Abstract
Raffinose family oligosaccharides (RFOs) have been implicated in mitigating the effects of environmental stresses on plants. In seeds, proposed roles for RFOs include protecting cellular integrity during desiccation and/or imbibition, extending longevity in the dehydrated state, and providing substrates for energy generation during germination. A gene encoding galactinol synthase (GOLS), the first committed enzyme in the biosynthesis of RFOs, was cloned from tomato (Lycopersicon esculentum Mill. cv Moneymaker) seeds, and its expression was characterized in tomato seeds and seedlings. GOLS (LeGOLS-1) mRNA accumulated in developing tomato seeds concomitant with maximum dry weight deposition and the acquisition of desiccation tolerance. LeGOLS-1 mRNA was present in mature, desiccated seeds but declined within 8 h of imbibition in wild-type seeds. However, LeGOLS-1 mRNA accumulated again in imbibed seeds prevented from completing germination by dormancy or water deficit. Gibberellin-deficient (gib-1) seeds maintained LeGOLS-1 mRNA amounts after imbibition unless supplied with gibberellin, whereas abscisic acid (ABA) did not prevent the loss of LeGOLS-1 mRNA from wild-type seeds. The presence of LeGOLS-1 mRNA in ABA-deficient (sitiens) tomato seeds indicated that wild-type amounts of ABA are not necessary for its accumulation during seed development. In all cases, LeGOLS-1 mRNA was most prevalent in the radicle tip. LeGOLS-1 mRNA accumulation was induced by dehydration but not by cold in germinating seeds, whereas both stresses induced LeGOLS-1 mRNA accumulation in seedling leaves. The physiological implications of LeGOLS-1 expression patterns in seeds and leaves are discussed in light of the hypothesized role of RFOs in plant stress tolerance.
The raffinose family oligosaccharides (RFOs) are soluble galactosyl-Suc carbohydrates that constitute a significant component of phloem-transported sugars in some plants (Haritatos et al., 2000). Their accumulation in plants is associated with stressful environmental conditions such as cold, heat, or dehydration (Santarius, 1973; Santarius and Milde, 1977; Hinesley et al., 1992; Ashworth et al., 1993; Wiemken and Ineichen, 1993; Bachmann et al., 1994; Taji et al., 2002). The potential role of RFOs in stress tolerance has been intensively studied in seeds, particularly with respect to desiccation tolerance and longevity in the dehydrated state. RFOs are abundant in most mature desiccation-tolerant (“orthodox”) seeds and are often rare or absent in “recalcitrant” seeds that cannot withstand desiccation (Lin and Huang, 1994; Sun et al., 1994). In seeds of many species, RFO accumulation coincides with the development of desiccation tolerance during seed maturation (Koster and Leopold, 1988; Leprince et al., 1993; Bewley and Black, 1994; Horbowicz and Obendorf, 1994; Black et al., 1996; Brenac et al., 1997a, 1997b), and RFO content has been positively correlated with seed longevity in storage (Bernal-Lugo and Leopold, 1992; Horbowicz and Obendorf, 1994; Lin and Huang, 1994; Bernal-Lugo and Leopold, 1995). Despite these correlations, large amounts of RFOs do not appear to be essential for desiccation tolerance (Hoekstra et al., 1994) or seed longevity in the dry state (Bentsink et al., 2000; Buitink et al., 2000; Gurusinghe and Bradford, 2001). On the other hand, raffinose/Suc mixtures are effective in protecting pea (Pisum sativum) embryo protoplasts from lethal dehydration stress (Xiao and Koster, 2001). RFOs may also accumulate in seeds primarily as a readily metabolizable carbohydrate source for energy generation during germination (Downie and Bewley, 2000). Thus, despite their abundance and widespread occurrence in mature seeds (Amuti and Pollard, 1977), the specific function(s) of RFOs in seed biology remains unknown.
RFOs are synthesized by donation of Gal from galactinol to Suc catalyzed by RAFFINOSE SYNTHASE (RAFS, EC 2.4.1.82), creating the trisaccharide raffinose. Subsequent additions of Gal units to raffinose result in stachyose, verbascose, and other RFOs. Galactinol is formed from UDP-Gal and myo-inositol by the action of GALACTINOL SYNTHASE (GOLS, EC 2.4.1.123), which is putatively the committed enzyme step in the RFO biosynthetic pathway (Pridham and Hassid, 1965; Lehle and Tanner, 1972; Saravitz et al., 1987; Peterbauer et al., 2001; Smith et al., 1991). Because the only known function for galactinol is in the formation of RFOs (Saravitz et al., 1987; Liu et al., 1995), galactinol synthesis is likely to be a regulated step in the biosynthesis of RFOs.
Liu et al. (1998) identified a GOLS transcript in kidney bean (Phaseolus vulgaris) seeds that increased in abundance in vegetative tissues when plants were exposed to cold stress. Takahashi et al. (1994) found that an mRNA present in rice (Oryza sativa) seedlings (WSI76), subsequently recognized as encoding a GOLS (Liu et al., 1997), accumulated in response to chilling at 4°C and to osmotic stress, but not to abscisic acid (ABA). Sprenger and Keller (2000) demonstrated that two distinct GOLS genes, both up-regulated by chilling, were transcribed in discrete locations (GOLS-1 in mesophyll cells and GOLS-2 in companion cells of the phloem) in the vegetative tissues of the RFO-translocating and frost-hardy species common bugle (Ajuga reptans). Seven AtGOLS genes were identified in Arabidopsis, and the mRNAs from at least three of these genes (AtGOLS1, AtGOLS2, and AtGOLS3) were preserved in mature, dry seeds. The expression of these three genes was characterized in vegetative tissues where AtGOLS1 and -2 were up-regulated in response to water and salinity stress but not cold, whereas AtGOLS3 was induced only during cold stress (Taji et al., 2002). Overexpression of AtGOLS2 improved the drought tolerance of the transgenic Arabidopsis plants (Taji et al., 2002).
As noted in a recent review by Peterbauer and Richter (2001), despite an abundance of information on RFO amounts in seeds and seed parts from a number of species during development and germination and despite a variety of hypotheses concerning their role(s) in seeds, there are no data on expression of GOLS genes in seeds during germination or during exposure of imbibed seeds to environmental stress. We report here the cloning of a GOLS gene (LeGOLS-1) from tomato (Lycopersicon esculentum Mill. cv Moneymaker) and characterization of its expression during seed maturation and during germination under various conditions. In addition, the availability of gibberellin (GA)- and ABA-deficient mutants in tomato allowed studies of the hormonal control of transcription of LeGOLS-1 in the absence of these endogenous hormones.
RESULTS
Cloning of the LeGOLS-1 Gene from Tomato
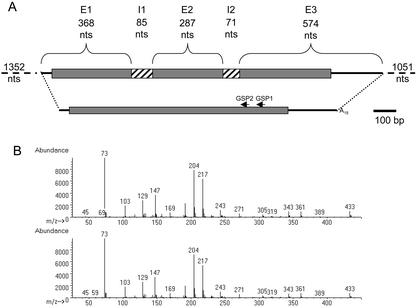
Using PCR and library screening methods (see “Materials and Methods”), a full-length cDNA of tomato LeGOLS-1 was isolated (accession no. AF311943) as well as the corresponding genomic sequence (accession no. AF447452; Fig. 1A). The deduced amino acid sequence (318 amino acids) of the tomato LeGOLS-1 protein was most homologous to an incomplete protein sequence from canola (Brassica napus; accession no. AF106954). The tomato protein was also highly homologous (59% identity, 81% similarity between the full-length proteins) to a putative GOLS from pea (accession no. AJ243815), to WS176 from rice (sequence annotated and deduced from 9845048; Takahashi et al., 1994), and to a protein sequence known to have galactinol synthesizing capability from common bugle (accession no. AJ237693; Sprenger and Keller, 2000). The conserved carboxy-terminal “PSAA,” present at the terminus of all GOLS genes identified to date (Sprenger and Keller, 2000; Taji et al., 2002), was also present at the carboxy terminus of the tomato sequence. However, the putative Ser phosphorylation site (Ser-263) identified in a subset of deduced GOLS proteins (Sprenger and Keller, 2000) and conserved in 14 of the 17 known full-length GOLS sequences (B. Downie, G. Chaiyaprasityhi, and T.-Y. Zhao, unpublished data) was replaced with an Ala (Ser→Ala-250) in LeGOLS-1. Analysis of the tomato protein predicted residence in the cytoplasm, consistent with published reports (Bachmann and Keller, 1995; Sprenger and Keller, 2000) and a mature protein devoid of a N-terminal signal peptide (Target P; Emanuelsson et al., 2000). The coding region, when expressed in Escherichia coli, produced a protein capable of synthesizing galactinol from UDP-Gal and myo-inositol (Fig. 1B).
Figure 1.
The gene and cDNA structure of LeGOLS-1 and recombinant protein activity. A, The gene and full-length LeGOLS-1 cDNA are depicted. Untranslated regions are lines, exons (E) are bracketed, and the hatched boxes represent introns (I). The gene-specific primers (GSP) used to retrieve the 5′ portion of the cDNA are indicated. A scale bar of 100 bp is beside the cDNA. The 1,352-nucleotide promoter region and 1,051 3′ nucleotides are not drawn to scale. B, Recombinant LeGOLS-1 protein synthesized a compound identical to that of galactinol. Upper spectrum was obtained from the product of UDP-Gal and myo-inositol incubated with recombinant LeGOLS-1. The lower spectrum is from authentic galactinol obtained from maize seeds. The quality of the match between the two spectra was 95%.
