Abstract
The Dunaliella salina photosynthetic apparatus organization and function was investigated in wild type (WT) and a mutant (zea1) lacking all β,β-epoxycarotenoids derived from zeaxanthin (Z). The zea1 mutant lacked antheraxanthin, violaxanthin, and neoxanthin from its thylakoid membranes but constitutively accumulated Z instead. It also lacked the so-called xanthophyll cycle, which, upon irradiance stress, reversibly converts violaxanthin to Z via a de-epoxidation reaction. Despite the pronounced difference observed in the composition of β,β-epoxycarotenoids between WT and zea1, no discernible difference could be observed between the two strains in terms of growth, photosynthesis, organization of the photosynthetic apparatus, photo-acclimation, sensitivity to photodamage, or recovery from photo-inhibition. WT and zea1 were probed for the above parameters over a broad range of growth irradiance and upon light shift experiments (low light to high light shift and vice versa). A constitutive accumulation of Z in the zea1 strain did not affect the acclimation of the photosynthetic apparatus to irradiance, as evidenced by indistinguishable irradiance-dependent adjustments in the chlorophyll antenna size and photosystem content of WT and zea1 strain. In addition, a constitutive accumulation of Z in the zea1 strain did not affect rates of photodamage or the recovery of the photosynthetic apparatus from photo-inhibition. However, Z in the WT accumulated in parallel with the accumulation of photodamaged PSII centers in the chloroplast thylakoids and decayed in tandem with a chloroplast recovery from photo-inhibition. These results suggest a role for Z in the protection of photodamaged and disassembled PSII reaction centers, apparently needed while PSII is in the process of degradation and replacement of the D1/32-kD reaction center protein.
Organisms of oxygenic photosynthesis convert the energy of sunlight into chemical energy, which supports most life on earth. In photosynthetic membranes of green algae and plants, incident irradiance is absorbed by chlorophyll (Chl)-binding light-harvesting antenna complexes (LHCs) associated with the reaction centers of PSII and PSI. However, when the photosynthetic apparatus absorbs irradiance in excess of that required for the saturation of photosynthesis, singlet oxygen is generated, and PSII is subject to an irreversible photooxidative damage (Vass et al., 1992; Telfer et al., 1994; Melis, 1999). This photodamage selectively impairs the function of the D1/32-kD reaction center protein of PSII and has the potential to lower rates of photosynthesis and diminish plant growth and productivity (Powles and Critchley, 1980; Powles, 1984).
The probability of photooxidative damage in chloroplasts depends on the oxidation reduction state of the primary electron-accepting plastoquinone of PSII (QA), which is the parameter that controls photodamage under a variety of physiological and environmental conditions. When QA is oxidized under continuous illumination, photochemical electron transport from the reaction center Chl (P680) converts excitation energy into chemical form. Under these conditions, there is a low probability of excitation transfer to molecular oxygen. When QA is reduced under continuous illumination, there is a relatively higher probability that exited Chl molecules in the triplet state would relax through energy transfer to oxygen, thus generating reactive singlet oxygen. Singlet oxygen adversely affects PSII by covalent modification of the photochemical reaction center Chl in the D1 protein (Aro et al., 1993). Under steady-state photosynthesis conditions, the reduction state of QA increases linearly with irradiance, thereby causing a correspondingly linear increase in the probability of photodamage (Huner et al., 1998; Melis, 1999). Organisms of oxygenic photosynthesis overcome this irreversible modification upon a molecular repair of the adversely affected PSII centers. The repair process entails disassembly of the PSII holocomplex and the selective removal and replacement of the photodamaged D1 protein (Mattoo and Edelman, 1987), constituting the so-called PSII damage-and-repair cycle (Guenther and Melis, 1990).
