Abstract
The acyclic polyol sorbitol is a primary photosynthetic product and the principal photosynthetic transport substance in many economically important members of the family Rosaceace (e.g. almond [Prunus dulcis (P. Mill.) D.A. Webber], apple [Malus pumila P. Mill.], cherry [Prunus spp.], peach [Prunus persica L. Batsch], and pear [Pyrus communis]). To understand key steps in long-distance transport and particularly partitioning and accumulation of sorbitol in sink tissues, we have cloned two sorbitol transporter genes (PcSOT1 and PcSOT2) from sour cherry (Prunus cerasus) fruit tissues that accumulate large quantities of sorbitol. Sorbitol uptake activities and other characteristics were measured by heterologous expression of PcSOT1 and PcSOT2 in yeast (Saccharomyces cerevisiae). Both genes encode proton-dependent, sorbitol-specific transporters with similar affinities (Km sorbitol of 0.81 mm for PcSOT1 and 0.64 mm for PcSOT2). Analyses of gene expression of these transporters, however, suggest different roles during leaf and fruit development. PcSOT1 is expressed throughout fruit development, but especially when growth and sorbitol accumulation rates are highest. In leaves, PcSOT1 expression is highest in young, expanding tissues, but substantially less in mature leaves. In contrast, PcSOT2 is mainly expressed only early in fruit development and not in leaves. Compositional analyses suggest that transport mediated by PcSOT1 and PcSOT2 plays a major role in sorbitol and dry matter accumulation in sour cherry fruits. Presence of these transporters and the high fruit sorbitol concentrations suggest that there is an apoplastic step during phloem unloading and accumulation in these sink tissues. Expression of PcSOT1 in young leaves before completion of the transition from sink to source is further evidence for a role in determining sink activity.
Sorbitol (an acyclic polyol) and Suc are the primary photosynthetic products and the major phloem-translocated components in a number of economically important taxa in the family Rosaceae, in particular in the subfamilies Pomoideae (e.g. apple [Malus pumila P. Mill.] and pear [Pyrus communis]) and Prunoideae (e.g. almond [Prunus dulcis (P. Mill.) D.A. Webber], cherry [Prunus spp.], peach [Prunus persica L. Batsch], and plum [Prunus spp.]) that collectively are the world's most important tree fruit crops (Loescher and Everard, 1996). Sorbitol is often the dominant translocated photosynthetic product. In mature apricot (Prunus armeniaca) leaves, for example, 65% to 75% of the translocated carbon is sorbitol (Bieleski and Redgwell, 1985). In these species, understanding the factors involved in facilitating and regulating sorbitol transport, including export from the leaf, long-distance distribution via the phloem network, and import into various sink tissues, is at least as important as those of Suc and other sugars.
In the past 10 years, sugar transporters have been extensively studied in various sink and source tissues with the isolation of two distinct families of sugar carriers: the disaccharide transporters that primarily catalyze Suc transport and the monosaccharide transporters that mediate transport of the hexose sugars (for review, see Weise et al., 2000; Williams et al., 2000). Active uptake of Suc across the plasma membrane in all known cases involves an H+-Suc symporter (Lemoine, 2000). The biochemical properties of the Suc transporters have mostly been elucidated through functional expression in yeast (Saccharomyces cerevisiae) cells where these transporters are pH dependent, electrogenic, and have a 1:1 stoichiometry (Buckhout and Tubbe, 1996; Lemoine, 2000). Expression of the Suc transporters in various plant tissues suggests essential roles in phloem loading for Suc translocation (Bürkle et al., 1998; Shakya and Sturm, 1998; Noiraud et al., 2000) and in growth, development, and reproduction (Gottwald et al., 2000). Although much of the Suc transport work has been focused on phloem loading, there has also been some limited work on both monosaccharide and disaccharide transporters in sink tissues where expression is often correlated with a high requirement for substrates for storage, synthesis, and metabolism (Williams et al., 2000).
In contrast to what we now know about the diversity of roles and regulation of the Suc and hexose transporters, very little is yet known about acyclic polyol transporters in either source or sink tissues of higher plants (Noiraud et al., 2001a). Recently, however, the cDNA of a mannitol transporter was isolated and characterized from celery (Apium graveolens) phloem tissues (Noiraud et al., 2001b). Transport of mannitol in transformed yeast cells indicated a proton symport mechanism, and mRNA expression data were consistent with a role in phloem loading of mannitol. Evidence of sorbitol transport is, however, much more limited, i.e. to studies of sorbitol uptake in apple fruit tissues (Yamaki and Asakura, 1988; Berüter, 1993; Berüter and Feusi, 1995) and peach leaf plasma membrane vesicles (Marquat et al., 1997). Although these results suggested a carrier-mediated process for sorbitol transport, there is no direct evidence for any potential transporters. None has yet been cloned, and nothing is otherwise known of the molecular mechanisms involved in sorbitol transport in either sink or source tissues.
Information on potential sorbitol transporters may be especially useful because of the limited data linking sorbitol metabolism and sink activity (especially in fruit development). Sorbitol normally does not accumulate in apple fruit tissues (Marlow and Loescher, 1984), and in peach fruit it is a minor component (Brooks et al., 1993), suggesting substantial metabolism to sugars and perhaps starch. Sorbitol is metabolized via an NAD-dependent sorbitol dehydrogenase (NAD-SorDH; Loescher et al., 1982) and an oxidase (SorOX; Lo Bianco and Rieger, 2002) that convert sorbitol into Fru or Glc, respectively. NAD-SorDH has been purified and characterized (Yamaguchi et al., 1994), and its cDNA has been cloned (Yamada et al., 1998), but in some fruits there are multiple isoforms (Park et al., 2002). NAD-SorDH activities are sometimes correlated with fruit development and with sugar accumulation (Yamaguchi et al., 1994, 1996; Lo Bianco et al., 1999); however, such studies may be confounded if there are multiple isoforms. Immunoblot analysis has shown that enzyme protein levels were low in young apple fruit but then increased as fruit matured (Yamaguchi et al., 1996), and mRNA similarly increased during several stages of fruit development (Yamada et al., 1998). However, a close relationship between NAD-SorDH activity and relative fruit growth rate could not be established in young apple tissues (Yamaguchi et al., 1996). Similarly, relationships between SorOX and fruit growth depend on stage of development (Lo Bianco et al., 1999). Thus, other enzymes, e.g. Suc synthase (SuSy) and various invertases, may be important to determining overall sink activity. However, data linking SuSy and invertases to sink activities are also difficult to interpret given the multiple forms of SuSy (Komatsu et al., 2002) and its control by phosphorylation status (Tanase et al., 2002) and the potential effects of invertase inhibitors (Greiner et al., 2000; Winter and Huber, 2000). All of this argues for further investigations of sink activities in these tissues with a focus on transporter identification and characterization.
