilibrated with 50 mm Tris (pH 7.5) and 25 mm NaCl.
Acid Extracts of 3-OMG-Fed Plant Materials
Water-soluble metabolites were extracted using perchloric acid (Brouquisse et al., 2001) from plant materials conditioned in the following way. For maize, 2,000 excised root tips were incubated for 48 h in medium A supplemented with 100 mm 3-OMG. For cells, 14 g of 5-d-old Arabidopsis cells and 9 g of 4-d-old tomato cells were incubated for 24 h in their own nutritive medium plus 100 mm 3-OMG. Plant materials were harvested by filtration and quickly rinsed with a 3-OMG-free medium before freezing in liquid N2. 13C NMR spectra were recorded first. For 31P NMR, CDTA was added to chelate divalent cations and the pH was adjusted to the desired value.
Ethanolic Extracts and Fractionation of Ethanol-Soluble Compounds
Ethanol-soluble compounds were extracted by the boiling ethanol/water method and resuspended in 1 mL of water after evaporation (Brouquisse et al., 1992). Neutral, anionic, and cationic compounds were then fractionated on cationic (AG 50W-X8 Superfine, NH4+ form, Bio-Rad Laboratories), and anionic (AG 1-X8 Superfine, OH− form, Bio-Rad Laboratories) columns according to the manufacturer's instructions.
14C Experiments
Two hundred excised maize root tips were incubated for 2 d in a 50-mL syringe containing 40 mL of medium A supplemented with antibiotic-antimycotics (A-7292, Sigma) and 100 mm [methyl-14C]3-OMG (0.37 MBq mmol−1). The syringe was connected on-line with four assay tubes, changed every 4 to 8 h, each containing 3 mL of 2% (w/v)KOH. More than 98% of the CO2 produced by the root tips was trapped in the KOH solution. After 24 and 48 h, samples of 100 root tips were harvested, thoroughly rinsed, and frozen at −193°C. Ethanol-soluble and -insoluble compounds, and then cationic, anionic, and neutral fractions, were prepared as described. The radioactivity in the KOH solution, and in neutral, anionic, cationic, and ethanol-insoluble fractions, was measured using a liquid scintillation analyzer (TRICARB 2000 CA, Hewlett-Packard, Palo Alto, CA). For each fraction, disintegrations per minute were converted to nanomoles of 3-OMG using the specific activity of [methyl-14C]3-OMG.
Synthesis and Purification of 3-OMG-6P
The phosphomonoester 3-OMG-6P was synthesized at 22°C from a 2.5-mL solution containing 100 mm 3-OMG, 50 mm KH2PO4, 10 mm MgCl2, 1 mm Mg-ATP, 100 mm phosphoenolpyruvate, 5 units of pyruvate kinase, and 10% (v/v) D2O at pH 7.7. The addition of 45 units of yeast (Saccharomyces cerevisiae) HXK (1426362, Roche Molecular Biochemicals, Grenoble, France) started the reaction, which was stopped after 7 d with boiling ethanol. The sample was evaporated and resuspended in water. Three steps of exclusion diffusion chromatography were used to isolate 3-OMG-6P. The extract was first chromatographed at 60°C on a BIO-GEL P2 column (1.5 × 200 cm, Bio-Rad Laboratories) equilibrated with water. The fractions containing 3-OMG-6P were concentrated and chromatographed again on the same column equilibrated with 50 mm NaNO3. New 3-OMG-6P fractions were desalted, using an HPLC system, through an HW 40-50 F column (50 × 2.1 cm, Interchim, Montluçon, France) equilibrated with water. The enrichment in 3-OMG-6P was assessed using 1H and 13C NMR spectroscopy. Commercial yeast HXK was checked for potential enzymatic contaminations. No phosphotransferase activity was detected. The only relevant contaminant activities were isomerase and phosphatase, each with a ratio (1:30–60) with respect to the formation rate of 3-OMG-6P.