When photooxidative damage to PSII occurs faster than its enzymatic repair, the photosynthetic capacity and quantum yield of photosynthesis are lowered, causing a condition known as photo-inhibition (Powles, 1984; Aro et al., 1993). To avoid or minimize photo-inhibition, photosynthetic organisms have evolved several strategies (Demmig-Adams and Adams, 1992; Horton et al., 1996; Niyogi et al., 1997b, 2001). These are distinguished between short- and long-term responses, aimed at diminishing overexcitation of the reaction centers. Short-term responses include a mechanism known as energy-dependent “non-photochemical quenching,” which can help dissipate excess absorbed light energy. In addition, within minutes upon exposure of plants to excessive irradiance, an irradiance-dependent xanthophyll cycle is activated, which involves reversible de-epoxidation of violaxanthin (V) and formation of zeaxanthin (Z) via antheraxanthin (A). Z is believed to play a photoprotective role via dissipation of excessive light energy as heat (Yamamoto, 1979; Demmig-Adams, 1990; Gilmore et al., 1995; Niyogi, 1999). When levels of absorbed irradiance become lower than those required for the saturation of photosynthesis, Z is converted back to V by the enzyme Z epoxidase (Hager, 1980). This xanthophyll cycle is a dynamically regulated and reversible interconversion of V to A to Z, occurring in the thylakoid membrane of photosynthesis (Demmig et al., 1987; Gilmore and Yamamoto, 1993; Demmig-Adams et al., 1996; Goss et al., 1998; Havaux and Niyogi, 1999). Another acclimation mechanism entails reversible changes in the Chl antenna size of the PSs. In this process, long-term high irradiance elicits modulation of gene expression, leading to a reduction in the amount of Chl and in the number of the LHC proteins in the photosynthetic apparatus (Melis, 1991). This causes the assembly of a smaller functional light-harvesting Chl antenna size in the chloroplast thylakoids, effectively diminishing overexcitation of the PSs (Smith et al., 1990; Neidhardt et al., 1998).
As a consequence, mutants with lesions in the Z epoxidase gene are deficient not only in A and V but also fail to synthesize neoxanthin (N; Rock and Zeevaart, 1991; Marin et al., 1996; Niyogi et al., 1997a; Jin et al., 2003). In addition, such mutants accumulate Z, even under low-light (LL) growth conditions. Higher plants with an impaired Z epoxidase are not affected in terms of photosynthesis (Rock et al., 1992; Tardy and Havaux, 1996; Hurry et al., 1997). Analogous mutations resulting in Z accumulation have been described in the green alga Scenedesmus obliquus (Bishop et al., 1998), Chlamydomonas reinhardtii (Niyogi et al., 1997a), and Dunaliella salina (Jin et al., 2003). Under LL growth conditions, no significant differences in the properties of photosynthesis in these organisms could be observed. Such earlier investigations suggested that Z could structurally and functionally replace the missing epoxy-carotenoids A, V, and N in mutants of both higher plant and green algae.
There is a sizable literature on the role of the reversible xanthophyll cycle in photoprotection (for review, see Demmig-Adams and Adams, 1992; Horton et al., 1996; Gilmore, 1997; Niyogi, 1999). The proposed photoprotective function of Z entails a direct quenching of excitation energy in the pigment bed of photosynthesis (Demmig-Adams, 1990; Horton et al., 1996), thereby protecting the photosynthetic apparatus from the consequences of overexcitation. However, the precise mechanism of photoprotection by Z is not well understood. It has been assumed that de-epoxidation of V to Z (Bugos and Yamamoto, 1996) helps to guard against a photooxidative damage to the PSII reaction center complex. Mechanistic aspects of this hypothesis were not rigorously investigated, though. Recent preliminary work from this laboratory suggested a role for Z after photodamage and while PSII occurred in the disassembled state before repair (Jin et al., 2001). In the present work, the role of Z in the protection of PSII was further investigated with the zea1 mutant of the green alga D. salina. This mutant is apparently aberrant in the Z epoxidase reaction and, irrespective of the growth or irradiance stress conditions, accumulates Z. Biochemical analyses showed that Z constitutively and quantitatively substituted for N, V, and A in the zea1 strain (Jin et al., 2003). These previous measurements also showed similar rates of growth and light saturation curves of photosynthesis for wild type (WT) and zea1 mutant in the light intensity range between 0 and 3,000 μmol photons m−2 s−1. Thus, this mutant offered an opportunity to rigorously study the role of Z in photo-acclimation and the PSII damage and repair properties of the cells. Results showed that a constitutive accumulation of Z in the thylakoid membrane does not alter the green alga photo-acclimation properties, sensitivity to irradiance stress, kinetics of photodamage, or recovery from photo-inhibition in D. salina. The results are discussed in terms of the accumulation of Z, which, in the WT, occurred in parallel with the accumulation of photodamaged PSII and the return of Z to V, which occurred in tandem with the recovery from photo-inhibition.