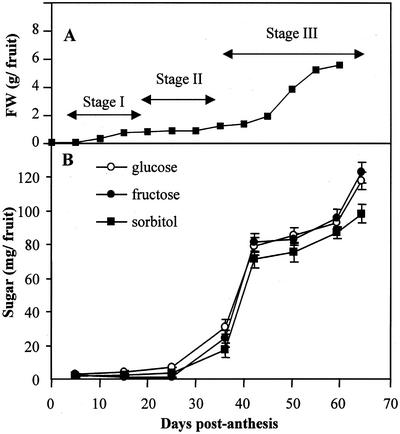
In cherries, fruit development is often described as consisting of three stages that are depicted as a double-sigmoid growth curve (Tukey and Young, 1939), i.e. with enlargement occurring during Stages I and III (for example, see Fig. 1A). Cell division and some growth occur early in Stage I, endocarp development (pit hardening), but little growth, occurs in Stage II, and maturation and ripening (as measured by softening and color development) begin early in stage III at the same time as most of total fruit growth and dry matter accumulation. Our preliminary data indicated that, unlike peach and apple, there is substantial sorbitol accumulation in sour cherry (Prunus cerasus) stage III fruit tissues with limited or little direct involvement of either SorDH or SorOX. Sweet cherry (Prunus avium) accumulates even larger amounts of sorbitol and other soluble carbohydrates in mature fruit (Roper and Loescher, 1987). Also, unlike apple or peach (Flore and Layne, 1996), there is no evidence that these species first accumulate starch. Such data suggest a major role for sorbitol transport in defining and controlling sink activity in cherry fruit development. Given that both Suc and mannitol transport are active, H+-driven, symport processes, we hypothesized that sorbitol transport and accumulation in cherry fruit tissues involve similar mechanisms. Here, we report the isolation and cloning and the heterologous expression and characterization in yeast of two sorbitol transporter genes from sour cherry fruit and developing leaf tissues.
Figure 1.
A, Sour cherry fruit growth and the three stages of fruit development. Each point is the mean of 20 to 60 fruit (ses are obscured by the symbols). B, Changes in Glc, Fru, and sorbitol content per fruit during development. Cherries were sampled at eight growth stages, from young to mature fruit (5, 16, 25, 36, 42, 50, 59, and 64 DPA). Each point is the mean of measurements with fruit from three different trees, with at least 6 g of fruit pericarp collected from each tree. Error bars = ses.
RESULTS
Sorbitol and Hexose Accumulation
Cherry fruit growth was typical, with a biphasic increase in fruit diameter and weight (Fig. 1A; Tukey and Young, 1939). Our fruit samples represented several stages in development, from young green fruit with soft endocarp tissues (pits; Stage I, 5–20 DPA) to straw-colored fruit with hardening pits (Stage II, 20–35 DPA), and then to pink and finally dark-red mature fruits (Stage III, 35–65 DPA; Fig. 1A). Glc, Fru, and sorbitol were the major carbohydrate components in both young and mature fruits. Fruit growth and accumulation of sorbitol and hexoses increased very rapidly after pit hardening when fruit changed in color from straw to pink (after 35 DPA) and then continued until the last harvest date (Fig. 1B). At 64 DPA, fruit (exclusive of pit and seed) typically contained approximately 120 mg of both Glc and Fru, and about 100 mg of sorbitol, for a total of 340 mg. Although trace amounts of inositol were invariably present, starch and Suc were not detected at any stage of fruit development.
Cloning of Sorbitol Transporters Genes
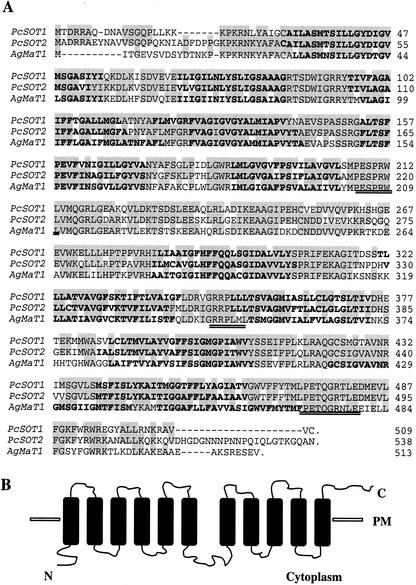
To identify potential sorbitol transporters, we used reverse transcriptase-PCR and degenerate primers corresponding to conserved regions of “sugar transporters.” From ripening fruit-derived cDNAs, this approach resulted in the isolation of an 820-bp cDNA with extensive sequence homology with a celery phloem mannitol transporter (AgMaT1; Noiraud et al., 2001b). A cDNA library screen isolated nine positive clones, and these were sequenced. Eight clones represented a single sequence and differed only in the length of the 5′- and 3′-untranslated regions. The longest cDNA was sequenced in its entirety. This cDNA was 1,927 bp long, potentially encoding a protein of 509 amino acid residues with a predicted molecular mass of 55 kD and pI of 8.38 (called PcSOT1 here, AF482011; Fig. 2A). The ninth isolated cDNA was distinct (called PcSOT2 here, AY100638) and was 2,032 bp long with a 1,617-bp coding region, potentially encoding a protein of 538 amino acid residues, a predicted molecular mass of 59 kD, and a pI of 9.26 (Fig. 2A). This PcSOT2 clone shared an 85% nucleotide sequence identity with PcSOT1 in the coding region and an amino acid similarity of 76%.
Figure 2.
A, Comparison of the deduced amino acid sequences of the sour cherry PcSOT1 and PcSOT2 cDNAs with a mannitol transporter AgMaT1 from celery. Shaded sequences are identical, the doubly underlined sequences correspond to those conserved in the sugar transporter subfamily of the major facilitator superfamily, and those in bold are putative membrane-spanning sequences. B, Putative transmembrane helical domains for PcSOT1 and PcSOT2. PM, Plasma membrane; N, N termini; C, C termini.