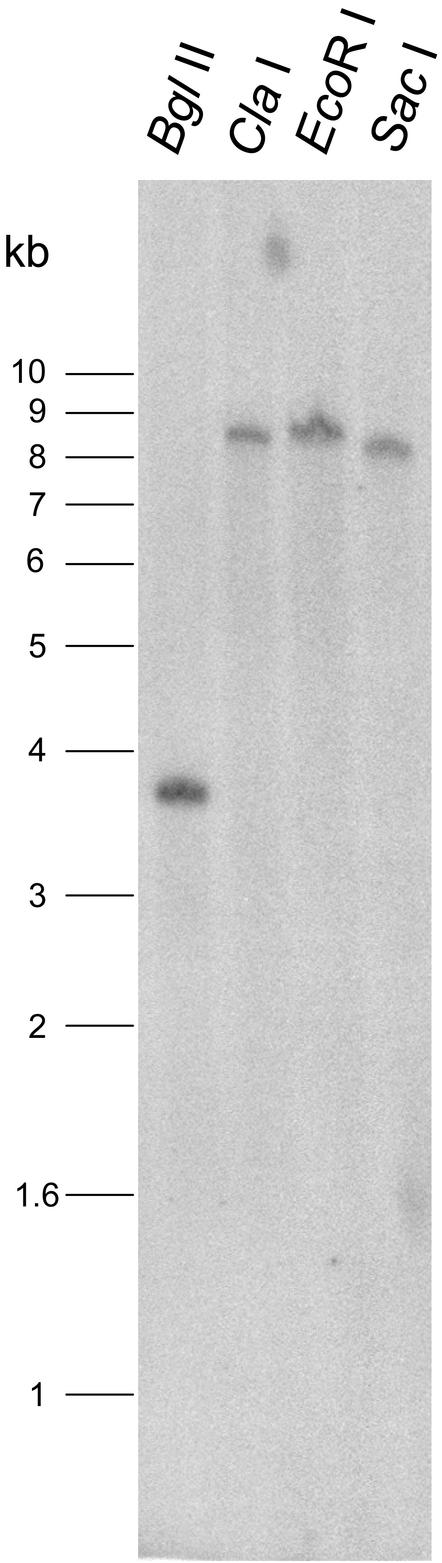
Southern hybridization of genomic DNA with the full-length LeGOLS-1 cDNA revealed only single bands, even when hybridized at low stringency (Fig. 2). The cloned LeGOLS-1 gene is apparently represented by a single copy in the genome or is quite distinct from other GOLS genes in tomato, in contrast to the situation in other species (Taji et al., 2002). The tomato GOLS gene had two introns (Fig. 1A) positioned in two highly conserved sites that are also interrupted in all reported GOLS genes, but a third intron, present in the seven Arabidopsis GOLS genes, was not present in tomato (B. Downie, G. Chaiyaprasityhi, and T.-Y. Zhao, unpublished data). The absence of sites for the four restriction enzymes used in Figure 2 in either the exons or introns of LeGOLS-1 was confirmed from the genomic sequence.
Figure 2.
Southern blot of tomato genomic DNA probed with the full coding region of LeGOLS-1. Only single hybridizing bands were detected, even when washed at low stringency (see “Materials and Methods”).
Expression of LeGOLS-1 mRNA during Seed Development and Germination and in Response to GA and ABA
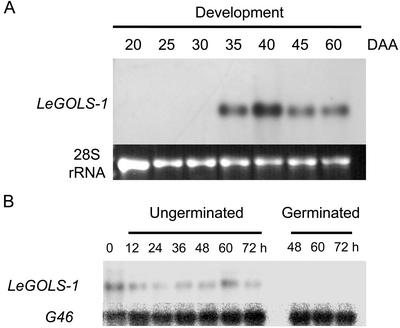
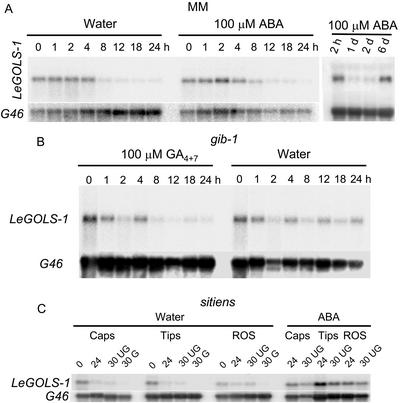
LeGOLS-1 mRNA was first detected in developing wild-type tomato seeds at 35 d after anthesis (DAA) and remained abundant throughout maturation (Fig. 3A). Detectable amounts of LeGOLS-1 mRNA coincided with maximum dry weight accumulation in the seeds (data not shown) and maximum amounts of planteose on a per seed or dry weight basis (Table I). Raffinose was present from the earliest time point sampled (20 DAA) and attained a maximum concentration before LeGOLS-1 mRNA became detectable (Table I). LeGOLS-1 mRNA was not detected in seeds that had completed germination (Fig. 3B). LeGOLS-1 mRNA was present in the endosperm caps, radicle tips, and the rest of the mature desiccated tomato seed, being most abundant in the radicle tips (Fig. 4A). LeGOLS-1 mRNA decreased within 24 h after imbibition (HAI) on water, on 100 μm GA4+7, or on 100 μm ABA in all seed parts examined (Fig. 4A). The decline in LeGOLS-1 mRNA in seed parts 24 HAI was more obvious than in whole seeds 24 HAI (Fig. 3B). The amount of transcript in seeds that had not completed germination at 48 HAI was greater than that in germinated seeds. The seeds that had not completed germination at 48 HAI also had greater amounts of transcript than the population of seeds at 24 HAI that included both seeds that would and would not complete germination within the next 24 h (Fig. 4A). Seeds imbibed on 100 μm ABA showed the same pattern even though none of these seeds would complete germination (Fig. 4A). LeGOLS-1 mRNA abundance was low in all seed parts after imbibition on 100 μm GA4+7, regardless of germination status (Fig. 4A).
Figure 3.
Northern blot of LeGOLS-1 transcript abundance during wild-type tomato seed maturation and germination. A, LeGOLS-1 expression was not detectable before 35 DAA, then increased and remained present throughout seed maturation. B, LeGOLS-1 expression during and after germination on water. After 48 h, seeds were separated into those that had or had not completed germination. G46 detects a constitutively expressed ribosomal protein mRNA used as a loading control (Cooley et al., 1999).
Table I.
Sugar amounts in developing tomato cv Moneymaker seeds on a per seed (upper) and dry weight (lower) basis
| Tomato cv Moneymaker | Dry Weight | Suc | Planteose | Raffinose | Stachyose | Verbascose |
|---|---|---|---|---|---|---|
| DAA | g | nmol seed−1 μmol g−1 dry wt | ||||
| 20 | 0.0005 | 13.2 ± 2.3 25.4 ± 4.4 | 0.0 0.0 | 0.9 ± 0.8 1.8 ± 1.5 | 0.1 ± 0.1 0.1 ± 0.1 | 0.0 0.0 |
| 25 | 0.0010 | 17.2 ± 1.29 16.9 ± 1.3 | 0.0 0.0 | 2.1 ± 0.1 1.8 ± 0.1 | 0.8 ± 0.6 0.7 ± 0.5 | 0.0 0.0 |
| 30 | 0.0016 | 17.6 ± 1.4 11.8 ± 1.3 | 2.7 ± 0.4 3.2 ± 1.7 | 3.1 ± 0.2 2.0 ± 0.2 | 0.1 ± 0.1 0.1 ± 0.1 | 0.0 0.0 |
| 35 | 0.0019 | 14.4 ± 1.3 7.4 ± 0.7 | 5.7 ± 1.2 3.0 ± 0.6 | 2.0 ± 0.2 1.0 ± 0.1 | 1.1 ± 0.1 0.6 ± 0.1 | 0.0 0.0 |
| 40 | 0.0022 | 19.5 ± 1.2 8.7 ± 0.5 | 12.5 ± 1.3 5.6 ± 0.6 | 0.9 ± 0.7 0.4 ± 0.3 | 2.7 ± 0.4 1.2 ± 0.2 | 0.0 0.0 |
| 60 | 0.0014 | 13.1 ± 1.1 9.4 ± 0.8 | 11.2 ± 0.8 8.0 ± 0.6 | 1.6 ± 0.3 1.2 ± 0.2 | 0.4 ± 0.1 0.3 ± 0.1 | 0.1 ± 0.1 0.1 ± 0.1 |
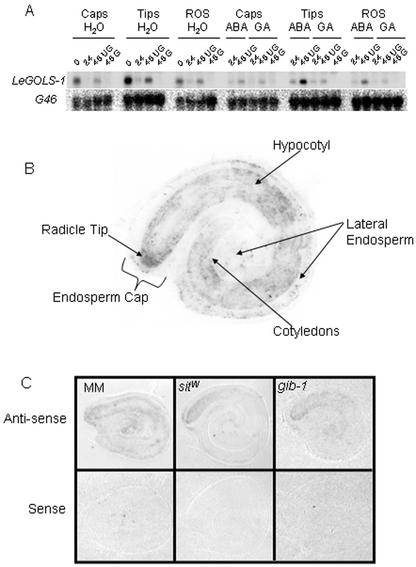
Figure 4.
Tissue-specific location of LeGOLS-1 expression in tomato seeds of various genotypes. A, Wild-type seeds were imbibed on water, 100 μm GA4+7, or 100 μm ABA for 0, 24, and 48 h; separated into those that had or had not completed germination when feasible (48 h only); and dissected into endosperm caps (Caps), radicle tips (Tips), and the rest of the seed (ROS). Total RNA was extracted from these seed parts and analyzed for LeGOLS-1 mRNA. G46 is a constitutively expressed loading control. B, A representative tissue print of a mature tomato seed imbibed on water for 3 h at 4°C. LeGOLS-1 mRNA is present throughout the embryo but is noticeably more abundant in the radicle tip. Some LeGOLS-1 mRNA is present in the cells at the periphery of the lateral and micropylar endosperm (endosperm cap). C, Antisense- and sense-probed tissue prints of wild-type, sitiens, and gib-1 mutant tomato seeds imbibed on water for 4 h at 25°C.