Partial Purification of HXK
One hundred grams of primary maize roots were homogenized in a blender (Waring, New Hartford, CT) using 500 mL of a 50 mm Tris buffer at pH 8.0, containing 5 mm MgCl2, 1 mm Na-EDTA, 5 mm β-mercaptoethanol, and 5% (w/v) glycerol (medium B), in the presence of 0.5% (w/v) polyvinylpolypyrrolidone. The homogenate was filtered through eight cheesecloth layers and centrifuged at 15,000g for 15 min. The supernatant was brought to 30% (w/v) saturation with ammonium sulfate, and centrifuged at 15,000g for 15 min. The resulting supernatant was brought to 80% (w/v) saturation with ammonium sulfate and centrifuged again. All the precipitated proteins were resuspended in medium B, and loaded, at a 1 mL min−1 flow rate, onto a Sephacryl S200-HR (Amersham Biosciences, Orsay, France) gel filtration column (2.5 × 70 cm) equilibrated with medium B. HXK containing fractions were then loaded, at a 1 mL min−1 flow rate, onto a Sepharose Q Fast Flow (Amersham Biosciences) column (1.6 × 40 cm) equilibrated with medium B. Bound proteins were eluted with a 0 to 1 m NaCl gradient. Active fractions were diluted three times with medium B and loaded onto an FPLC MONO-Q 5/5 HR (Amersham Biosciences) column equilibrated with medium B, at a flow rate of 0.5 mL min−1. HXK activity was separated from phosphatase activity through the use of the following discontinuous NaCl gradient: from 0 to 150 mm NaCl during 10 min, 150 mm NaCl for 6 min, from 150 to 250 mm NaCl during 14 min, and from 250 mm to 1 m NaCl during 20 min. Active fractions were diluted three times with medium B and the MONO-Q fractionation step was performed once again. After this last step, fractions with a HXK/phosphatase activity ratio >100 were pooled together and used for HXK kinetic parameter determination. Purified fractions typically yielded the following HXK activities: 2.5 ± 0.3 μmol Glc min−1 mg protein−1, and 0.81 ± 0.04 μmol Fru min−1 mg protein−1. The fructokinase activity was assayed with 40 mm Fru.
Enzyme Activity Assays
Endopeptidase activity was measured, at pH 6.1, against azocasein (James et al., 1993). The azocaseinase activity was calculated using the extinction coefficient E1% (w/v) azocaseine in 1 m NaOH, 440 = 37 L cm−1g−1. HXK activity assay medium with Glc and Man contained the enzymatic extract, 50 mm Tris (pH 7.5), 5 mm MgCl2, 3 mm dithiothreitol (DTT), 2 mm ATP, 1 mm NAD, and 5 units of G6P dehydrogenase (from leuconostoc mesenteroides). Reactions, run at 25°C, were routinely initiated with 2 mm Glc or Man and monitored at 340 nm. When Man was used as a substrate, the reaction medium was supplemented with 5 units of phospho-Man isomerase and 10 units of phospho-Glc isomerase. To assess the HXK activity with 3-OMG, the assay medium (final volume = 2 mL) contained 0.5 to 1.5 units of purified enzyme, 200 mm Tris (pH 7.5), 3 mm DTT, 2 mm ATP, 5 mm MgCl2, 1% (v/v) phosphatase inhibitor cocktail II (P5726, Sigma), and 20 mm KH2PO4. Reaction was initiated by the addition of [methyl-14C]3-OMG (4 MBq mmol−1) at varying concentrations (10, 20, 50, and 100 mm), and run at 25°C. Reactions were stopped by adding 3 mL of boiling ethanol to the 600-μL samples after 0, 90, and 180 min. Samples were dried in a rotary evaporator and the residue resuspended in 1 mL of water. The molecules 3-OMG and 3-OMG-6P were separated through a 1.5-mL anionic column (AG-1, Cl− form). Radioactivity was measured in the anionic fraction and disintegrations per minute were converted to nanomoles using the specific radioactivity of [methyl-14C]3-OMG. Neutral phosphatase assay was conducted in 0.5 m Tris (pH 7.5). The reaction, measured at 25°C, was initiated with 5 mm p-nitrophenyl phosphate and the appearance of p-nitrophenol was monitored at 420 nm (Ep-nitrophenol,420 = 75.75 cm−1 mm−1). G6P phosphatase assay medium (1 mL at 25°C) contained 250 to 500 μL of desalted extract in 50 mm Tris (pH 7.5), 25 μm chymostatin, and 5 mm G6P. Aliquots of 200 μL were sampled every 10 min and the reaction stopped by adding 500 μL of boiling water. After 5 min at 100°C, samples were dried in a rotary evaporator and the residues resuspended in 1 mL of water. Residual G6P in the samples was measured at 340 nm, in a 50 mm Tris buffer (pH 7.5) containing 3 mm MgCl2, 3 mm DTT, 0.5 mm NAD, and 5 units of G6P dehydrogenase. The Glc produced was measured subsequently after addition of 2 mm ATP and 3 units of yeast HXK.