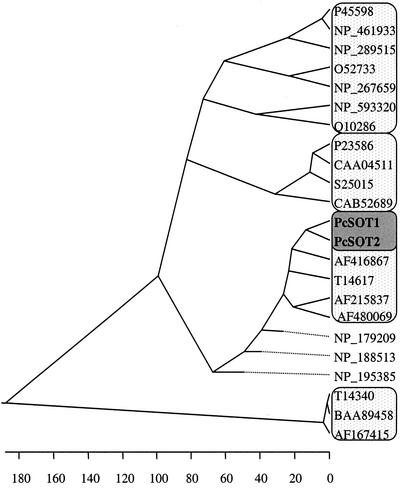
In contrast with many sugar transport proteins with a 6 + 6 arrangement of transmembrane domains (Buckhout and Tubbe, 1996), the deduced amino acid sequence of both PcSOT1 and PcSOT2 genes here appeared to have 11 putative membrane-spanning domains with a 6 + 5 arrangement. However, the sequences responsible for these differences were nearly identical to that of AgMaT1, which has a 6 + 6 arrangement; thus, the 6 + 5 model may be an artifact of the hydropathy analysis. The N termini and central loops are located on the cytoplasmic side, but in the 6 + 5 model the C termini loops are located on the outside (Fig. 2B). The consensus sequences for sugar transporters, PESPRWL, GRRPLLL, and PETQGRTLE, are all present. BLAST results showed that the sequences of PcSOT1 and PcSOT2 are also similar to an uncharacterized putative sugar transporter from Arabidopsis (accession no. NP-188513), the celery phloem mannitol transporter (accession no. AF-215837; Noiraud et al., 2001b), and two putative sugar transporters from sugar beet (Beta vulgaris; accession nos. T-14617 and T-14606; Chiou and Bush, 1996). A phylogenetic tree was constructed from a Clustal alignment of 23 different sugar transporters (Fig. 3). The 23-amino acid sequences are divided into four major subgroups. The PcSOT1 and PcSOT2 are closest to a group of putative sugar transporters in which only the mannitol transporter (accession no. AF-215837) has a defined function. PcSOT1 and PcSOT2 do not appear to be closely related to known Suc transporters (accession nos. T-14340, BAA-89458, and AF-167415) or hexose transporters (accession nos. CAA-04511, CAB-52689, S-25015, P-23586, NP-267659, NP-289515, and NP-461933), and these sorbitol transporters are also quite distinct from myo-inositol transporters (accession nos. NP-593320 and Q-10286; Fig. 3). Nonetheless, like the mannitol transporter from celery plants (Noiraud et al., 2001b), PcSOT1 and PcSOT2 are both clearly members of the major facilitator superfamily (Marger and Saier, 1993), and the hydrophobicity analyses indicate that, like the disaccharide and monosaccharide transporters, they are highly hydrophobic integral membrane proteins.
Figure 3.
Phylogenetic tree of related sequences. Alignment was performed with DNASTAR-MegAlign (GCG Inc., Madison, WI). GenBank accession numbers for the amino acid sequences are: P-45598 and NP-461933, putative Ara transporters; NP-289515, a putative Gal transporter; O-52733 and NP-267659, putative Xyl transporters; NP-593320 and Q-10286, myo-inositol transporters; P-23586, CAA-04511, S-25015, and CAB-52689, hexose transporters; AF-416867, a putative rice (Oryza sativa) sugar transporter; T-14617, a putative sugar beet sugar transporter; AF-215837 and AF-480069, the celery mannitol transporters; NP-179209, NP-188513, and NP-195385, putative Arabidopsis sugar transporters; and T-14340, BAA-89458, and AF-167415, Suc transporters. Dotted lines indicate a negative branch length.
Characterization of PcSOT1 and PcSOT2 in Yeast
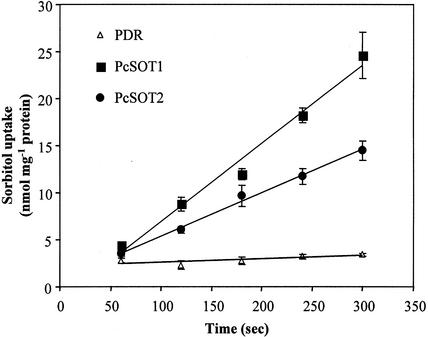
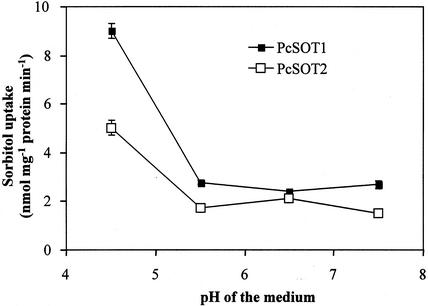
To determine the transport functions of PcSOT1 and PcSOT2, the yeast strain RS453 was transformed with the recombinant plasmid pDR196 containing either PcSOT1 or PcSOT2. RS 453 is unable to grow on synthetic complete (SC) medium with sorbitol as a sole carbon source, and endogenous sorbitol dehydrogenase activity is undetectable (Z. Gao, R. Lemoine, and W. Loescher, unpublished data). To reduce Glc suppression of the expression of PcSOT1 and PcSOT2 in yeast, the transformed yeast cells were first inoculated overnight on SC-Glc medium and then inoculated on SC-glycerol medium supplemented with 0.05% (w/v) Glc. Using 14C-labeled sorbitol to perform uptake experiments, yeast cells expressing PcSOT1 or PcSOT2 showed 5- to 6-fold higher rates of uptake of 14C-labeled sorbitol than yeast transformed with the empty pDR196 plasmid (Fig. 4). All the uptake experiments were run on three independent yeast clones with similar results. The average slopes were 5.0 and 2.8 nmol sorbitol taken up min−1 mg protein−1 for PcSOT1 and PcSOT2, respectively, compared with 0.22 for cells transformed with the control pDR 196 plasmid (Fig. 4). Sorbitol uptake by both cherry fruit transporters decreased rapidly from pH 4.5 to 5.5 with little activity remaining above pH 5.5 (Fig. 5). Using radiolabeled Suc and mannitol, we also compared, in yeast expressing these two transporters, uptake of mannitol, an isomer of sorbitol, and uptake of Suc, which is also transported in cherry fruit. These results showed that the cherry fruit transporters were relatively specific, with uptake rates for mannitol and Suc of 3.6% and 12.5%, respectively, of that for sorbitol. The proton uncoupler carbonyl cyanide m-chlorophenylhydrazone (CCCP) markedly inhibited sorbitol uptake (Table I), indicating that sorbitol transport is linked to the proton electrochemical potential across the plasma membrane. However, sorbitol uptake was not inhibited by the presence of the thiol reagent p-chloro-mercuribenzene-sulphoic acid (PCMBS; Table I). Other sugars and polyols at a 10-fold external concentration showed different effects on sorbitol transport (Table I). The presence of Suc or xylitol indicated only a little effect on transport by PcSOT1 and did not inhibit transport by PcSOT2. Man, Glc, Fru, and mannitol all strongly inhibited uptake of sorbitol by PcSOT1, but PcSOT2 was less affected (Table I).
Figure 4.