RESULTS
Pigment Composition and PS Stoichiometry
The zea1 mutant of D. salina was unable to synthesize detectable amounts of the epoxy-xanthophylls A, V, and N but constitutively accumulated Z (Jin et al., 2003). When cells were grown under 100 μmol photons m−2 s−1 (LL growth conditions), the Chl content (approximately 3 × 10−15 mol cell−1) and the Chl a/b ratio (approximately 4:1) were about the same in WT and zea1 mutant (Table I). Total Car content (approximately 1.5 × 10−15 mol cell−1) was also similar in WT and zea1 mutant. However, the Z content of the mutant (Z, approximately 6.9 × 10−16 mol cell−1) was about 23-fold greater than that in the WT (Z, approximately 0.3 × 10−16 mol cell−1, Table I). The de-epoxidation state ([Z]/[V + A + Z] ratio) was 0.18:1 in the WT and 1:1 in the mutant. These results suggest a quantitative substitution of A, V, and N by Z in the thylakoid membrane of the zea1 strain.
Table I.
Pigment content, Chl a/b ratio, de-epoxidation state, and photosystem stoichiometry in D. salina wild type and zea1 mutant
| Parameter | WT | zea1 |
|---|---|---|
| mol Chl cell−1 × 10−15 | 2.56 ± 0.2 | 2.82 ± 0.12 |
| Chl a/b | 4.2 ± 0.1 | 4.1 ± 0.2 |
| mol carotenoid (Car)/cell × 10−15 | 1.5 ± 0.21 | 1.49 ± 0.1 |
| mol Z cell−1 × 10−16 | 0.3 ± 0.1 | 6.9 ± 0.1 |
| Z/(V + A + Z) | 0.18 ± 0.01 | 1 ± 0.0 |
| mmol QA mol Chl−1 | 2.6 ± 0.07 | 2.8 ± 0.15 |
| mmol P700 mol Chl−1 | 2.1 ± 0.04 | 2.07 ± 0.15 |
| PSII/PSI (QA/P700) | 1.23 | 1.35 |
Cells were grown under 100 μmol photons m−2 s−1 (LL).
Photochemical apparatus organization in the two strains was compared upon analysis of PS concentration. Light-induced absorbance difference measurements were used to determine the concentrations of QA and P700 as a measure of functional PSII and PSI reaction centers, respectively. Table I shows that, under LL growth conditions, WT and the zea1 mutant had similar PSII and PSI concentrations either on a per Chl or on a per cell basis. Similar Chl a/b and PSII/PSI ratios in the thylakoid membranes of WT and zea1 mutant suggest that the level of LHCII and LHCI were about the same in the two strains (Neidhardt et al., 1998; Jin et al., 2001). Taken together, these results show that absence of the epoxy-xanthophylls A, V, and N and constitutive expression of Z did not bring about changes in the PSII/PSI ratio, level of the LHC proteins, or functional Chl antenna size of the PSs in the chloroplast thylakoids.
Photosynthesis Characteristics
The quantum yield and productivity of photosynthesis in WT and zea1 were assessed upon comparative analysis of the light saturation curve of photosynthesis in the two strains. In such presentation, the rate of O2 evolution was measured and plotted as a function of incident light intensity, thus obtaining the photosynthesis versus irradiance curve. From the slope of the initial linear part of the light saturation curve of photosynthesis (not shown), information was obtained about the relative quantum yield of photosynthesis (Φ) in WT and zea1 (Table II). The light-saturated rate of oxygen evolution (Pmax) defined the capacity of photosynthesis in the two strains. As shown in Table II, WT (Φ = 0.36) and zea1 (Φ = 0.36) had similar Φ. However, the photosynthetic capacity of the mutant (Pmax = 95 ± 7.2 mmol O2 mol Chl−1 s−1) was about 15% greater than that of the WT (Pmax = 80 ± 3.2 mmol O2 mol Chl−1 s−1). Chl fluorescence activity of WT and zea1 was measured in vivo. The fluorescence parameter (Fv/Fm) offers a nonintrusive method for the measurement of photochemical charge separation efficiency at PSII. Table II shows that PSII photochemical charge separation efficiency of the mutant (Fv/Fm = 0.68) was slightly higher than that of the WT (Fv/Fm = 0.62). These results provide evidence that constitutive expression and assembly of Z in the thylakoid membrane of D. salina, occurring instead of A, V, and N, does not bring about a permanent quenching of excitation in the pigment bed or otherwise affect the function of photosynthesis.