Other Analytical Methods
Proteins were quantified (Bradford, 1976) using the Bio-Rad microassay reagent. Bovine γ-globulin was used as the standard. SDS-PAGE, western-blot, and immunoprecipitation experiments were performed as described (Brouquisse et al., 2001). AdNs were extracted by the cold diethyl-ether/trichloracetic acid procedure and determined by the bioluminescence method (Saglio and Pradet, 1980). Because AMP was too low to be confidently measured by this method, it was calculated from the adenylate kinase mass action ratio, Keq = (ATP × AMP)/(ADP)2, using the measured mean values of ATP and ADP, and assuming that Keq = 0.8 (Pradet and Raymond, 1983). The total AdN content, Σ AdN = (ATP + ADP + AMP), and AEC = (ATP + 0.5ADP)/(ATP + ADP + AMP), were then calculated.
NMR Spectroscopy
All the NMR measurements were performed using an AMX400 WB spectrometer (Bruker S.A., Wissembourg, France): 31P and 13C NMR spectra were acquired at 162 and 100.6 MHz, respectively. During in vivo experiments, they were recorded alternatively for 10 and 50 min, respectively, using a dual electronically switched 25-mm probe. Acid extracts were analyzed using a 10-mm broadband probe. General acquisition conditions have been described (Brouquisse et al., 2001) and specific acquisition parameters are given in the figure legends.
A solution composed of 50 mm methylenediphosphonic acid (MDP; 9508, Sigma) and 8 m ethanol contained in a concentric capillary provided the chemical shifts and intensity references for all spectra. The resonance assignment of metabolites was based upon chemical shifts. The concentration time course of a given metabolite was obtained in vivo in the following way. First, the intensity of its characteristic resonance was measured in each spectrum, eventually corrected to take sample growth out of the detection coil into account (normalization to the initial number of root tips within the detection coil), as well as spectral fluctuations, and plotted as a function of time. Second, the curve obtained was calibrated against concentration as described below for each isotope.
Carbon NMR
The chemical shifts were referenced to tetramethylsilane using the Suc fructosyl C2 resonance (C2f) fixed at 104.4 ppm as a secondary internal reference. In the absence of Suc resonances in a spectrum, the resonances of ethanol contained in the capillary mixture were used as secondary external reference (their chemical shifts were measured at 58.25 and 18.03 ppm). The Suc amount was derived from the intensity of the C2f resonance, the 3-OMG amount was derived from the sum of the intensities of the C3β (86.2 ppm), and C3α (83.6 ppm) resonances and Asn amount was derived from the intensity of the βCH resonance at 35.6 ppm. These intensities were corrected for the saturation effect arising from the fast acquisition procedure and for the nuclear Overhauser enhancement developed during the relaxation delay (the correction factor was calculated from three interleaved standard and relaxed spectra). The absolute metabolite amounts were calculated from the corrected intensities using the ethanol CH2 resonance intensity calibrated itself in moles.