Uptake of sorbitol by transgenic yeast cells. RS453 cells were grown to the early logarithmic phase. Uptake of 0.5 mm [14C]sorbitol was measured at an external pH of 4.5. Three independent yeast clones transformed with PcSOT1 or PcSOT2 were tested (all with similar results) and compared with one clone expressing empty pDR196. The results are the means ± se of four replicates.
Figure 5.
pH dependence of sorbitol transport in transgenic yeast cells expressing PcSOT1 or PcSOT2. Measurements were performed at 0.5 mm sorbitol in SC medium containing 25 mm MES buffered at the indicated pH. Incubation time was 2 min. The results are the means ± se of four replicates.
Table I.
Sensitivity of the sorbitol transporter (PcSOT1 and PcSOT2) activity to inhibitor (at the indicated concentration) and other sugar or sugar alcohol substances (5 mm)
| Added Compound | Relative Uptake Rate
|
|
|---|---|---|
| PcSOT1 | PcSOT2 | |
| % | ||
| None | 100 | 100 |
| 50 μm CCCP | 3.2 | 7.6 |
| 100 μm PCMBS | 93.4 | 83.7 |
| Man | 19.1 | 53.7 |
| Suc | 78.3 | 93.7 |
| Glc | 6.1 | 26.1 |
| Fru | 16.1 | 35.5 |
| Xylitol | 75.7 | 96.1 |
| Mannitol | 21.3 | 48.5 |
The substances were added 30 s prior to 14C- or 3H-labeled sorbitol. All uptake tests were performed at 0.5 mm sorbitol (pH 4.5), and incubation time was 3 min. Control activities (100%) were 5.19 and 4.03 nmol mg−1 protein min−1 for PcSOT1 and PcSOT2, respectively. The results are the means of four replicates expressed as relative uptake rate to sorbitol.
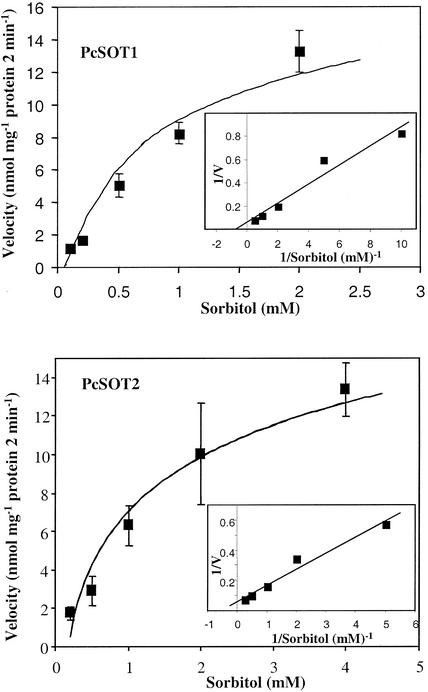
When uptake of sorbitol by PcSOT1 and PcSOT2 in yeast was assayed at concentrations ranging from 0.1 to 4 mm, we obtained Michaelis-Menten saturation plots (Fig. 6). The two transporters showed similar apparent affinities for sorbitol (Km of 0.81 mm for PcSOT1 and 0.64 mm for PcSOT2 at pH 4.5). These yeast expression results collectively confirmed that both PcSOT1 and PcSOT2 function as sorbitol transporters.
Figure 6.
Concentration dependence of sorbitol transport in transgenic yeast cells expressing PcSOT1 (A) or PcSOT2 (B). The results are means ± se of four replicates. The inset shows a Lineweaver-Burk plot of the uptake data.
Expression of PcSOT1 and PcSOT2
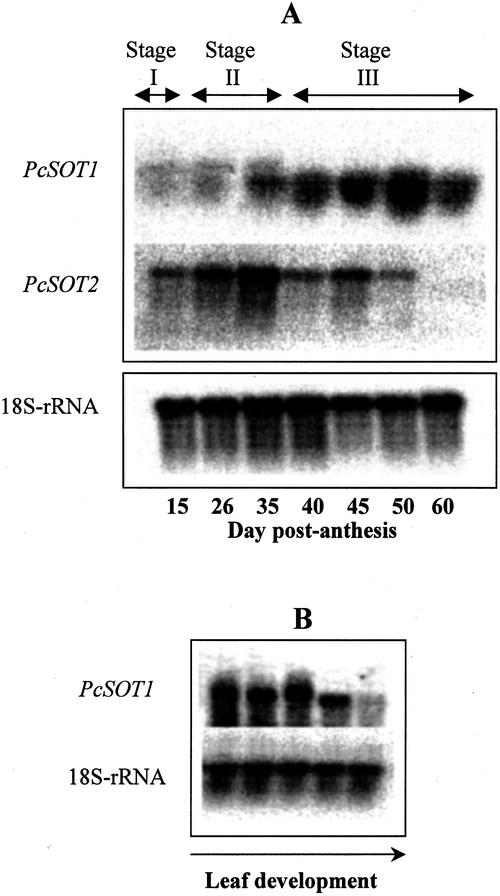
Because the PcSOT1 shares 85% nucleotide sequence identity with the PcSOT2 cDNA, a risk of cross-hybridization had to be avoided. Therefore, we PCR amplified a 400-bp unique nucleotide sequence found in the 3′-untranslated region of PcSOT1 and a 485-bp sequence in the 3′-untranslated region of PcSOT2. The two 3′ sequences share only a 31% level of identity and, thus, should be gene-specific probes for PcSOT1 and PcSOT2, respectively. RNAs hybridizing with these probes were investigated at various stages of fruit and leaf development (Fig. 7). Although detectable throughout fruit development, RNAs hybridizing with the PcSOT1 probe increased, first late in pit hardening (Stage II), and then quite substantially with onset of high sorbitol accumulation rates at 40 DPA (early in Stage III). During the later stages of ripening in Stage III, the abundance of these RNAs slightly decreased (Fig. 7A). RNAs hybridizing with the PcSOT1 probe were also high in young and expanding leaves but were substantially decreased in fully mature leaf tissues (Fig. 7B). In contrast, RNAs hybridizing with the PcSOT2 probe were mainly evident in young fruit (Stages I and II) before the onset of sorbitol accumulation (Fig. 7A), with the highest expression during pit hardening and then decreasing as fruit developed. PcSOT2 expression, as measured with the PcSOT2 probe, was not detected in any leaf tissues (data not shown).
Figure 7.