To confirm the localization of LeGOLS-1 expression, tissue prints were conducted with mature tomato cv Moneymaker (MM) seeds imbibed on water at 4°C for 3 h or wild-type, ABA-deficient sitiens (sitW), or GA-deficient gib-1 tomato seeds imbibed on water at 25°C for 4 h (Fig. 4, B and C, respectively). LeGOLS-1 mRNA was present throughout the embryo and was also detectable to a lesser extent in the endosperm (Fig. 4B). The greatest amount of mRNA was present in the radicle tip regardless of genotype (Fig. 4, B and C), verifying results from northern-blot analysis of seed parts (Fig. 4A). Sense probe of a specific activity equal to that of the antisense probe did not hybridize to any seed tissues (Fig. 4C).
Northern analysis of tomato seed parts indicated that the majority of LeGOLS-1 mRNA was lost within 24 HAI (Fig. 4A). To further characterize this decline in abundance, seeds were sampled more frequently within the first 24 HAI. LeGOLS-1 mRNA abundance decreased substantially in wild-type seeds between 4 and 8 HAI on water and between 8 and 12 HAI on 100 μm ABA (Fig. 5A). However, if seeds were maintained in the presence of ABA for extended periods (unable to complete germination), LeGOLS-1 mRNA accumulated again after a considerable delay (Fig. 5A). When GA-deficient gib-1 seeds were imbibed on 100 μm GA4+7, LeGOLS-1 mRNA declined between 4 and 8 HAI, but the mRNA remained more abundant in gib-1 seeds imbibed on water (a condition under which the mutant seeds do not complete germination; Fig. 5B).
Figure 5.
The effect of ABA and GA on the maintenance of LeGOLS-1 mRNA abundance in imbibed tomato seeds. A, Wild-type tomato cv Moneymaker seeds were imbibed on either water or 100 μm ABA. LeGOLS-1 transcript abundance decreased substantially after 4 h (water-imbibed) or 8 h (ABA-imbibed). ABA did not maintain LeGOLS-1 transcript abundance, but message was again present after 6 d on ABA, possibly due to the induction of secondary dormancy. B, GA-deficient gib-1 mutant seeds, which require supplemental GA to complete germination, were imbibed on either 100 μm GA4+7 or water. Total RNA extracted after different times of imbibition was hybridized to LeGOLS-1 antisense riboprobe. LeGOLS-1 mRNA abundance decreased substantially within 12 h in GA-imbibed seeds but remained higher when imbibed on water. C, Seeds of the ABA-deficient sitiens mutant were imbibed on water for 0, 24, and 30 h, when they were separated into those seeds that had or had not completed germination. Despite having very low amounts of ABA (Groot and Karssen, 1992), sitiens seeds accumulated LeGOLS-1 transcript during development and maturation desiccation, accounting for its presence in dry seeds. Upon imbibition, transcript amounts declined as they did in wild-type seeds. When sitiens seeds were imbibed on 100 μm ABA, LeGOLS-1 mRNA amounts remained high for at least 30 h, although amounts were declining (compare 24 and 30 h). G46 is a constitutively expressed loading control.
Because ABA did not maintain LeGOLS-1 mRNA in 24-h-imbibed mature tomato seeds but the mRNA accumulated again after longer incubation on ABA (Figs. 4A and 5A), we asked whether ABA might be involved in LeGOLS-1 accumulation during seed development. Despite having very low ABA contents (Groot and Karssen, 1992), sitW seeds were capable of accumulating LeGOLS-1 mRNA during development and/or dehydration, because LeGOLS-1 mRNA was present in dry seed parts before imbibition (Figs. 4C and 5C). Upon imbibition on water, transcript amounts declined as they did in wild-type seeds (Fig. 5C), whereas imbibition on 100 μm ABA maintained LeGOLS-1 mRNA abundance in sitW seeds (Fig. 5C).
Expression of LeGOLS-1 mRNA in Response to Water and Temperature Stress
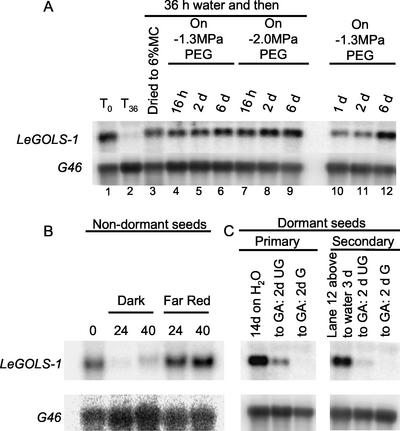
The effects of environmental stresses on LeGOLS-1 mRNA abundance were assessed in MM seeds imbibed on water or on −1.3 or −2.0 MPa polyethylene glycol (PEG) solutions. After a decline in mRNA amounts at 36 HAI on water (Fig. 6A, lane 2), LeGOLS-1 mRNA accumulated in response to slow desiccation or imposed osmotic stress (Fig. 6A, lanes 3 and 4–9). Seeds initially imbibed at low water potential also maintained or accumulated the transcript (Fig. 6A, lanes 10–12).
Figure 6.
Water stress induces LeGOLS-1 transcription. A, Wild-type tomato cv Moneymaker seeds were imbibed on water for 36 h and then either dried or transferred to −1.3 or −2.0 MPa PEG solutions. Seeds were alternatively imbibed directly on −1.3 MPa PEG. Total RNA was extracted and analyzed for LeGOLS-1 abundance. After a decline in mRNA amounts upon imbibition for 36 h on water, LeGOLS-1 transcript accumulated again in response to desiccation or osmotic stress. Seeds initially imbibed at low water potential also maintained or accumulated the transcript. B, Wild-type tomato cv Moneymaker seeds were imbibed for 24 or 40 h in the dark or under continuous far-red illumination, which prevents completion of germination. Far-red illumination maintained LeGOLS-1 transcript abundance in wild-type tomato cv Moneymaker seeds. C, LeGOLS-1 transcript abundance was also maintained or accumulated in seeds that were in primary dormancy (ungerminated after 14 d on water) or secondary dormancy (imbibed on −1.3 MPa PEG for 6 d, then transferred to water 3 d, radicle not protruded). GA effectively stimulated germination of seeds exhibiting both types of dormancy and decreased LeGOLS-1 mRNA abundance in these seeds.
The completion of germination of wild-type seeds was prevented by far-red illumination, and this treatment also prevented the decline of mRNA amounts during imbibition (Fig. 6B). Seeds that were in primary dormancy (Fig. 6C; without radicle protrusion for 14 d on water) or secondary dormancy (Fig. 6C; −1.3 MPa PEG for 6 d, transferred to water for 3 d without radicle protrusion) contained large amounts of LeGOLS-1 mRNA. GA effectively alleviated both primary and secondary dormancy in most seeds and decreased LeGOLS-1 mRNA abundance (Fig. 6C).
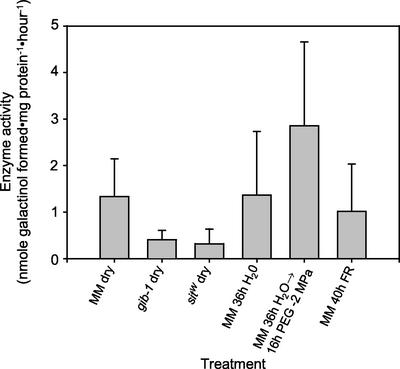
Similar GOLS enzyme activity was detected in extracts from mature, desiccated wild-type seeds and from 36-h-imbibed seeds (Fig. 7). Less GOLS activity was detected in seeds of desiccated gib-1 and sitW seeds than in those of the wild type (Fig. 7). Enzyme activity increased in seeds imbibed for 36 h on water and then transferred to −2 MPa PEG for 16 h; however, enzyme activity did not increase (above that present in desiccated seeds) in seeds imbibed for 40 h under far-red light (Fig. 7).
Figure 7.
GOLS enzyme activity from tomato seeds after various treatments. Desiccated seeds of both gib-1 and sitW mutants had less GOLS activity than did desiccated wild-type seeds. Despite a decline in mRNA amounts after imbibition for 36 h, GOLS enzyme activity did not decrease relative to that present in desiccated seeds. Treatments that prevented the completion of germination maintained GOLS enzyme activity.
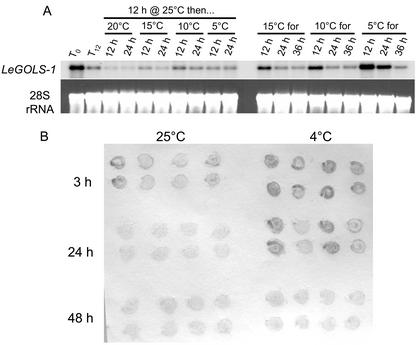
We examined whether chilling was capable of stimulating LeGOLS-1 mRNA accumulation or delaying the decline of transcript abundance in seeds. Both northern blots and tissue prints confirmed that LeGOLS-1 mRNA declined more slowly as the imbibition temperature decreased, but the mRNA had declined to low amounts by 36 to 48 h even at temperatures where germination was prevented (5°C or 4°C; Fig. 8, A and B).
Figure 8.