Table II.
Photosynthetic capacity, relative photon use efficiency, and PSII efficiency of D. salina wild type and zea1 mutant
| Parameter Measured | LL
|
|
|---|---|---|
| WT | zea1 | |
| Photon use efficiency (Φ), arbitrary units | 0.36 ± 0.02 | 0.36 ± 0.02 |
| Photosynthetic capacity (Pmax), mmol O2 mol Chl−1 s−1 | 80 ± 3.2 | 95 ± 7.2 |
| Fv/Fm | 0.62 ± 0.04 | 0.68 ± 0.02 |
Photo-Acclimation of D. salina WT and zea1 Mutant
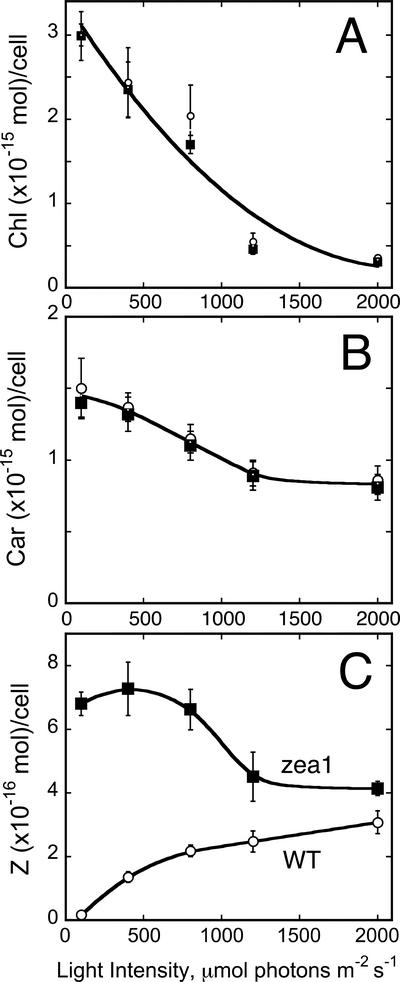
Under the LL growth conditions (100 μmol photons m−2 s−1) employed in this work, WT and zea1 were indistinguishable on the basis of their photosynthesis characteristics. Measurements were extended to include the photo-acclimation of the cells to different levels of growth irradiance, in the range of 100 to 2,000 μmol photons m−2 s−1. Figure 1, A and B, show the pigment (Chl and total Car) content in the two strains as a function of growth irradiance. Levels of Chl in both WT and mutant declined as a function of growth irradiance from 3 × 10−15 to 0.3 × 10−15 mol cell−1 (Fig. 1A). Total Car in the cells also declined as a function of growth irradiance, from 1.5 × 10−15 to 0.8 × 10−15 mol cell−1 (Fig. 1B). It is noteworthy that cellular Chl content decreased considerably more than that of Car as a function of growth irradiance. No significant difference could be detected in the irradiance-dependent adjustment of Chl and total Car content between WT and zea1 mutant, suggesting that the mutation did not affect the ability of the cells to acclimate to the level of irradiance.
Figure 1.
Chl and Car content of D. salina cultures. The effect of growth irradiance on the cellular Chl content (A), total Car content (B), and Z content (C) of WT (white circle) and zea1 mutant (squares) is shown.