A, RNA gel-blot analysis of sorbitol transporters (PcSOT1 and PcSOT2) genes in developing cherry fruit at different stages (I–III, as shown at the top of the blots) and also at different days after flowering (as shown on the bottom of blots). B, RNA gel-blot analysis of a sorbitol transporter (PcSOT1) in developing cherry leaves, from quite immature leaves, just beginning to expand (on the left) to fully expanded (on the right). In A and B, the probes used for hybridization are indicated to the left of each blot. PcSOT1 and PcSOT2 were gene-specific probes from 3′-untranslated regions, prepared by PCR. Loading of each lane, 20 μg of total RNA, was verified by reprobing the blot with an 18S rRNA probe from Arabidopsis.
DISCUSSION
Both monosaccharide and disaccharide transporters have now been characterized in many plants. The technique used for characterization of these and other transporters is almost invariably heterologous expression in yeast and similar systems (Dreyer et al., 1999). Some of these transporters have been reported in various sink tissues including those of roots, floral organs, and developing and germinating seeds, and several are also associated with plant-microbial interactions (Williams et al., 2000). However, with one exception (Fillion et al., 1999), there has been practically no work on identification and characterization of sugar transporters in perennial fruit crops where carbohydrate accumulation in sink (fruit) tissues is often a critically important component of crop productivity and quality. As a further consideration, many of these plants produce, transport, and metabolize not only Suc, but also other photosynthetic products, e.g. an acyclic polyol such as mannitol or sorbitol or one or more members of the raffinose family of oligosaccharides. Of the acyclic polyols, only one transporter has thus far been characterized, the AgMaT1 mannitol transporter from celery phloem (Noiraud et al., 2001b). Many members of the Rosaceae (the pome and stone fruits, in the subfamilies Pomoideae and Prunoideae, respectively) and several other families transport and accumulate sorbitol, but the mechanisms involved in sorbitol membrane transport have not been defined. We have now isolated and characterized by heterologous expression two putative sorbitol transporters (PcSOT1 and PcSOT2) from sour cherry fruit tissues that accumulate high levels of sorbitol, especially at the later stages of fruit maturation. Yeast cells expressing PcSOT1 and PcSOT2 take up sorbitol quite efficiently and specifically when compared with cells containing only the vector (Fig. 4). The two transporters in the yeast system were H+ dependent, with optimal activities at acidic pH levels, similar to Suc/H+ symporters. In addition, the proton uncoupler, CCCP, completely inhibited sorbitol uptake. These results suggest that the two transporters both act as proton/sorbitol cotransporters, with the proton pump ATPase creating the pH and potential differences across the plasma membrane to drive sorbitol transport. The two transporters, however, were not sensitive to the addition of the thiol group reagent PCMBS, which is in contrast to the high sensitivity of Suc transporters (Noiraud et al., 2000), but similar to the mannitol transporter AgMaT1 isolated from celery phloem (Noiraud et al., 2001b). The Km of 0.6 to 0.8 mm sorbitol for PcSOT1 and PcSOT2 in yeast indicates a 60- to 70-fold higher affinity compared with the Km of 35 to 55 mm sorbitol obtained from uptake studies of apple tissues (Berüter, 1993) but it is consistent with the Km of 0.67 mm sorbitol reported for peach leaf plasma membrane vesicles (Marquat et al., 1997). The Km values here are also in the same range as those reported for mannitol and most Suc carriers (Noiraud et al., 2001a, 2001b).
PcSOT1 and PcSOT2 were both quite specific for sorbitol: The mannitol transport rates were quite low, only 3% that of sorbitol. In contrast, the celery mannitol transporter, AgMaT1, transports a variety of acyclic polyols, among them sorbitol, although rates are lower than those for mannitol (L. Maurousset and R. Lemoine, unpublished data). Mannitol, despite its low uptake rates, did, however, inhibit transport of sorbitol by both PcSOT1 and PcSOT2, presumably by competing for the active site. Despite its conformational similarities to sorbitol, xylitol had little effect on sorbitol transport. The inhibitory effects of Glc and Fru are similar to those observed with AgMaT1 (Noiraud et al., 2001b), but these may be an artifact of heterologous expression in yeast where transporter expression is often repressed with addition of Glc or Fru (Horak and Wolf, 1997; Noiraud et al., 2001b). Thus, comparisons of Glc and Fru uptake in relation to PcSOT1 and PcSOT2 may not be applicable to this yeast system. Nonetheless, as in the case with the mannitol transporter (Noiraud et al., 2001b), the presence of either sorbitol transporter, PcSOT1 or PcSOT2, did not significantly enhance radiolabeled Glc uptake in RS 453 cells (Z. Gao, R. Lemoine, and W. Loescher, unpublished data). Further, Glc or Fru effects on sorbitol transporter activity in cherry fruit seem unlikely given the quantities of Glc, Fru, and sorbitol in Stage III cherry fruit storage parenchyma (Fig. 1A); however, the transporter may not be influenced by these sugars due to compartmentation.
Because sorbitol and mannitol are isomers, a high degree of homology between the cDNAs of the sorbitol and mannitol carriers might be expected. PcSOT1 and PcSOT2 cDNA sequences are quite similar to AgMaT1; however, the two cherry transporters do not otherwise have close sequence relationships to the known hexose and Suc transporters (Fig. 3). Although the functions of those genes (sequences) closely grouped with the acyclic polyol transporters in Figure 3 (e.g. from sugar beet, rice, and Arabidopsis) remain to be determined, the similarities to the two sorbitol transporters may provide further arguments for considering that these may encode acyclic polyol transporters. As such, the results here may also indicate that these transporters represent a distinct group of transporters.
Consistent with its function in yeast and with its proposed role as a sorbitol transporter, PcSOT1 was expressed mainly later in fruit development (Fig. 7A), coincident with the high rates of sorbitol accumulation and fruit growth during Stage III. Thus, the data here are not only consistent with PcSOT1 as a sorbitol transporter, but also with the hypothesis that it may play an important role in sorbitol accumulation, particularly later in sour cherry fruit development. Similar results, i.e. correlations of expression with sugar accumulation, have been observed for a hexose transporter in ripening grape (Vitis vinifera; Fillion et al., 1999). The expression pattern of PcSOT2 (Fig. 7A) indicates that although this transporter may not be directly involved in the later stages of sorbitol accumulation, it may have a specific function earlier in fruit growth at or before pit hardening (at Stage II) or about the time when the fruit experiences the first increases in growth and dry matter accumulation (during Stage I).
Given that we measured only relatively low levels of SorDH gene expression and enzyme activities throughout development (data not shown), a major role for SorDH in cherry fruit growth and dry matter accumulation seems unlikely. The dramatic increases in sorbitol accumulation during Stage III of fruit development would instead appear to result primarily from sorbitol transport and not SorDH activity. However, variability in the sorbitol to Suc ratio in mature leaves of different Prunus spp. has been observed (Moing et al., 1997), and it is possible that the cherry fruit import relatively more sorbitol, compared with Suc, at the later stages of development. Or, as is more likely, Suc is hydrolyzed to Glc and Fru by invertase, leaving sorbitol to accumulate in the absence of substantial SorDH activity or detectable SorOX.