LeGOLS-1 is not up-regulated by chilling in seeds. A, Wild-type tomato seeds were imbibed at 25°C for 12 h and then either harvested for RNA extraction or transferred to 20°C, 15°C, 10°C, or 5°C for an additional 12 or 24 h before harvest. Seeds were also imbibed on water at 15°C, 10°C, or 5°C and sampled at 12, 24, and 36 h. LeGOLS-1 transcript was present in all seeds regardless of treatment type or duration. The colder temperatures delayed the decrease in message amounts as imbibition time increased. Temperatures as low as 5°C were not sufficient to increase message abundance beyond that present in mature, desiccated seeds over the brief time course. B, Tissue prints of wild-type tomato seeds imbibed at either 25°C or 4°C for up to 2 d that had not completed germination confirmed that cold temperature was not sufficient to increase LeGOLS-1 mRNA abundance in imbibing seeds but served only to delay its decrease.
To compare with published accounts of GOLS gene expression in vegetative tissues of other species, detached tomato leaves or whole seedlings were subjected to several stresses. LeGOLS-1 transcript abundance was initially low in leaves (Fig. 9, lane 5), but increased slightly when leaves were detached and allowed to dehydrate (Fig. 9, lanes 1–4). The marked increase in LeGOLS-1 mRNA abundance between attached and detached leaves (Fig. 9, lanes 5 and 1) may be due to wounding or slight dehydration while in the plastic bag for 6 h (the fresh weight was the same as when the leaf was removed from the plant). Dehydration for 20 min before being placed in a plastic bag increased LeGOLS-1 mRNA abundance only slightly, whereas prolonged dehydration for up to 60 min did not result in any further increases in LeGOLS-1 mRNA quantity (Fig. 9). A one-time foliar application of 100 μm ABA to intact seedlings also resulted in a transient increase in LeGOLS-1 transcript abundance (Fig. 9, lanes 5–8). Subjecting seedlings to 4°C stimulated LeGOLS-1 gene expression by 48 h, and this was reversed by subsequently moving the chilled seedlings to 25°C (Fig. 9). Heat stress also stimulated slight but detectable LeGOLS-1 gene expression in seedlings within 12 h but continuing to stress seedlings at 37°C beyond 12 h killed them (Fig. 9).
Figure 9.
LeGOLS-1 transcript accumulates in dehydration-stressed leaves and chilling-stressed seedlings. Mature leaves were detached from 18-d-old tomato plants and dehydrated to different moisture contents. The excised leaves were placed immediately in a plastic bag (0 min, 100% of initial fresh weight) or were allowed to lose water on the bench top for up to 1 h (20 min = 92%, 40 min = 88%, and 60 min = 88% of initial fresh weight) and then were sealed in plastic bags for 6 h before freezing and extraction of RNA. Plants were also sprayed once to run-off with 100 μm ABA and leaves harvested at 0, 6, 12, and 24 h after ABA application. Some plants were subjected to 4°C for 12, 24, and 48 h, and the leaves from some plants harvested, whereas other plants were moved to 25°C for an additional 48 h before the leaves were harvested. Some plants were placed at 37°C for 4, 8, and 12 h and leaves harvested at each time.
DISCUSSION
On the basis of Southern-blot analysis, the tomato LeGOLS-1 gene is present as a single copy in the tomato genome or is highly divergent in sequence from other GOLS genes in tomato (Fig. 2). The presence of a single GOLS gene would distinguish tomato from Arabidopsis (at least seven genes; Taji et al., 2002) and common bugle (at least two; Sprenger and Keller, 2000). The presence of additional GOLS genes in tomato with sequences dissimilar enough to prevent hybridization even at low stringency (Fig. 2A) would alternatively be unusual for this conserved gene family. Resolution of this question awaits additional cloning or eventual sequencing of the tomato genome.
Detectable amounts of LeGOLS-1 mRNA were present on and after 35 DAA during tomato seed development, corresponding to the time when tomato seeds achieve maximum dry weight and become tolerant of desiccation (Berry and Bewley, 1991). This expression pattern during development was similar to that observed for PeaGS1, a GOLS gene expressed during pea seed development (Peterbauer et al., 2001). LeGOLS-1 mRNA was present in the mature, desiccated seeds of every tomato genotype examined. Mature tomato seeds contain small amounts of raffinose and verbascose (Gurusinghe and Bradford, 2001), which presumably require galactinol, and therefore GOLS, for their synthesis. However, the presence of LeGOLS-1 mRNA and raffinose was poorly correlated in developing seeds (Fig. 2A; Table I). The major oligosaccharide in tomato seeds (in addition to Suc) conversely is O-α-d-galactopyranosyl-(1–6)-β-d-fructofuranosyl-α-d-glucopyranoside (planteose; Duke, 1992; Gurusinghe and Bradford, 2001), which is putatively synthesized from Suc and UDP-Gal rather than galactinol (Kandler and Hopf, 1982). The maximum accumulation of planteose coincided well with the maximum abundance of LeGOLS-1 at 40 DAA. However, recombinant LeGOLS-1 had no detectable planteose-synthesizing capacity (data not shown), although it is capable of synthesizing galactinol (Fig. 1B). A reduced dependence on the raffinose series oligosaccharides in tomato relative to other sucrosyl oligosaccharides could be related to the apparent lack of diversity of GOLS genes in this species.
The presence of LeGOLS-1 mRNA in mature, desiccated seeds of both the sitiens and gib-1 mutants indicates that neither normal amounts of ABA nor normal amounts of GA are required for the transcription of this gene in developing seeds. In both cases, the mutant seeds were grown on plants that received small amounts of either ABA or GA to improve their growth and fertility, so the possibility that low amounts of these hormones may have been present cannot be excluded. However, the phenotypic features characteristic of sitW seeds (lack of dormancy, relative insensitivity to osmotic stress, and far-red light and rapid completion of germination; Downie et al., 1999) and gib-1 seeds (failure to complete germination on water; Groot and Karssen, 1987) make it unlikely that the hormone applications significantly affected seed development. Desiccated seeds of both mutants had less enzyme activity than did desiccated wild-type seeds (Fig. 7).
The amount of LeGOLS-1 mRNA in tomato seeds declined after imbibition on water (Figs. 4A and 5A), although this was not always obvious (Fig. 3B). The small amount of RFOs in the tomato micropylar region (radicle tip and surrounding endosperm tissue) varied little during this period, whereas planteose amounts declined by 50% from 0 to 24 HAI (Gurusinghe and Bradford, 2001). In some cases, LeGOLS-1 mRNA appeared to increase in abundance between 24 and 48 h in seeds that had not completed germination (Figs. 3B and 4A). The apparent increase could be due to the subdivision of each sample at 48 HAI into seeds that had or had not completed germination. The samples that had not completed germination would be enriched in seeds that were dormant or slow to germinate and that also maintained higher amounts of LeGOLS-1 transcript (Fig. 4A). However, this cannot explain the increase in LeGOLS-1 mRNA amounts between 24 and 48 HAI for seeds on 100 μm ABA (Fig. 4A). At this ABA concentration, none of the seeds had completed germination at 48 HAI and so no selection had occurred. Rather, after an initial decline in message abundance (Fig. 5A), LeGOLS-1 mRNA accumulated in tomato seeds that were prevented from completing germination by high concentrations of ABA (Figs. 4A and 5A), osmoticum (Fig. 6A), far-red light (Fig. 6B), or dormancy (Fig. 6C). Transfer of 36-h-imbibed seeds to PEG resulted in an increase in LeGOLS-1 mRNA (Fig. 6A) and GOLS enzyme activity (Fig. 7). In addition, tomato seeds that had been imbibed in osmotic solutions accumulated raffinose under some conditions (Gurusinghe and Bradford, 2001). In nature, seeds that fail to complete germination due to dormancy or adverse environmental conditions may be subjected to desiccation at any time. If Suc and RFOs are involved in the desiccation tolerance and longevity of seeds in the dry state (Brenac et al., 1997a; Buitink et al., 2000), the re-expression of LeGOLS-1 in imbibed seeds that will not complete germination for various reasons (ABA, dormancy, osmotic stress, or far-red light) would be a sound survival strategy.
GA-deficient mutant seeds maintained LeGOLS-1 mRNA after imbibition unless they were supplied with exogenous GA4+7, which resulted in a decline in LeGOLS-1 mRNA abundance (Fig. 5B). ABA, on the other hand, which is known to antagonize GA action, delayed the decline of LeGOLS-1 mRNA amounts in sitw seeds (Fig. 5C) and the mRNA re-accumulated in wild-type seeds maintained for an extended period in ABA (Fig. 5A). In addition, continuous irradiation of wild-type seeds with far-red light, known to inhibit both the biosynthesis of and sensitivity to GA (Yamaguchi et al., 1998), maintained LeGOLS-1 mRNA abundance (Fig. 6B). Progression toward the completion of seed germination (radicle protrusion) is often depicted as being dependent upon a balance between the stimulatory effects of GA and the repressive action of ABA (Koornneef et al., 1982). Our data suggest that LeGOLS-1 gene expression in seeds may not be directly sensitive to ABA, but that any factor that delays completion of germination long enough will result in renewed expression of the gene. GA may directly enhance breakdown of LeGOLS-1 mRNA, or loss of the message may simply accompany the general turnover of mRNAs and switch in gene expression patterns accompanying the initiation of germination (Berry and Bewley, 1991).