Acclimation of photosynthetic organisms to irradiance also entails changes in Z content. The cellular content of Z in the WT increased as a function of growth irradiance from 0.3 to 3.2 × 10−16 mol cell−1 (Fig. 1C), reflecting a shift in the de-epoxidation state of the Car present, more toward Z. In the zea1 mutant, Z per cell was constant as a function of growth irradiance in the 100 to 400 μmol photons m−2 s−1 (about 7 × 10−16 mol cell−1). It gradually declined at higher light intensities to about 4 × 10−16 mol cell−1 at 2,000 μmol photons m−2 s−1 (Fig. 1C), consistent with the overall decline of total Car per cell as a function of growth irradiance.
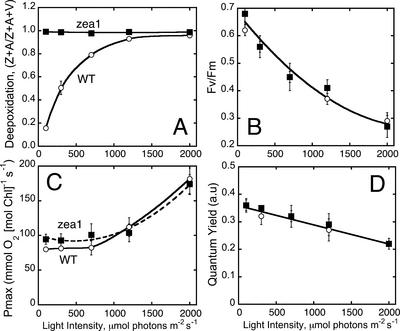
The de-epoxidation state of the xanthophyll cycle Car was also determined in cells grown under different levels of irradiance. In the WT, the de-epoxidation state increased as a function of growth irradiance from 0.18 to 0.95 (Fig. 2A). In the zea1 mutant, the de-epoxidation state remained at 1.0 irrespective of the growth irradiance regime. The latter is consistent with a zea1 lesion in the Z epoxidase gene, which prevents the epoxidation of Z to V and also renders the xanthophyll cycle inoperative. Figure 2B shows the Fv/Fm ratio of dark-adapted WT and zea1 cells grown under different irradiance regimes. The ratio declined as a function of growth irradiance from Fv/Fm = 0.65 at 100 μmol photons m−2 s−1 to Fv/Fm = 0.25 at 2,000 μmol photons m−2 s−1. This response, attributed to the slowly reversible excitation quenching, was identical in WT and zea1 mutant, showing that constitutive accumulation of Z in the mutant did not affect in any way the development of this excitation quenching as a function of growth irradiance.
Figure 2.
Effects of growth irradiance on de-epoxidation state of the xanthophyll cycle (A); PSII photochemical charge separation efficiency, as measured by the Chl fluorescence Fv/Fm ratio (B); Pmax of cellular photosynthesis (C); and Φ of photosynthesis (D) in WT (white circles) and zea1 mutant (squares) of D. salina.
To further probe the effect of a constitutive accumulation of Z on the photo-acclimation properties of D. salina, the light-saturated rate and the Φ were measured as a function of growth irradiance. Figure 2C shows that, in the range from 100 to 2,000 μmol photons m−2 s−1, Pmax increased as a function of growth irradiance from about 90 to about 180 mmol O2 mol Chl−1 s−1, whereas the Φ declined from 0.36 to 0.22 (Fig. 2D, arbitrary units). The increase in Pmax is attributed to a lowering of the Chl content (Pmax measurement is on a per Chl basis). The lowering of the Φ is attributed to steady-state photo-inhibition of photosynthesis in these green algae, which is accentuated with growth irradiance (Vasilikiotis and Melis, 1994; Baroli and Melis, 1996). It is concluded that a constitutive accumulation of Z in D. salina does not alter the photo-acclimation response of the cells.
The above analyses showed a substantially different Z content between WT and zea1 mutant (Figs. 1C and 2A). However, there were no discernible differences between the two strains in PSII photochemical charge separation efficiency of the acclimated cells (Fig. 2B) or photo-inhibition of photosynthesis as a function of growth irradiance (Fig. 2D). Considering the proposed role of Z in photoprotection (Demmig et al., 1987; Gilmore and Yamamoto, 1993; Demmig-Adams et al., 1996; Goss et al., 1998; Havaux and Niyogi, 1999), these results indicate that a constitutive presence of Z does not confer enhanced resistance to photooxidative damage in the zea1 mutant. To more rigorously assess the role of Z in the protection against photooxidative damage of PSII in chloroplasts, further detailed analysis was undertaken at the thylakoid membrane and molecular levels.