Phosphorous NMR
Chemical shifts were referenced to 85% (w/v) H3PO4 using internal glycerylphosphorylcholine as a secondary reference (−0.05 ppm) for in vitro spectra and internal UDPG (−12.65 ppm) for in vivo spectra (the chemical shift of MDP contained in the capillary mixture was measured at 16.72 ppm). Quantification of acid extract spectra was done by integration of the resolved resonances and calibration of the intensities was achieved using the resonance intensity of MDP. The in vivo data were calibrated using the intensities of a relaxed spectrum of maize root tips fed on 200 mm Glc. The intensities, devoid of nuclear Overhauser enhancement, were calibrated against concentrations using the MDP reference. The amounts of G6P and 3-OMG-6P could not be determined separately from in vivo spectra because there is an overlap of their resonances.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
ACKNOWLEDGMENTS
We thank Marie Hélène Andrieu and Nathalie Pochon who helped us to excise and analyze about 40,000 maize root tips necessary for this study, Monique Gaudillère for her help in AdN extraction and analysis, Pierre Carol and Eliane Charpentier for the Arabidopsis cells and advice, and Jean-Luc Le Bail for his dedicated technical assistance with the NMR spectrometer and perifusion system. Discussions arising from in-depth criticism of the work by the referees added value to our report. We are sincerely grateful to Jan A. Miernyk for his thorough reading of the manuscript, sharp language corrections, and pertinent suggestions to improve the original version.
Footnotes
This work was supported by the Centre National de la Recherche Scientifique, by the Commissariat à l'Energie Atomique, by the Institut National de la Recherche Agronomique, by the Université Victor Segalen in Bordeaux, by the Université Joseph Fourier in Grenoble, in part by the EC (grant no. BIO4–CT96–0311), and by the Ministère de l'Education Nationale, de la Recherche, et de la Technologie (grants to S.C. and A.E.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010538.
LITERATURE CITED
- ap Rees T. Hexose phosphate metabolism by non-photosynthetic tissues of higher plants. In: Preiss J, editor. The Biochemistry of Plants. Vol. 14. New York: Academic Press; 1988. pp. 1–33. [Google Scholar]
- Axelos M, Curie C, Mazzolini L, Bardet C, Lescure B. A protocol for transient gene expression in Arabidopsis thaliana protoplasts isolated from cell suspension cultures. Plant Physiol Biochem. 1992;30:123–128. [Google Scholar]
- Bock K, Pedersen C. Carbon-13 nuclear magnetic resonance spectroscopy of monosaccharides. In: Tipson RS, Horton D, editors. Advances in Carbohydrates Chemistry and Biochemistry. New York: Academic Press; 1983. pp. 27–66. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brouquisse R, Evrard A, Rolin D, Raymond P, Roby C. Regulation of protein degradation and protease expression by mannose in maize root tips. Pi sequestration by mannose may hinder the study of its signaling properties. Plant Physiol. 2001;125:1485–1498. doi: 10.1104/pp.125.3.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouquisse R, James F, Pradet A, Raymond P. Asparagine metabolism and nitrogen distribution during protein degradation in sugar-starved maize root tips. Planta. 1992;188:384–395. doi: 10.1007/BF00192806. [DOI] [PubMed] [Google Scholar]
- Brouquisse R, James F, Raymond P, Pradet A. Study of glucose starvation in excised maize root tips. Plant Physiol. 1991;96:619–626. doi: 10.1104/pp.96.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier C, Bourgeois E, Pradet A, Raymond P. Molecular cloning and characterization of six cDNAs expressed during glucose starvation in excised maize (Zea mays L.) root tips. Plant Mol Biol. 1995;28:473–485. doi: 10.1007/BF00020395. [DOI] [PubMed] [Google Scholar]