The response of LeGOLS-1 gene expression to cold was tissue specific. In tomato seeds, cold temperature did not stimulate GOLS transcription (Fig. 8), whereas in vegetative tissues, it did (Fig. 9). In rice seedlings, GOLS transcription was strongly up-regulated by osmoticum in both cold-tolerant and cold-sensitive cultivars, but chilling induced GOLS transcription most strongly in the cold-tolerant cultivar (Takahashi et al., 1994). In common bugle, GOLS gene expression in intact vegetative tissues was stimulated by chilling only after a 1- to 2-week lag period (Sprenger and Keller, 2000). In contrast, GOLS-2 expression was up-regulated by chilling within 8 h in excised leaves (Sprenger and Keller, 2000). AtGOLS expression was induced by cold within 8 to 12 h in Arabidopsis plants (Taji et al., 2002), with expression being greater in stems and leaves than in siliques (Liu et al., 1998). LeGOLS-1 message abundance increased after only 48 h at 4°C in leaves of intact tomato plants (Fig. 9), whereas cold temperatures only delayed the decline of LeGOLS-1 message in seeds (Fig. 8, A and B). One possible reason for the inability of cold temperatures to induce GOLS gene expression in seeds may be that, even in the imbibed state, many seeds are resistant to this stress. When held at 4°C for as long as 4 months, white spruce (Picea glauca [Moench.] Voss.) seeds eventually completed germination and mobilized their stored reserves of RFOs, suggesting that any protective capacity of RFOs was subservient to their utility as energy reserves (Downie and Bewley, 2000). However, the vegetative tissues of the plant are more susceptible to chilling injury, and RFOs may ameliorate this stress. Hence, one manifestation of overexpressing a transcriptional activator of cold acclimation (CBF3) in Arabidopsis was the accumulation of Suc and raffinose in leaves (Gilmour et al., 2000).
In contrast to the insensitivity of LeGOLS-1 gene expression to ABA, dehydration of previously imbibed seeds by desiccation or osmotic stress induced LeGOLS-1 gene expression in tomato seeds (Fig. 6). In seeds, induction of LeGOLS-1 expression was most pronounced after osmotic or dehydration stress, an attribute shared with the GOLS genes in vegetative tissues of rice and Arabidopsis (Takahashi et al., 1994; Taji et al., 2002). This may also be the case in tomato vegetative tissues (Fig. 9), although the effects of dehydration may be confounded with wounding and leaf detachment (Fig. 9, lanes 5 and 1). Although LeGOLS1 is up-regulated in vegetative tissues by cold, and in seed and possibly vegetative tissue by dehydration, there is no recognizable C-repeat/dehydration responsive element conserved core motif (Thomashow, 2001) in the promoter.
The imposition of an osmotic stress sufficient to prevent radicle protrusion is in common use in the seed industry to prepare a variety of vegetable and flower seeds for synchronous radicle protrusion (priming). Despite priming increasing LeGOLS-1 mRNA abundance, priming can result in a decline of RFO content in some seeds (Hoekstra et al., 1994), including tomato (Hilhorst and Downie, 1996), as well as a decline in seed longevity (Tarquis and Bradford, 1992; Saracco et al., 1995; Gurusinghe and Bradford, 2001). Thus, at least in seeds of some species, GOLS gene expression may not correlate with an accumulation of raffinose, but either RAFS gene expression or posttranslational regulation imposed on one or both enzymes (GOLS and/or RAFS) could determine the amount of raffinose manufactured by the seed (Peterbauer et al., 2001). Posttranslational regulation may explain why no GOLS activity was detected in MM seeds imbibed 36 h and then dried (data not shown), although these seeds had detectable amounts of LeGOLS-1 mRNA (Fig. 6A). Sprenger and Keller (2000) documented a positive association between GOLS-2 message abundance, GOLS activity, and RFO accumulation in source leaves of common bugle grown at either warm or cold temperatures. Therefore, RFO biosynthesis may be under different levels of control in either different tissues within the same species or similar tissues in different species.
The occurrence of LeGOLS-1 in greatest amounts in the radicle tips of embryos from mature, desiccated seeds correlates well with presence of raffinose in the radicle tips of tomato seeds (Gurusinghe and Bradford, 2001) and the abundance of RFOs in the radicle tips of seeds of numerous species (Koster and Leopold, 1988). In addition, Kuo et al. (1997) documented significantly greater GOLS activity and RFO amounts in the embryonic axis relative to the cotyledons of developing soybean (Glycine max) seeds. The radicle is the part of the seed that first loses desiccation tolerance as germination progresses, which was attributed to an earlier decline in RFO amounts in this tissue relative to tissues capable of withstanding water loss (Koster and Leopold, 1988). The radicle is also the seed part most susceptible to death due to aging in dry storage (Golovina et al., 1997), a phenomenon also potentially associated with the presence of Suc and oligosaccharides (Ooms et al., 1993, 1994; Wolkers et al., 1998a, 1998b). A T-DNA knockout of one of at least four seed imbibition proteins (SIPs; possibly encoding proteins with raffinose synthetic capacity) in Arabidopsis resulted in lower Suc and verbascose amounts in the leaves, whereas raffinose amounts remained unchanged. This decreased the Suc to raffinose ratio in the leaves of the mutant, which also exhibited an increase in dehydration tolerance (Anderson and Kohorn, 2001). A similar phenotype has been documented in Arabidopsis plants overexpressing AtGOLS2 (Taji et al., 2002). How these perturbations in RFO metabolism have led to enhanced drought tolerance in vegetative tissues remains unknown.
MATERIALS AND METHODS
Plant Material
Tomato (Lycopersicon esculentum Mill. cv Moneymaker) seeds were obtained from plants grown in the field in 1995 and developing seeds were obtained in 2000. For the developmental time course, open flowers were tagged every day and seeds recovered from fruit every 5 d throughout development from 20 to 45 DAA and at maturity (60 DAA). Immature seeds were immediately recovered from the fruit, washed to remove the locular tissue, frozen in liquid nitrogen, and stored at −80°C until use.
ABA-deficient sitiens and GA-deficient gib-1 mutant seeds in the tomato cv Moneymaker background were obtained from fruit from plants grown in a greenhouse under 16 h light at 25°C/20°C day and night temperatures, respectively. Leaves of the sitiens plants were sprayed twice weekly with a solution of 10 μm cis-trans ABA (Sigma-Aldrich, St. Louis), whereas leaves of gib-1 plants were sprayed twice weekly with a solution of 10 μm GA4+7 (Abbott Laboratories, North Chicago). The seeds were cleaned after release from the fruit by incubating them in 0.1 m HCl for 1 h before washing with tap water. Seeds were dried to 5% moisture content (fresh weight basis) before storage at −20°C.
Germination Conditions and Treatments
Germination conditions were according to Dahal et al. (1997). Fifteen milliliters of distilled, deionized water, 100 μm GA4+7 (Abbott Laboratories), 100 μm cis-trans ABA (Sigma-Aldrich), or concentrations of PEG 8000 (aerated and replaced daily) sufficient to create an osmotic potential of −1.3 or −2.0 MPa at the temperature of germination (Michel and Kaufmann, 1973) were placed on two 8.5-cm diameter blotting paper discs (Grade 628, Stults Scientific Eng., Springfield, IL) in a petri dish. One gram of seeds was then sown on the blotters. Dishes were placed at 25°C in the dark inside sealed containers that also enclosed water-saturated paper towels. Seeds were imbibed on water for various durations at 5°C, 10°C, 15°C, 20°C, or 25°C. In addition, seeds were initially sown on water at 25°C for various durations and subsequently moved to solutions of low osmotic potential (−1.3 or −2.0 MPa for 16, 48, or 144 h), transferred to colder germination temperatures (4°C), placed on 100 μm GA4+7, or redried (29°C for 60 h to 6% moisture content fresh weight). Seeds harvested from wild-type tomato cv Moneymaker plants grown in the greenhouse initially completed germination to only 30% due to primary dormancy. These seeds were initially imbibed on water for 14 d, and the dormant seeds were subsequently moved to 100 μm GA4+7. Mature, desiccated seeds initially imbibed directly on PEG solutions (−1.3 or −2.0 MPa for 16, 48, or 144 h) were subsequently moved to water and then to 100 μm GA4+7. Seeds imbibed on PEG solutions were thoroughly rinsed for 5 min in running tap water and vacuum dried for 1 min to remove surface water before being transferred or frozen. ABA-deficient sitiens seeds on water were harvested at 30 h rather than 48 h due to their more rapid completion of germination. Seed samples were stored at −80°C until RNA extraction.
Whole wild-type seeds were germinated at 20°C for up to 40 h either in the dark or under continuous FR illumination in a custom-made FR chamber (Lagarias et al., 1997; Downie et al., 1999).
Seedlings were grown in pots in a greenhouse for 16 d and allowed to acclimatize to room temperature for 2 d. On the 18th d, leaves (upper two leaves from each plant) were excised and placed immediately in a plastic bag or were allowed to lose water for up to 1 h before being placed in a plastic bag. Samples were then held for 6 h before weighing and freezing in liquid nitrogen. In a second experiment, ABA (100 μm) was applied once as a spray to intact seedlings. Intact seedlings were also incubated at 4°C or 37°C. Some seedlings that had been held for 2 d at 4°C were moved back to 25°C for 12 h and then sampled. All seedling treatments were applied under continuous light.