Thus, all these data, and especially those indicating relatively high expression of PcSOT1 at the later stages of fruit development when sorbitol accumulation rates are highest, are consistent with a significant role for a sorbitol transporter in sorbitol accumulation in maturing sour cherry fruit. Similarly, the presence of PcSOT2 at earlier stages of fruit development is also consistent with a role in fruit growth and dry matter accumulation. Presence of these transporters and the high fruit sorbitol concentrations suggest that there is an apoplastic step during phloem unloading and accumulation in these sink tissues. In addition, expression of PcSOT1 in developing leaf tissues indicates one or more additional roles in sink tissues, e.g. in utilization, storage, or compartmentation in leaf tissues as these undergo the transition from sink to source. Expression of PcSOT1 and PcSOT2 is obviously tightly regulated, which has several implications for regulation of sink activities and partitioning mechanisms in sour cherry tissues. Although these proteins are presumably targeted to the plasma membrane in yeast, further confirmation of roles for these transporters in defining sink activity will require information on their upstream regulatory sequences and on their precise localization at the cell and membrane level in planta.
MATERIALS AND METHODS
Sour cherry (Prunus cerasus L. cv Montmorency) fruits were collected from 5 to 64 DPA from trees located at the Horticulture Research Center (Michigan State University, East Lansing). The fruit samples represented all the important stages in fruit phenology, with the last sample consisting of fully mature, dark-red fruit. All fruits were immediately pitted and frozen in liquid nitrogen before storage at −80°C. Leaves were sampled mid-season from developing shoots that provided a range of leaf ages from buds and young sink leaves to fully expanded, fully photosynthetically competent source leaves. All leaf samples were frozen in liquid nitrogen and then stored at −80°C.
Analysis of Soluble Sugars and Starch
Fruit samples (about 2 g fresh weight) were extracted three times with 10 mL of 80% (v/v) ethanol for 1 h at 70°C, and the extracts were pooled. Triplicate samples were prepared for each stage of fruit development. They were then vacuum evaporated to dryness at 60°C, redissolved in 1 mL of distilled water, and 0.5 mL of the solution was passed through a Sep-Pak C18 column (Waters, Milford, MA) and a 0.22-μm filter (Gelman Sciences, Ann Arbor, MI). Aliquots (10 μL) were injected into a Waters HPLC system equipped with a Shodex SC1011 carbohydrate column (6.5 × 300 mm, Shoko Co., Ltd., Tokyo) and a refractive index detector. The sugars were eluted (with water at 1.0 mL min−1) and identified and quantified by comparison of retention times and peak areas with standards of known sugars. All the data were processed with an HPLC on-line computer using Waters Millennium32 software (version 3.05). Starch was analyzed by digesting the residues with amyloglucosidase followed by quantification of the resulting Glc using Glc oxidase.
cDNA Library Construction and Gene Cloning
Total RNA was isolated as previously described by Hunter and Reid (2001). Poly(A+) RNA was isolated from total RNA by oligo(dT)-cellulose column chromatography (CLONTECH Laboratories, Inc., Palo Alto, CA). Four cDNA libraries (two from each stage of development) were constructed in a Uni-Zap XR vector according to the manufacturer's instructions (Stratagene, La Jolla, CA) using the poly(A+) mRNA from quite immature green (about 12 DPA) and fully mature red (53 DPA) fruit mesocarp tissues. The primary libraries were amplified according to the manufacturer's protocols and represented at least 1 × 106 clones. Analysis of the sequences was carried out at the Genomics Technology Support Facility at Michigan State University. Sequence analyses were performed using DNASTAR (GCG Inc.), and data comparisons were through the BLAST server at the National Center for Biotechnology Information (Bethesda, MD). Transmembrane regions were predicted using Hidden Markov Model Topology Prediction (Tusnady and Simon, 1998).
A putative sorbitol transporter PcSOT cDNA was amplified from ripening fruit by reverse transcriptase-PCR. Degenerate primers were designed based on a sugar transporter region conserved among numerous plants (Noiraud et al., 2001a). The primers were G62 [forward 5′.GA(A/G)TC(T/C/A/G)CC(T/C/A/G)CG(T/C/A/G) TGGCT.3′] and G60 [reverse 5′.CG(T/C/A/G)CC(T/C)TG(T/C/A/G)GT(T/C)TC(T/C)GG.3′]. The forward primer corresponds to an ESPRWL conserved motif, and the reverse primer corresponds to an RGQTEP conserved motif. This cDNA was cloned to pGEM easy vector (Promega, Madison, WI) according to the manufacturer's instructions and sequenced. The cDNA was then used as a probe to screen approximately 40 × 104 recombinant phages from the ripening fruit-derived cDNA library according to the manufacturer's instructions. Putative PcSOT clones were isolated and sequenced at the Genomics Technology Support Facility.
Expression and Characterization of PcSOT1 and PcSOT2 in Yeast (Saccharomyces cerevisiae)
The PcSOT1 and PcSOT2 cDNAs were ligated into the EcoRI-XhoI sites of the yeast shuttle vector pDR196 (Rentsch et al., 1995). This vector allows expression of full-length cDNA under the control of the yeast PMA1 promoter. Yeast strain RS 453 (Sauer and Stadler, 1993), which lacks the uracil gene for selection of recombinant cells, was transformed with the cDNAs according to the method of Dohmen et al. (1991). Cells transformed with empty pDR196 plasmid were used as controls.