- Csaky TZ, Wilson JE. The fate of 3-O-14CH3-glucose in the rat. Biochim Biophys Acta. 1956;22:185–186. doi: 10.1016/0006-3002(56)90237-2. [DOI] [PubMed] [Google Scholar]
- da-Silva WS, Rezende GL, Galina A. Subcellular distribution and kinetic properties of cytosolic and non-cytosolic hexokinases in maize seedling roots: implication for hexose phosphorylation. J Exp Bot. 2001;52:1191–1201. [PubMed] [Google Scholar]
- Fan TW-M. Recent advances in profiling plant metabolites by multinuclear and multidimensional NMR. In: Shachar-Hill Y, Pfeffer P, editors. Nuclear Magnetic Resonance in Plant Biology. Rockville, MD: American Society of Plant Biologists; 1996. pp. 181–254. [Google Scholar]
- Fujiki Y, Ito M, Nishida I, Watanabe A. Multiple signaling pathways in gene expression during sugar starvation. Phamacological analysis of din gene expression in suspension-cultured cells of Arabidopsis. Plant Physiol. 2000;124:1139–1147. doi: 10.1104/pp.124.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galina A, da-Silva WS. HXK activity alters sugar-nucleotide formation in maize root homogenate. Phytochemistry. 2000;53:29–37. doi: 10.1016/s0031-9422(99)00456-2. [DOI] [PubMed] [Google Scholar]
- Galina A, Reis M, Albuquerque MC, Puyou AG, Puyou MTG, De Meis L. Different properties of the mitochondrial and cytosolic HXKs in maize roots. Biochem J. 1995;309:105–112. doi: 10.1042/bj3090105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancedo C, Gancedo JM. Phosphorylation of 3-O-methyl-d-glucose and catabolite repression in yeast. Eur J Biochem. 1984;148:593–597. doi: 10.1111/j.1432-1033.1985.tb08881.x. [DOI] [PubMed] [Google Scholar]
- Gatley SJ, Holden JE, Halama JR, DeGrado TR, Bernstein DR, Ng CK. Phosphorylation of glucose analog 3-O-methyl-d-glucose by rat heart. Biochem Biophys Res Commun. 1984;119:1008–1014. doi: 10.1016/0006-291x(84)90874-x. [DOI] [PubMed] [Google Scholar]
- Genix P, Bligny R, Martin J-B, Douce R. Transient accumulation of asparagine in sycamore cells after a long period of sucrose starvation. Plant Physiol. 1990;94:717–722. doi: 10.1104/pp.94.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt DE, Riegel A, Roitsch T. Regulation of sucrose synthase expression in Chenopodium rubrum: characterization of sugar induced expression in photoautotrophic suspension cultures and sink tissue specific expression in plants. J Plant Physiol. 1995;146:231–238. [Google Scholar]
- Gogarten JP, Bentrup F-W. Fluxes and compartmentation of 3-O-methyl-d-glucose in Riccia fluitans. Planta. 1983;159:423–431. doi: 10.1007/BF00392078. [DOI] [PubMed] [Google Scholar]
- Gogarten JP, Bentrup F-W. Substrate specificity of the hexose carrier in the plasmalemma of Chenopodium suspension cells probed by transmembrane exchange diffusion. Planta. 1989;178:52–60. doi: 10.1007/BF00392526. [DOI] [PubMed] [Google Scholar]
- Graham IA, Denby KJ, Leaver CJ. Carbon catabolite repression regulates glyoxylate cycle gene expression in cucumber. Plant Cell. 1994;6:761–772. doi: 10.1105/tpc.6.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford NG, Purcell PC, Hardie DG. Is hexokinase really a sugar sensor in plants? Trends Plant Sci. 1999;4:117–120. doi: 10.1016/s1360-1385(99)01377-1. [DOI] [PubMed] [Google Scholar]
- Herold A, Lewis DH. Mannose and green plants: occurrence, physiology and metabolism, and use as a tool to study the role of orthophosphate. New Phytol. 1977;79:1–40. [Google Scholar]