PCR Amplification, cDNA Library Screening, and 5′-RACE Procedure
A 503-bp fragment of a tomato GOLS cDNA was amplified from reverse-transcribed RNA obtained from mature MM seeds imbibed at 4°C for 1 week using degenerate primers fashioned from amino acid sequence alignments of known GOLS cDNAs (Krenz, 1997; Liu et al., 1997). Degenerate forward primers were synthesized (Genset, La Jolla, CA) to include nucleotides coding for amino acids 108 to 114 (RIWKFVE; 5′-GIA THT GGR ART TYG TIG-3′) and degenerate reverse-complemented primers were prepared to include amino acids 281 to 287 (EDIKMLV; 5′-CIA RCA TYT TDA TRT CYT C-3′). The amplicon from these primers included an internal conserved site for amino acids 185 to 196 [YFNAG(M/F) F(V/L)(Y/H/F) EP(N/D/G)] to verify the identity of the amplicon. Touch-down PCR was used starting at 57°C and decreasing the annealing temperature 1°C each round to 42°C. Forty additional rounds of PCR were subsequently performed annealing at 42°C. The 500-bp amplicon was ligated into pCR2.1 (Invitrogen, San Diego; Fig. 1). The cloned amplicon was digested with EcoRI, isolated through an agarose gel, excised, and purified using a kit (Qiagen, Valencia, CA). The amplicon was labeled (Feinburg and Vogelstein, 1983) using [α-32P]dATP (3,000 Ci mmol−1, New England Nuclear Life Science Products, Boston) and Klenow DNA Polymerase I (Roche Diagnostics, Indianapolis). Using the labeled amplicon as a probe, a partial length cDNA (LeGOLS-1) encoding 731 bp tailed with 18 adenine residues was identified from a λ-ZAP Express cDNA library (Stratagene, La Jolla, CA) prepared from 40 h, water-imbibed gib-1 mutant tomato seeds.
To obtain the 5′ portion of the gene, RACE was performed using a kit according to the manufacturer's instructions (Invitrogen). The amplicon was cloned into pCR 2.1 (Invitrogen) and sequenced. The full coding region of LeGOLS-1 was spliced together from the 5′-truncated clone in pBK-CMV and the 5′-RACE product in pCR2.1 by overlap-extension-PCR (Slack, 1998) using the thermal stable DNA polymerase, Pfu (Stratagene).
Sequencing of the amplicon, partial length cDNA, 5′-RACE fragment, and spliced full coding region was performed at either the Advanced Plant Genetics Facility (University of California, Davis) or the Macromolecular Structure Analysis Laboratory (University of Kentucky, Lexington). Both facilities used an ABI Prism 377 DNA Sequencer (Applied Biosystems, Foster City, CA) and dye termination chemistry with AmpliTaq DNA polymerase, FS (Taq; FS; Applied Biosystems) to read cycle-sequencing reactions employing a combination of universal and gene-specific primers (Genset Corporation, Operon Technologies, Alameda, CA).
Genomic DNA Isolation and Analysis
Genomic DNA was isolated from expanding tomato leaves according to Murray and Thompson (1980). Genomic DNA (5 μg per lane) was exhaustively digested with restriction endonucleases, electrophoresed through a 0.8% (w/v) agarose gel in 1× Tris-acetate EDTA (Sambrook et al., 1989) and transferred to GeneScreen membrane (New England Nuclear Life Science Products, Inc., Boston). The digested DNA was cross-linked to the membrane and prehybridized at 65°C for 12 h in 6× SSPE, 5× Denhardt's solution (Denhardt, 1966), 0.5% (w/v) SDS, and 100 μg mL−1 boiled, sheared salmon sperm DNA. Thereafter, the blot was hybridized with the radiolabeled, 957-bp coding region. The blot was first washed twice, 15 min each time, at low stringency (2× SSC, 0.1% [w/v] SDS at 65°C) and exposed to a phosphor screen for 2 d. The image was captured using a Storm (Molecular Dynamics, Sunnyvale, CA), and the blots were rewashed at high stringency (0.2× SSC and 0.1% [w/v] SDS, 65°C, 1× 30 min) and re-exposed to the phosphor screen for 5 d.
RNA Isolation and Analysis
Total RNA from seeds and seed parts, regardless of treatment or genotype, was extracted according to Cooley et al. (1999). Total RNA (5 μg per lane) from seeds or seed parts was transferred onto positively charged nylon membranes (Amersham Life Science Inc., Arlington Heights, IL) in 10× SSC (1× SSC is 150 mm NaCl and 15 mm sodium citrate, pH 7) overnight and UV cross-linked at 120 000 μJoules cm−2 on a FB-UVXL-1000 Stratalinker (Fisher Scientific, Santa Clara, CA). After rinsing the membranes for 5 min in 2× SSC, they were placed in prehybridization solution (50% [v/v] formamide, 5× Denhardt's solution [Denhardt, 1966], 100 μg mL−1 boiled, sheared salmon sperm DNA, 0.2% [w/v] SDS, and 6× SSC, pH 7.0, [Sambrook et al., 1989]) for 4 to 6 h at 60°C. Radiolabeled antisense RNA probes from the cDNA were generated by linearizing the plasmid with XhoI (Fig. 1) and incubating the template at 37°C with T7 DNA-dependent, RNA polymerase (Amersham Biosciences, Piscataway, NJ) in a run-off transcription reaction in the presence of [α-32P]UTP (3000 Ci mmol−1, New England Nuclear). The probe was added to the prehybridization solution described above, and the membrane was probed for at least 12 h at the same temperature as prehybridization. The primary wash was done in 2× SSC and 0.1% (w/v) SDS, at room temperature for 5 min, and repeated but at 65°C for 30 min. The two final high-stringency washes were at 0.2× SSC and 0.1% (w/v) SDS, 65°C for 30 min each. The hybridized probe was detected by autoradiography using Kodak X-OMAT x-ray film (Eastman-Kodak Ltd.) or on a phosphor imaging screen using a Storm (Molecular Dynamics). Tissue prints of seeds imbibed 3, 4, 24, and 48 h at either 25°C or 4°C were performed as described by Nonogaki et al. (2000).
Genomic Clone Isolation
Five hundred thousand recombinants from an EMBL3 genomic library (Budelier et al., 1990) were screened, and three genomic clones of the tomato GOLS were recovered. The recombinants were purified in subsequent screens, and viral DNA was recovered, cleaved in single digests with EcoRI or BamHI or in double digests with SalI and EcoRI or SalI and BamHI, subcloned into pBSII KS (Invitrogen) cut with the appropriate enzyme(s), and dephosphorylated if necessary. The subcloned fragments of genomic DNA comprising and encompassing the LeGOLS-1 gene were identified by Southern blot and sequenced at the Macromolecular Structure Analysis Laboratory (University of Kentucky) using T7, T3, and gene-specific primers (Operon Technologies).
In addition, two gene-specific primers for LeGOLS-1 cDNA were used to amplify most of the LeGOLS-1 gene from tomato genomic DNA. Because the BglII fragmentation of tomato genomic DNA appeared, from Southern-blot analysis, to encompass the whole LeGOLS-1 gene and to result in a relatively small restriction fragment, inverse PCR was simultaneously used on BglII-cut, self-ligated genomic DNA. The resulting amplicon was cloned into pCR2.1 (Invitrogen) and sequenced.
Enzyme Assay and Recombinant Protein Expression
Seeds were pulverized in liquid nitrogen in a mortar and pestle and homogenized in ice-cold extraction buffer (50 mm HEPES, 1 mm dithiothreitol [DTT], 50 mm ascorbic acid, 10% [v/v] ethylene glycol, and 1 mm MnCl2, pH 7.5; Liu et al., 1995) for 1 min with a Polytron. After centrifugation (10,000g for 20 min), the supernatant was assayed directly for GOLS activity in a 25-μL reaction in microfuge tubes. Aliquots (10 μL) of the seed supernatant or recombinant protein were added to assay buffer (50 mm HEPES, 4 mm MnCl2, 2 mm DTT, and 4 μg bovine serum albumin; Liu et al., 1995), which was then pre-incubated for 15 min at 30°C before the addition of 0.2 μCi of Uridine-5′-Diphospho-Gal-(Gal-6-3H) (Sigma-Aldrich; 16.8 Ci mmol−1), 2.5 μL of 40 mm cold UDP-Gal (the final assay was 4 mm with respect to UDP-Gal), and, to one of two duplicate reactions per sample, 20 mm myo-inositol, whereas to the other, water was added to the reaction (Bachmann et al., 1994). Assays proceeded for 2 h and were terminated by the addition of 50 μL of 100% (v/v) ethanol, vortexed, and centrifuged. Un-reacted UDP-Gal was removed by the addition of 100 mg of Dowex-1 resin (formate form prepared according to Dawson et al. [1986]) added to each tube and incubated for 20 min before the addition of 1 mL of water. After vortexing and centrifugation, 500 μL was added to scintillant, and the amount of radioactivity determined. Samples to which water rather than seed extract had been added were used to determine the effectiveness of the resin in removing un-reacted UDP-Gal from the supernatant. Similar samples without added resin were used to determine assay-specific activity for the determination of enzyme activity based on the amount of product formed. Duplicate reactions using only cold UDP-Gal were run with some extracts, and the presence of galactinol was verified using gas chromatography-mass spectrometry (GC-MS; see below). Protein concentration in the samples was determined using the Bradford assay (Bradford, 1976) due to its compatibility with the DTT in the extraction buffer.
The coding region of the tomato GOLS was cloned into pET43.1 (Novagen, Madison, WI) using XmaI/BspE I (vector/insert) and EcoRI. After sequence confirmation, plasmid was inserted into BL21(DE3) RIL cells (Stratagene) grown in Luria-Bertani, 100 μg mL−1 ampicillin, and 34 μg mL−1 chloramphenicol to an A600 of 0.4 before induction with isopropylthio-β-galactoside at a final concentration of 1 mm and expressed for 16 h at 25°C. The cells were lysed using a French press, the presence of soluble protein was confirmed, and the lysate was used in the enzyme assay directly or the recombinant protein was purified on a HiTrap chelating column (Amersham Biosciences) according to the manufacturer's instructions and used in the assay.