Photo-Inhibition Status as a Function of Growth Irradiance
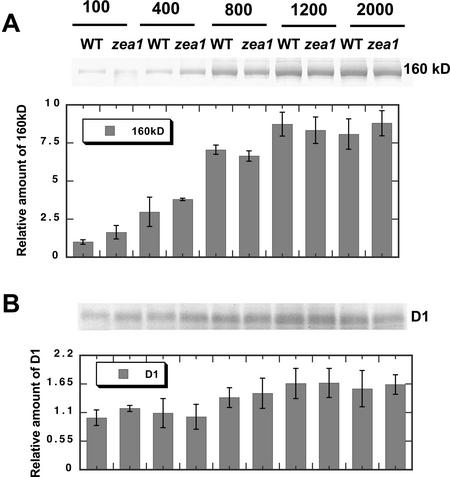
To further analyze the effect of growth irradiance on the photosynthetic apparatus of WT and zea1 mutant, thylakoid membranes were isolated from WT and zea1 cells grown under different levels of irradiance. SDS-PAGE analysis of the thylakoid membrane proteins was performed with samples loaded on an equal Chl basis, followed by western-blot analyses with specific polyclonal antibodies. Figure 3 shows western blots with specific polyclonal antibodies against the D1 reaction center protein of PSII (Kim et al., 1993) or against a 160-kD PSII repair intermediate (Kim et al., 1993; Melis and Nemson, 1995; Yokthongwattana et al., 2001). The 160-kD protein complex is known to contain a photodamaged but as yet undegraded D1 protein (Kim et al., 1993) and the D2 protein of the PSII reaction center (Melis and Nemson, 1995). This unusual property has permitted, for the first time to our knowledge, an SDS-PAGE-based quantitation of photodamaged versus active D1 in chloroplast thylakoids (Baroli and Melis, 1996). It was postulated that formation of such a 160-kD protein complex might reflect PSII conformational changes that occur as a direct consequence of photodamage and the ensuing partial disassembly of PSII (Yokthongwattana et al., 2001). The quantitative measurement of the 160-kD protein complex provides a convenient way by which to assess the extent of the in vivo photo-inhibition in D. salina. Results from such quantitative western-blot analysis in Figure 3A show increasing steady-state levels of the 160-kD complex as a function of growth irradiance. However, there were no obvious differences in the amount of the 160-kD protein complex accumulating in the zea1 thylakoid membrane versus that of the WT. Moreover, levels of the active D1 protein in the zea1 thylakoids showed no significant difference compared with those of the WT (Fig. 3B). It is concluded that a constitutive accumulation of Z in the zea1 mutant does not confer enhanced advantage over the WT in terms of protection from photo-inhibition.
Figure 3.
Quantitative western-blot analysis of thylakoid membrane proteins from D. salina WT and zea1 mutant grown under different light intensities. Proteins were probed with specific polyclonal antibodies against the 160-kD PSII repair intermediate (A) or against the PSII D1/32-kD reaction center protein (B). The corresponding densitometric quantitations of the bands are given as a bar graph on the bottom of each panel. Lanes were loaded on an equal Chl basis (4 nmol Chl lane−1).
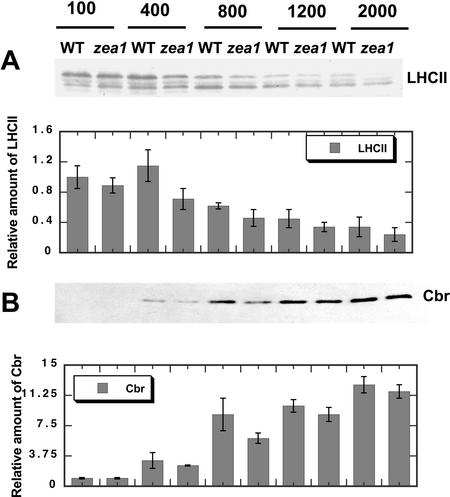
The amount and composition of the LHCII and Cbr proteins in WT and the zea1 mutant were estimated from western-blot analyses with specific polyclonal antibodies raised against the apoproteins of the LHCII and against the Cbr protein (Fig. 4). LHCII proteins in both WT and the zea1 mutant declined as a function of growth irradiance (Fig. 5A). Among four distinct bands of the LHCII (termed according to electrophoretic mobility as LHCII-1–4), the amount of LHCII-1 declined faster than LHCII-2 to 4 as a function of growth irradiance. This is consistent with the photo-acclimation response of the photosynthetic apparatus (Anderson, 1986; Tanaka and Melis, 1997). This response appeared to be similar in WT and zea1 mutant.