Uptake of Radiolabeled Sorbitol
The uptake of sorbitol was followed as described by Noiraud et al. (2001b). Yeast cells were initially grown overnight in SC medium supplemented with 2% (w/v) Glc and then transferred to SC medium supplemented with 3% (w/v) glycerol and 0.05% (w/v) Glc. The cell were grown for 16 h to the early logarithmic phase, washed with distilled water, and resuspended to 1% (w/v) in SC medium containing 25 mm MES (pH 4.5). For each sorbitol uptake assay, 100 μL of the cell suspension was mixed with 100 μL of suspension buffer containing 0.5 mm sorbitol at 14C-sorbitol-specific activity (11.5 GBq mmol−1), although 3H-sorbitol was sometimes used (with a similar specific activity). Cells were incubated at 28°C for 0, 1, 2, 3, and 5 min on a water bath. Sorbitol uptake was stopped by the addition of 4 mL of ice-cold water and filtered on glass fiber filters in a vacuum filtration apparatus. Cells were rapidly washed three times with 4 mL of ice-cold water and transferred to liquid scintillation vials and counted. To determine relative uptake rates of mannitol and Suc by the transformed yeast cells, 0.5 mm mannitol at 3H-mannitol-specific activity (14.8 MBq mmol−1) or 0.5 mm Suc at 14C-Suc at specific activity (14.8 MBq mmol−1) was used for uptake. For inhibition studies, a protonophore (50 μm CCCP) or a thiol reagent (100 μm p-chloro-mercuribenzenesulphonic acid) was added 30 s before the sorbitol uptake assay. Similarly, for the sugar and acyclic polyol competition studies, 5 mm of each compound was added 30 s before the assay. To measure the Km values of PcSOT1 and PcSOT2, sorbitol concentrations of 0.1, 0.2, 0.5, 1, 2, and 4 mm and an uptake time of 2 or 4 min were used. The pH dependence of sorbitol uptake was also determined. The yeast cells were grown and treated as described above but were resuspended in SC medium containing 25 mm MES at pH 4.5, 5.5, 6.5, or 7.5. All the uptake experiments were repeated at least four times with the pDR 196 empty vector-transformed cells as controls.
RNA Gel-Blot Hybridization
Twenty micrograms of total RNA was separated on 1.3% (w/v) agarose gel under denaturing (formaldehyde) conditions. RNA was capillary transferred (Sambrook et al., 1989) to Hybond N+ nylon membranes (Amersham, Buckinghamshire, UK) overnight in 20× SSC and fixed to the membrane at 80°C for 1.5 h. Gene-specific DNA probes were prepared for PcSOT1 and PcSOT2 by PCR. The primers were 5′ CAGAAACAAAGGGCCGTCG 3′ and T7 for PcSOT1 and 5′ GCAAGTTGATCATGGTGATG 3′ and T7 for PcSOT2. Random priming was used to α-32P-label cDNA of PcSOT1 and PcSOT2 using a NEBlot kit (New England Biolabs, Inc., Beverly, MA) according to the manufacturer's instructions. Membranes were hybridized and washed with low-salt buffer as described previously (Gao and Loescher, 2000). The mRNA abundances were captured on a PhosphorImaging screen (Molecular Dynamics, Sunnyvale, CA). The same blots were washed at 90°C for 30 min in a solution containing 2% (w/v) SDS, 0.5 m Tris (pH 7), and 0.1 mm EDTA to strip off the probes, and rehybridized with a 32P-labeled probe of 18S rRNA from Arabidopsis to standardize for RNA loading.
Naming of PcSOT1 and PcSOT2 followed a search of the Mendel CPGN database, and no SOT synonyms exist.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Footnotes
This work was supported by Michigan State University (Project GREEEN) and by the Centre National de la Recherche Scientifique (France; special fellowship to Z.G.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.016725.
LITERATURE CITED
- Berüter J. Characterization of the permeability of excised apple tissue for sorbitol. J Exp Bot. 1993;44:519–528. [Google Scholar]
- Berüter OJ, Feusi MES. Comparison of sorbitol transport in excised tissue discs and cortex tissue of intact apple fruit. J Plant Physiol. 1995;146:95–102. [Google Scholar]
- Bieleski RL, Redgwell RJ. Sorbitol versus sucrose as photosynthesis and translocation products in developing apricot leaves. Aust J Plant Physiol. 1985;12:657–668. [Google Scholar]
- Brooks SJ, Moore JN, Murphy JB. Quantitative and qualitative changes in sugar content of peach genotypes [Prunus persica (L.) Batsch.] J Am Soc Hortic Sci. 1993;118:97–100. [Google Scholar]
- Buckhout TJ, Tubbe A. Structure, mechanisms of catalysis, and regulation of sugar transporters in plants. In: Zamski E, Schaffer AA, editors. Photoassimilate Distribution in Plant and Crops: Source-Sink Relationships. New York: Marcel Dekker; 1996. pp. 229–260. [Google Scholar]
- Bürkle L, Hibberd JM, Quick WP, Kühn C, Hirner B, Frommer WB. The H+-sucrose cotransporter NtSUT1 is essential for sugar export from tobacco leaves. Plant Physiol. 1998;118:59–68. doi: 10.1104/pp.118.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou TJ, Bush DR. Molecular cloning, immunochemical localization to the vacuole, and expression in transgenic yeast and tobacco of a putative sugar transporter from sugar beet. Plant Physiol. 1996;110:511–520. doi: 10.1104/pp.110.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmen RJ, Strasser AWM, Honer CB, Hollenberg CP. An efficient transformation procedure enabling long term storage of competent cells of various yeast genera. Yeast. 1991;7:691–692. doi: 10.1002/yea.320070704. [DOI] [PubMed] [Google Scholar]
- Dreyer I, Horeau C, Lemaillet G, Zimmermann S, Bush DR, Rodriguez-Navarro A, Schachtman DP, Spalding EP, Sentenac H, Gaber RF. Identification and characterization of plant transporters using heterologous expression systems. J Exp Bot. 1999;50:1073–1087. [Google Scholar]
- Fillion L, Ageorges A, Picaud S, Coutos-Thévenot P, Lemoine R, Romieu C, Delrot S. Cloning and expression of a hexose transporter gene expressed during the ripening of grape berry. Plant Physiol. 1999;120:1083–1093. doi: 10.1104/pp.120.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flore JA, Layne DR. Prunus. In: Zamski E, Schaffer AA, editors. Photoassimilate Distribution in Plant and Crops: Source-Sink Relationships. New York: Marcel Dekker; 1996. pp. 825–849. [Google Scholar]
- Gao ZF, Loescher WH. NADPH supply and mannitol biosynthesis: characterization, cloning, and regulation of the non-reversible glyceraldehyde-3-phosphate dehydrogenase in celery leaves. Plant Physiol. 2000;124:321–330. doi: 10.1104/pp.124.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald JR, Krysan PJ, Young JC, Evert RF, Sussman MR. Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc Natl Acad Sci USA. 2000;97:13979–13984. doi: 10.1073/pnas.250473797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner S, Koster U, Lauer K, Rosenkranz H, Vogel T, Rausch T. Plant invertase inhibitors: expression in cell culture and during plant development. Aust J Plant Physiol. 2000;27:807–814. [Google Scholar]