GOLS assays were conducted as described above for seed extracts but in a 250-μL reaction mixture with 4 mm cold UDP-Gal only, and the products were recovered in ethanol, lyopholized to dryness, derivatized, and analyzed using GC-MS as described below.
GC-MS Detection of Galactinol
Lyopholized samples of ethanol-soluble sugars from enzyme assays were dissolved in 0.5 mL of 80:20 (v/v) methanol:water and vortexed until the pellet was dissolved. An aliquot (100 μL) of the sample was pipetted into 13- × 100-mm glass tubes, and blown to dryness with UHP N2 (Scott-Gross Co., Lexington, KY). Immediately after drying, 250 μL of pyridine (Sigma-Aldrich) was added, and the sample was vortexed. A 150-μL aliquot of HMDS-trimethylchlorosilane, 3:1 silylation reagent (Supelco, Bellefonte, PA) was added to the sample, briefly vortexed, and heated to 95°C for 10 min. Tubes were topped with glass marbles to reduce evaporation. After 10 min, samples were vortexed and taken to dryness using UHP N2; 250 μL of hexane (Fisher Optima, Santa Clara, CA) was added. After vortexing, samples were filtered through a 0.2-μm PTFE membrane (Pall Gelman, Ann Arbor, MI) into a 1.5-mL sample vial, and an aliquot (1 μL) was injected onto a gas chromatograph (5890, Hewlett Packard, Palo Alto, CA) equipped with flame ionization detector detection and a 30-m OV-5 column (5% [w/w] diphenyl- and 95% [w/w] dimethyl-polysiloxane). The column flow rate of He was 2.3 mL min−1. The injector temperature was 250°C, and that of the detector was 300°C. The initial column temperature was 100°C, and the run consisted of a 1-min hold at 100°C, ramping 6°C min−1 to 175°C, and then ramped at 10°C min−1 to 300°C, which was held for an additional 32 min. After GC, a Hewlett Packard GCD Plus mass spectrometer was used to identify the galactinol peaks in the elution profile. Conditions for separation on the GC-MS were identical to those for the GC-flame ionization detector. Mass spectra of the putative galactinol peak from the recombinant tomato protein were matched with spectra obtained from galactinol from a variety of plant sources and were compared with that for pure galactinol provided by Dr. Franca Marinone Albini as published by Ferrarotti (1996).
Sugar Quantification
Sugars were isolated and detected according to Gurusinghe and Bradford (2001).
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
ACKNOWLEDGMENTS
We thank Prof. Clark Lagarius (University of California, Davis) who kindly allowed us the use of his far-red light source. Prof. Charles Gasser (University of California, Davis) provided an aliquot of the tomato genomic library. Abbott Laboratories provided us with GA4+7. Prof. Robert Houtz (University of Kentucky) allowed the use of his FPLC for recombinant protein purification, and Prof. George Wagner (University of Kentucky) the use of his French Press. The Department of Plant Pathology, University of Kentucky allowed access to their gel documentation and phosphor imaging systems.
Footnotes
This work was supported in part by the U.S. Department of Agriculture National Research Initiative-Competitive Grants program (grant no. 98–35100–6082), by the Western Regional Seed Physiology Research Group, and by Regional Research Project W–168.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.016386.
LITERATURE CITED
- Amuti KS, Pollard CJ. Soluble carbohydrates of dry and developing seeds. Phytochemistry. 1977;16:529–532. [Google Scholar]
- Anderson CM, Kohorn BD. Inactivation of Arabidopsis SIP1 leads to reduced levels of sugars and drought tolerance. J Plant Physiol. 2001;158:1215–1219. [Google Scholar]
- Ashworth EN, Stirm VE, Volenec JJ. Seasonal variations in soluble sugars and starch within woody stems of Cornus sericea L. Tree Physiol. 1993;13:379–388. doi: 10.1093/treephys/13.4.379. [DOI] [PubMed] [Google Scholar]
- Bachmann M, Keller F. Metabolism of the raffinose family oligosaccharides in leaves of Ajuga reptans L.: inter- and intracellular compartmentation. Plant Physiol. 1995;109:991–998. doi: 10.1104/pp.109.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M, Matile P, Keller F. Metabolism of the raffinose family oligosaccharides in leaves of Ajuga reptans L. Cold acclimation, translocation, sink to source transition: discovery of chain elongation enzyme. Plant Physiol. 1994;105:1335–1345. doi: 10.1104/pp.105.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentsink L, Alonso BC, Vreugdenhil D, Tesnier K, Groot SPC, Koornneef M. Genetic analysis of seed-soluble oligosaccharides in relation to seed storability of Arabidopsis. Plant Physiol. 2000;124:1595–1604. doi: 10.1104/pp.124.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal-Lugo I, Leopold AC. Changes in soluble carbohydrates during seed storage. Plant Physiol. 1992;98:1207–1210. doi: 10.1104/pp.98.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal-Lugo I, Leopold AC. Seed stability during storage: raffinose content and seed glassy state. Seed Sci Res. 1995;5:75–80. [Google Scholar]
- Berry T, Bewley JD. Seeds of tomato (Lycopersicon esculentum Mill.) which develop in a fully hydrated environment in the fruit switch from a developmental to a germinative mode without a requirement for desiccation. Planta. 1991;186:27–34. doi: 10.1007/BF00201494. [DOI] [PubMed] [Google Scholar]
- Bewley JD, Black M. Seeds. Physiology of Development and Germination. Ed 2. New York: Plenum Press; 1994. pp. 126–128. , 137. [Google Scholar]
- Black M, Corbineau F, Grzesik M, Guy P, Come D. Carbohydrate metabolism in the developing and maturing wheat embryo in relation to its desiccation tolerance. J Exp Bot. 1996;47:161–169. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brenac P, Horbowicz M, Downer SM, Dickerman AM, Smith ME, Obendorf RL. Raffinose accumulation related to desiccation tolerance during maize (Zea mays L.) seed development and maturation. J Plant Physiol. 1997a;150:481–488. [Google Scholar]
- Brenac P, Smith ME, Obendorf RL. Raffinose accumulation in maize embryos in the absence of a fully functional Vp1 gene product. Planta. 1997b;203:222–228. [Google Scholar]
- Budelier KA, Smith AG, Gasser CS. Regulation of a stylar transmitting tissue-specific gene in wild-type and transgenic tomato and tobacco. Mol Gen Genet. 1990;224:183–192. doi: 10.1007/BF00271551. [DOI] [PubMed] [Google Scholar]
- Buitink J, Hemminga MA, Hoekstra FA. Is there a role for oligosaccharides in seed longevity? An assessment of intracellular glass stability. Plant Physiol. 2000;122:1217–1224. doi: 10.1104/pp.122.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley MB, Yang H, Dahal P, Mella RA, Downie AB, Haigh AM, Bradford KJ. Vacuolar H+-ATPase is expressed in response to gibberellin during tomato seed germination. Plant Physiol. 1999;121:1339–1347. doi: 10.1104/pp.121.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahal P, Nevins DJ, Bradford KJ. Relationship of endo-β-mannanase activity and cell wall hydrolysis in tomato endosperm to germination rates. Plant Physiol. 1997;113:1243–1252. doi: 10.1104/pp.113.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson RMC, Elliott DC, Elliott WH, Jones KM. Data for Biochemical Research. Ed 3. Oxford: Oxford University Press; 1986. pp. 504–505. [Google Scholar]
- Denhardt DT. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966;23:641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Downie B, Bewley JD. Soluble sugar content of white spruce (Picea glauca) seeds during and after germination. Physiol Plant. 2000;110:1–12. [Google Scholar]
- Downie B, Gurusinghe S, Bradford KJ. Internal anatomy of individual tomato seeds: relationship to abscisic acid and germination physiology. Seed Sci Res. 1999;9:117–128. [Google Scholar]
- Duke JA. Handbook of Phytochemical Constituents of GRAS Herbs and Other Economic Plants. Boca Raton, FL: CRC Press; 1992. [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol. 2000;300:1005–1016. doi: 10.1006/jmbi.2000.3903. [DOI] [PubMed] [Google Scholar]
- Feinburg AP, Vogelstein B. A technique for radiolabelling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ferrarotti M. La disidratazione nella pianta “resurrection” Boea hygroscopica. PhD thesis. Pavia, Italy: University of Pavia; 1996. [Google Scholar]
- Gilmour SJ, Sebolt AM, Salazar MP, Everard JD, Thomashow MF. Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol. 2000;124:1854–1865. doi: 10.1104/pp.124.4.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovina EA, Tiknonov AN, Hoekstra FA. An electron paramagnetic resonance spin-probe study of membrane-permeability changes with seed aging. Plant Physiol. 1997;114:383–389. doi: 10.1104/pp.114.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot SPC, Karssen CM. Gibberellins regulate seed germination in tomato by endosperm weakening: a study with gibberellin-deficient mutants. Planta. 1987;171:525–531. doi: 10.1007/BF00392302. [DOI] [PubMed] [Google Scholar]
- Groot SPC, Karssen CM. Dormancy and germination of abscisic acid-deficient tomato seeds: studies with the sitiens mutant. Plant Physiol. 1992;99:952–958. doi: 10.1104/pp.99.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurusinghe S, Bradford KJ. Galactosyl-sucrose oligosaccharides and potential longevity of primed seeds. Seed Sci Res. 2001;11:121–133. [Google Scholar]