- Ho S-L, Chao Y-C, Tong W-F, Yu S-M. Sugar coordinately and differentially regulates growth- and stress-related gene expression via a complex signal transduction network and multiple control mechanisms. Plant Physiol. 2001;125:877–890. doi: 10.1104/pp.125.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann S, Winderickx J, de Winde JH, Valckx D, Cobbaert P, Luyten K, de Meirsman C, Ramos J, Thevelein JM. Novel alleles of yeast hexokinase PII with distinct effects on catalytic activity and catabolite repression of SUC2. Microbiology. 1999;145:703–714. doi: 10.1099/13500872-145-3-703. [DOI] [PubMed] [Google Scholar]
- Ichimura K, Kohata K, Goto R. Soluble carbohydrates in Delphinium and their influence on sepal abscission in cut flowers. Physiol Plant. 2000;108:307–313. [Google Scholar]
- James F, Brouquisse R, Pradet A, Raymond P. Changes in proteolytic activities in glucose-starved maize root tips. Regulation by sugars. Plant Physiol Biochem. 1993;31:845–856. [Google Scholar]
- James F, Brouquisse R, Suire C, Pradet A, Raymond P. Purification and biochemical characterization of a vacuolar serine endopeptidase induced by glucose starvation in maize roots. Biochem J. 1996;320:283–292. doi: 10.1042/bj3200283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J-C, Sheen J. Sugar sensing in higher plants. Plant Cell. 1994;6:1665–1679. doi: 10.1105/tpc.6.11.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J-C, Sheen J. Sugar sensing in higher plants. Trends Plant Sci. 1997;2:208–214. [Google Scholar]
- Johnston M. Feasting, fasting and fermenting. Glucose sensing in yeast and other cells. Trends Genet. 1999;15:29–33. doi: 10.1016/s0168-9525(98)01637-0. [DOI] [PubMed] [Google Scholar]
- Journet EP, Bligny R, Douce R. Biochemical changes during sucrose deprivation in higher plant cells. J Biol Chem. 1986;261:3193–3199. [PubMed] [Google Scholar]
- King GA, Woollard DC, Irving DE, Borst WM. Physiological changes in asparagus spear tips after harvest. Physiol Plant. 1990;80:393–400. [Google Scholar]
- Koch KE. Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Kraakman LS, Winderickx J, Thevelein JM, de Winde JH. Structure-function analysis of yeast hexokinase: structural requirement for triggering cAMP signalling catabolite repression. Biochem J. 1999;343:159–168. [PMC free article] [PubMed] [Google Scholar]
- Lalonde S, Boles E, Hellmann H, Barker L, Patrick JW, Frommer WB, Ward JM. The dual function of sugar carriers: transport and sugar sensing. Plant Cell. 1999;11:707–726. doi: 10.1105/tpc.11.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse-Lagae F, Giroix M-H, Sener A, Malaisse WJ. Phosphorylation of 3-O-methyl-d-glucose by yeast and beef HXK. FEBS Lett. 1986;198:292–294. doi: 10.1016/0014-5793(86)80423-9. [DOI] [PubMed] [Google Scholar]
- Martin T, Hellman H, Schmidt R, Willmitzer L, Frommer WB. Identification of mutants in metabolically regulated gene expression. Plant J. 1997;11:53–62. doi: 10.1046/j.1365-313x.1997.11010053.x. [DOI] [PubMed] [Google Scholar]
- Moriyasu Y, Ohsumi Y. Autophagy in tobacco suspension-cultured cells in response to sucrose starvation. Plant Physiol. 1996;111:1233–1241. doi: 10.1104/pp.111.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt C, Gross W. Different sugar kinases are involved in the sugar sensing of Galdieria sulphuraria. Plant Physiol. 2002;128:291–299. [PMC free article] [PubMed] [Google Scholar]
- Peoples MB, Dalling MJ. The interplay between proteolysis and amino acid metabolism during senescence and nitrogen reallocation. In: Noodén LD, Leopold AC, editors. Senescence and Aging in Plants. San Diego: Academic Press Inc.; 1988. pp. 181–217. [Google Scholar]