- Horak J, Wolf DH. Catabolite inactivation of the galactose transporter in the yeast Saccharomyces cerevisiae: ubiquitination, endocytosis, and degradation in the vacuole. J Bacteriol. 1997;179:1541–1549. doi: 10.1128/jb.179.5.1541-1549.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter DA, Reid MS. A simple and rapid method for isolating high quality RNA from flower petals. Acta Hortic. 2001;543:147–152. [Google Scholar]
- Komatsu OA, Moriguchi T, Koyama K, Omura M, Akihama T. Analysis of sucrose synthase genes in citrus suggests different roles and phylogenetic relationships. J Exp Bot. 2002;53:61–71. [PubMed] [Google Scholar]
- Lemoine R. Sucrose transporters in plants: update on function and structure. Biochim Biophys Acta. 2000;1465:246–262. doi: 10.1016/s0005-2736(00)00142-5. [DOI] [PubMed] [Google Scholar]
- Lo Bianco R, Rieger M. Roles of sorbitol and sucrose in growth and respiration of “Encore” peaches at the three developmental stages. J Am Soc Hortic Sci. 2002;127:297–302. [Google Scholar]
- Lo Bianco R, Rieger M, Sung SS. Carbohydrate metabolism of vegetative and reproductive sinks in the late-maturing peach cultivar “Encore.”. Tree Physiol. 1999;19:103–109. doi: 10.1093/treephys/19.2.103. [DOI] [PubMed] [Google Scholar]
- Loescher WH, Everard JD. Sugar alcohol metabolism in sinks and sources. In: Zamski E, Schaffer AA, editors. Photoassimilate Distribution in Plant and Crops: Source-Sink Relationships. New York: Marcel Dekker; 1996. pp. 185–207. [Google Scholar]
- Loescher WH, Marlow GC, Kennedy RA. Sorbitol metabolism and sink-source interconversions in developing apple leaves. Plant Physiol. 1982;70:335–339. doi: 10.1104/pp.70.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marger MD, Saier JMH. A major superfamily of transmembrane facilitators that catalyze uniport, symport and antiport. Trends Biochem Sci. 1993;18:13–20. doi: 10.1016/0968-0004(93)90081-w. [DOI] [PubMed] [Google Scholar]
- Marlow G, Loescher WH. Watercore. Hortic Rev. 1984;6:189–251. [Google Scholar]
- Marquat C, Petel G, Gendraud M. Saccharose and sorbitol transporters from plasmalemma membrane vesicles of peach tree leaves. Biol Plant. 1997;39:369–378. [Google Scholar]
- Moing A, Langlois N, Svanella L, Zanetto A, Gaudillere JP. Variability in sorbitol: sucrose ratio in mature leaves of different Prunus species. J Am Soc Hortic Sci. 1997;122:83–90. [Google Scholar]
- Noiraud N, Delrot S, Lemoine R. The sucrose transporter of celery: identification and expression during salt stress. Plant Physiol. 2000;122:1447–1455. doi: 10.1104/pp.122.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noiraud N, Maurousset L, Lemoine R. Transport of polyols in higher plants. Plant Physiol Biochem. 2001a;39:717–728. [Google Scholar]
- Noiraud N, Maurousset L, Lemoine R. Identification of a mannitol transporter, AgMaT1, in celery phloem. Plant Cell. 2001b;13:695–705. doi: 10.1105/tpc.13.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Song KJ, Kim MY, Hwang JH, Shin YU, Kim WC, Chung WI. Molecular cloning and characterization of four cDNAs encoding the isoforms of NAD-dependent sorbitol dehydrogenase from the Fuji apple. Plant Sci. 2002;162:513–519. [Google Scholar]
- Rentsch D, Laloi M, Rouhara I, Scmelzer E, Delrot S, Frommer WB. NTR1 encodes a high affinity oligopeptide transporter in Arabidopsis. FEBS Lett. 1995;370:264–268. doi: 10.1016/0014-5793(95)00853-2. [DOI] [PubMed] [Google Scholar]
- Roper TR, Loescher WH. Relationships between leaf area per fruit and fruit quality in “Bing” sweet cherry. HortScience. 1987;22:1273–1276. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sauer N, Stadler R. A sink-specific H+/monosaccharide co-transporter from Nicotiana tabacum: cloning and heterologous expression in baker's yeast. Plant J. 1993;4:601–610. doi: 10.1046/j.1365-313x.1993.04040601.x. [DOI] [PubMed] [Google Scholar]
- Shakya R, Sturm A. Characterization of source- and sink-specific sucrose/H+ symporters from carrot. Plant Physiol. 1998;118:1473–1480. doi: 10.1104/pp.118.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanase K, Shiratake K, Mori H, Yamaki S. Changes in the phosphorylation state of sucrose synthase during development of Japanese pear fruit. Physiol Plant. 2002;114:21–26. doi: 10.1046/j.0031-9317.2001.10137.x. [DOI] [PubMed] [Google Scholar]
- Tukey HB, Young JO. Histological study of the developing fruit of the sour cherry. Bot Gaz. 1939;100:723–749. [Google Scholar]
- Tusnady GE, Simon I. Principles governing amino acid composition of integral membrane proteins: application to topology prediction. J Mol Biol. 1998;283:489–506. doi: 10.1006/jmbi.1998.2107. [DOI] [PubMed] [Google Scholar]
- Weise A, Barker L, Kuehn C, Lalonde S, Buschmann H, Frommer WB, Ward JM. A new subfamily of sucrose transporters, SUT4, with low affinity/high capacity localized in enucleate sieve elements of plants. Plant Cell. 2000;12:1345–1355. doi: 10.1105/tpc.12.8.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LE, Lemoine R, Sauer N. Sugar transporters in higher plants: a diversity of roles and complex regulation. Trends Plant Sci. 2000;5:283–290. doi: 10.1016/s1360-1385(00)01681-2. [DOI] [PubMed] [Google Scholar]
- Winter H, Huber SC. Regulation of sucrose metabolism in higher plants: localization and regulation of activity of key enzymes. Crit Rev Biochem Mol Biol. 2000;35:253–289. doi: 10.1080/10409230008984165. [DOI] [PubMed] [Google Scholar]
- Yamada K, Oura Y, Mori H, Yamaki S. Cloning of NAD-dependent sorbitol dehydrogenase from apple fruit and gene expression. Plant Cell Physiol. 1998;39:1357–1361. doi: 10.1093/oxfordjournals.pcp.a029345. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Kanayama Y, Soejima J, Yamaki S. Changes in the amounts of the NAD-dependent sorbitol dehydrogenase and its involvement in the development of apple fruit. J Am Soc Hortic Sci. 1996;121:848–852. [Google Scholar]
- Yamaguchi H, Kanayama Y, Yamaki S. Purification and properties of NAD-dependent sorbitol dehydrogenase from apple fruit. Plant Cell Physiol. 1994;35:887–892. [Google Scholar]
- Yamaki S, Asakura T. Energy coupled transport of sorbitol and other sugars into the protoplast isolated from apple fruit flesh. Plant Cell Physiol. 1988;29:961–968. [Google Scholar]