- Haritatos E, Medville R, Turgeon R. Minor vein structure and sugar transport in Arabidopsis thaliana. Planta. 2000;211:105–111. doi: 10.1007/s004250000268. [DOI] [PubMed] [Google Scholar]
- Hilhorst HWM, Downie B. Primary dormancy in tomato (Lycopersicon esculentum cv. Moneymaker): studies with the sitiens mutant. J Exp Bot. 1996;47:89–97. [Google Scholar]
- Hinesley LE, Pharr DM, Snelling LK, Funderburk SR. Foliar raffinose and sucrose in four conifer species: relationship to seasonal temperature. J Am Soc Hortic Sci. 1992;117:852–855. [Google Scholar]
- Hoekstra FA, Haigh AM, Tetteroo FAA, Van Roekel T. Changes in soluble sugars in relation to desiccation tolerance in cauliflower seeds. Seed Sci Res. 1994;4:143–147. [Google Scholar]
- Horbowicz M, Obendorf RL. Seed desiccation tolerance and storability: dependence of flatulence-producing oligosaccharides and cyclitols. Review and survey. Seed Sci Res. 1994;4:385–405. [Google Scholar]
- Kandler O, Hopf H. Oligosaccharides based on sucrose (sucrosyl oligosaccharides) In: Loewus FA, Tanner W, editors. Plant Carbohydrates 1. Encyclopedia of Plant Physiology, New Series. 13 A. Berlin: Springer-Verlag; 1982. pp. 384–393. [Google Scholar]
- Koornneef M, Jorna ML, Brinkhorst-van der Swan DLC, Karssen CM. The isolation of abscisic acid (ABA) deficient mutants by selection of induced revertants in non-germinating gibberellin sensitive lines of Arabidopsis thaliana (L.) Heynh. Theor Appl Genet. 1982;61:385–393. doi: 10.1007/BF00272861. [DOI] [PubMed] [Google Scholar]
- Koster KL, Leopold AC. Sugars and desiccation tolerance in seeds. Plant Physiol. 1988;88:829–832. doi: 10.1104/pp.88.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenz D. Galactinol synthase (GS), a key enzyme in biosynthesis of raffinose family oligosaccharides (RF): activation of enzyme activity and induction of gene expression by cold and desiccation. In: Lucas WJ, Ryan C, editors. Information Processing Systems in Plants: Their Evolution and Function. Proceedings of the Spring Symposium. Davis: University of California; 1997. p. 100. [Google Scholar]
- Kuo T-M, Lowell CA, Smith PT. Changes in soluble carbohydrates and enzymic activities in maturing soybean seed tissues. Plant Sci. 1997;125:1–11. [Google Scholar]
- Lagarias DM, Crepeau MW, Maines MD, Lagarias JC. Regulation of photomorphogenesis by expression of mammalian biliverdin reductase in transgenic Arabidopsis plants. Plant Cell. 1997;9:675–688. doi: 10.1105/tpc.9.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehle L, Tanner W. Synthesis of raffinose-type sugars. In: Ginsberg V, editor. Methods in Enzymology. Complex Carbohydrates: Part B. Vol. 28. New York: Academic Press; 1972. pp. 522–529. [Google Scholar]
- Leprince O, Hendry GAF, McKersie BD. The mechanisms of desiccation tolerance in developing seeds. Seed Sci Res. 1993;3:231–246. [Google Scholar]
- Lin T-P, Huang N-H. The relationship between carbohydrate composition of some tree seeds and their longevity. J Exp Bot. 1994;45:1289–1294. [Google Scholar]
- Liu J-J, Galvez AF, Krenz DC, de Lumen BO. Galactinol synthase (GS), a key enzyme in biosynthesis of raffinose family oligosaccharides (RFO): activation of enzyme activity and induction of gene expression by cold and desiccation. Plant Physiol Suppl. 1997;114:130. [Google Scholar]
- Liu J-J, Krenz DC, Glavez AF, de Lumen BO. Galactinol synthase (GS): increased enzyme activity and levels of mRNA due to cold and desiccation. Plant Sci. 1998;134:11–20. [Google Scholar]
- Liu J-J, Odegard W, de Lumen BO. Galactinol synthase from kidney bean cotyledon and zucchini leaf. Plant Physiol. 1995;109:505–511. doi: 10.1104/pp.109.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel BE, Kaufmann RM. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973;51:914–916. doi: 10.1104/pp.51.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MG, Thompson WF. Rapid isolation of high molecular weight DNA. Nucleic Acids Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonogaki H, Gee OH, Bradford KJ. A germination-specific endo-β-mannanase gene is expressed in the micropylar endosperm cap of tomato seeds. Plant Physiol. 2000;123:1235–1246. doi: 10.1104/pp.123.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms JJJ, Léon-Kloosterziel KM, Bartels D, Koornneef M, Karssen CM. Acquisition of desiccation tolerance and longevity in seeds of Arabidopsis thaliana: a comparative study using ABA-insensitive abi3 mutants. Plant Physiol. 1993;102:1185–1191. doi: 10.1104/pp.102.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms JJJ, Wilmer JA, Karssen CM. Carbohydrates are not the sole factor determining desiccation tolerance in seeds of Arabidopsis thaliana. Physiol Plant. 1994;90:431–436. [Google Scholar]
- Peterbauer T, Lahuta LB, Blöchl A, Mucha J, Jones DA, Hedley CL, Gòrecki RJ, Richter A. Analysis of the raffinose family oligosaccharide pathway in pea seeds with contrasting carbohydrate composition. Plant Physiol. 2001;127:1764–1772. [PMC free article] [PubMed] [Google Scholar]
- Peterbauer T, Richter A. Biochemistry and physiology of raffinose family oligosaccharides and galactosyl cyclitols in seeds. Seed Sci Res. 2001;11:185–197. [Google Scholar]
- Pridham JB, Hassid WZ. Biosynthesis of raffinose. Plant Physiol. 1965;40:984–986. doi: 10.1104/pp.40.6.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Santarius KA. The protective effect of sugars on chloroplast membranes during temperature and water stress and its relationship to frost, desiccation and heat resistance. Planta. 1973;113:105–114. doi: 10.1007/BF00388196. [DOI] [PubMed] [Google Scholar]
- Santarius KA, Milde H. Sugar compartmentation in frost-hardy and partially dehardened cabbage leaf cells. Planta. 1977;136:163–166. doi: 10.1007/BF00396193. [DOI] [PubMed] [Google Scholar]
- Saracco F, Bino RJ, Bergervoet JHW, Lanteri S. Influence of priming-induced nuclear replication activity on storability of pepper (Capsicum annuum L.) seed. Seed Sci Res. 1995;5:25–29. [Google Scholar]
- Saravitz DM, Pharr DM, Carter TE. Galactinol synthase activity and soluble sugars in developing seeds of four soybean genotypes. Plant Physiol. 1987;83:185–189. doi: 10.1104/pp.83.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack F. Subscriber's notebook. In: Chanda VB, editor. The Red Book Bulletin, Current Protocols in Molecular Biology, Supplement 42. New York: John Wiley & Sons; 1998. pp. 1–2. [Google Scholar]
- Smith PT, Kuo TM, Crawford CG. Purification and characterization of galactinol synthase from mature zucchini squash leaves. Plant Physiol. 1991;96:693–698. doi: 10.1104/pp.96.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger N, Keller F. Allocation of raffinose family oligosaccharides to transport and storage pools in Ajuga reptans: the roles of two distinct galactinol synthases. Plant J. 2000;21:249–258. doi: 10.1046/j.1365-313x.2000.00671.x. [DOI] [PubMed] [Google Scholar]
- Sun WQ, Irving TC, Leopold AC. The role of sugar, vitrification and membrane phase transition in seed desiccation tolerance. Physiol Plant. 1994;90:621–628. [Google Scholar]
- Taji T, Ohsumi C, Iuchi S, Seki M, Kasuga M, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K. Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J. 2002;29:417–426. doi: 10.1046/j.0960-7412.2001.01227.x. [DOI] [PubMed] [Google Scholar]
- Takahashi R, Joshee N, Kitagawa Y. Induction of chilling resistance by water stress, and cDNA sequence analysis and expression of water stress-regulated genes in rice. Plant Mol Biol. 1994;26:339–352. doi: 10.1007/BF00039544. [DOI] [PubMed] [Google Scholar]
- Tarquis AM, Bradford KJ. Prehydration and priming treatments that advance germination also increase the rate of deterioration of lettuce seeds. J Exp Bot. 1992;43:307–317. [Google Scholar]
- Thomashow MF. So what's new in the field of plant cold acclimation? Lots! Plant Physiol. 2001;125:89–93. doi: 10.1104/pp.125.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemken V, Ineichen K. Effect of temperature and photoperiod on the raffinose content of spruce roots. Planta. 1993;190:387–392. [Google Scholar]
- Wolkers WF, Alberda M, Koornneef M, Léon-Kloosterziel KM, Hoekstra FA. Properties of proteins and the glassy matrix in maturation-defective mutant seeds of Arabidopsis thaliana. Plant J. 1998a;16:133–143. doi: 10.1046/j.1365-313x.1998.00277.x. [DOI] [PubMed] [Google Scholar]
- Wolkers WF, Oldenhof H, Alberda M, Hoekstra FA. A Fourier transform infrared microspectroscopy study of sugar glasses: application to anhydrobiotic higher plant cells. Biochim Biophys Acta. 1998b;1379:83–96. doi: 10.1016/s0304-4165(97)00085-8. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Smith MW, Brown RGS, Kamiya Y, Sun T-P. Phytochrome regulation and differential expression of gibberellin 3β-hydroxylase genes in germinating Arabidopsis seeds. Plant Cell. 1998;10:2115–2126. doi: 10.1105/tpc.10.12.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Koster KL. Desiccation tolerance of protoplasts isolated from pea embryos. J Exp Bot. 2001;364:2105–2114. doi: 10.1093/jexbot/52.364.2105. [DOI] [PubMed] [Google Scholar]