- Plaxton W. Metabolic aspects of phosphate starvation in plants. In: Lynch JP, Deikman J, editors. Phosphorus in Plant Biology: Regulatory Roles in Molecular, Cellular, Organismic and Ecosystem Processes. Rockville, MD: American Society of Plant Biologists; 1998. pp. 229–241. [Google Scholar]
- Pradet A, Raymond P. Adenine nucleotide ratios and adenylate energy charge in energy metabolism. Annu Rev Plant Physiol. 1983;34:199–224. [Google Scholar]
- Raghotama KG. Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:665–693. doi: 10.1146/annurev.arplant.50.1.665. [DOI] [PubMed] [Google Scholar]
- Reinhold L, Eshhar Z. Transport of 3-O-methylglucose into and out of storage cells of Daucus carota. Plant Physiol. 1968;63:1023–1030. doi: 10.1104/pp.43.7.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille P-ML, Robitaille PA, Brown GG, Jr, Brown GG. An analysis of the pH-dependent chemical-shift behavior of phosphorous-containing metabolites. J Magn Reson. 1991;92:73–84. [Google Scholar]
- Roby C, Martin J-B, Bligny R, Douce R. Biochemical changes during sucrose deprivation in higher plant cells. Phosphorus-31 nuclear magnetic resonance studies. J Biol Chem. 1987;262:5000–5007. [PubMed] [Google Scholar]
- Roitsch T, Bittner M, Godt DE. Induction of apoplastic invertase of Chenopodium rubrum by d-glucose and a glucose analog and tissue specific expression suggest a role in sink-source regulation. Plant Physiol. 1995;108:285–294. doi: 10.1104/pp.108.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland F, Moore B, Sheen J (2002) Sugar sensing and signaling in plants. Plant Cell S185–S205 [DOI] [PMC free article] [PubMed]
- Roscher A, Emsley L, Raymond P, Roby C. Unidirectional steady state rates of central metabolism enzymes measured simultaneously in a living plant tissue. J Biol Chem. 1998;273:25053–25061. doi: 10.1074/jbc.273.39.25053. [DOI] [PubMed] [Google Scholar]
- Saglio P, Pradet A. Soluble sugars, respiration, and energy charge during aging of excised maize root tips. Plant Physiol. 1980;66:516–519. doi: 10.1104/pp.66.3.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Ges V, Roby C, Bligny R, Pradet A, Douce R. Kinetic studies of the variations of cytoplasmic pH, nucleotide triphosphates (31P-NMR) and lactate during normoxic and anoxic transitions in maize root tips. Eur J Biochem. 1991;200:477–482. doi: 10.1111/j.1432-1033.1991.tb16207.x. [DOI] [PubMed] [Google Scholar]
- Sauer N, Stadler R. A sink-specific H+ monosaccharide cotransporter from Nicotiana tabacum: cloning and heterologous expression in baker's yeast. Plant J. 1993;4:601–610. doi: 10.1046/j.1365-313x.1993.04040601.x. [DOI] [PubMed] [Google Scholar]
- Sheen J, Zhou L, Jang J-C. Sugars as signaling molecules. Curr Opin Plant Biol. 1999;2:410–418. doi: 10.1016/s1369-5266(99)00014-x. [DOI] [PubMed] [Google Scholar]
- Smeekens S. Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:49–81. doi: 10.1146/annurev.arplant.51.1.49. [DOI] [PubMed] [Google Scholar]
- Tarshis MA, Bekkouzjin AG, Ladygina VG, Panchenko LF. Properties of 3-O-methyl-d-glucose transport system in Acholeplasma laidlawii. J Bacteriol. 1976;125:1–7. doi: 10.1128/jb.125.1.1-7.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassi F, Maestri E, Restivo FM, Marmiloni N. The effects of carbon starvation on cellular metabolism and protein and RNA synthesis in Gerbera callus culture. Plant Sci. 1992;83:127–136. [Google Scholar]
- Wiese J, Kleber R, Hampp R, Nehls U. Functional characterization of the Amanita muscaria monosaccharide transporter. Plant Biol. 2000;2:278–282. [Google Scholar]
- Yu S-M. Cellular and genetic responses of plants to sugar starvation. Plant Physiol. 1999;121:687–693. doi: 10.1104/pp.121.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]