Figure 4.
Quantitative western-blot analysis of thylakoid membrane proteins from D. salina WT and zea1 mutant grown under different light intensities. Proteins were probed with specific polyclonal antibodies raised against the LHC-II (A) and Cbr protein (B). The corresponding densitometric quantification of the bands is given as a bar graph on the bottom of each panel. Lanes were loaded on an equal Chl basis (4 nmol Chl lane−1).
Figure 5.
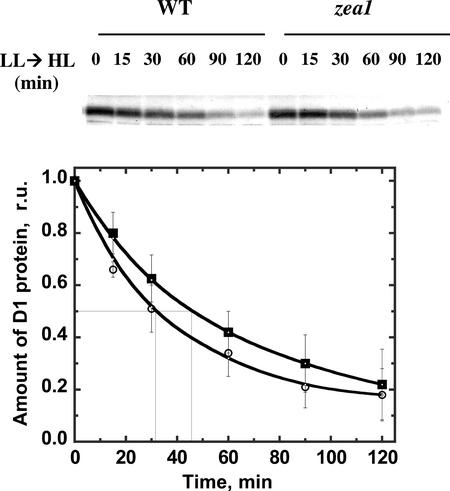
Time course for the loss of the D1 protein from its 32-kD position after an LL → high light (HL) shift of the cultures. D. salina WT (white circles) and zea1 mutant (squares) were grown under LL to the late log phase. Cells were suspended in the presence of lincomycin immediately before an LL → HL shift. Western blots probed with polyclonal antibodies against the D1 protein are show in the upper panel. Densitometric quantification of the corresponding western blots for WT (white circles) and zea1 mutant (squares) are shown in the lower panel. The half time of the 32-kD protein loss was 32 ± 12 min for the WT and 45 ± 15 min for the zea1 mutant.
The Cbr protein is homologous to higher plant ELIP proteins and belongs to the LHC super family (Green and Kühlbrandt, 1995; Banet et al., 2000). Cbr proteins accumulate when D. salina cells are stressed by HL. Therefore, the Cbr protein is thought to be an indicator of irradiance stress (Lers et al., 1991; Levy et al., 1992, 1993). Thylakoid membrane proteins of WT and zea1 cells were probed with a Cbr antibody, and levels of the Cbr protein in WT and mutant were quantified from western-blot analyses. Figure 4B shows that Cbr proteins were practically absent in both WT and zea1 mutant when grown at 100 μmol photons m−2 s−1, whereas the levels of Cbr increased substantially with growth irradiance. This increase in the level of the Cbr protein was nearly identical in the WT and zea1 mutant (Fig. 4B).
Photodamage and Photo-Inhibition after an LL → HL Shift
To gain a better insight into the potential role of a constitutive accumulation of Z in the thylakoid membrane, light shift experiments were performed and the effect on rate of photodamage or recovery from photo-inhibition were recorded. The rate of PSII photodamage was compared in WT and zea1 mutant after an LL → HL shift of the cultures. In such measurements, the amount of D1 protein was quantified as a function of time in the presence of lincomycin, a chloroplast protein biosynthesis inhibitor. In the presence of lincomycin, de novo biosynthesis of the D1 protein is prevented and, therefore, the repair process is inhibited. After photodamage, there is a loss to the D1 protein from the 32-kD position because the latter is converted into a 160-kD protein complex (Kim et al., 1993; Baroli and Melis, 1996). This process can be monitored by western-blot analysis from the kinetics of the D1 loss from the 32-kD position (Kim et al., 1993). Figure 5 shows results from such measurements. WT and zea1 mutant cells were transferred from LL → HL at 0 min. Samples were harvested at the indicated times, and thylakoid membranes were isolated and subjected to western-blot analysis (Fig. 5, upper). Quantitation of the D1 decay kinetics (Fig. 5, lower) showed half times of about 32 ± 12 min for WT and 45 ± 15 min for the zea1 mutant. These results may suggest that a constitutive accumulation of Z in the thylakoid membrane would cause somewhat slower kinetics in the processing of the inert D1 protein after photodamage.
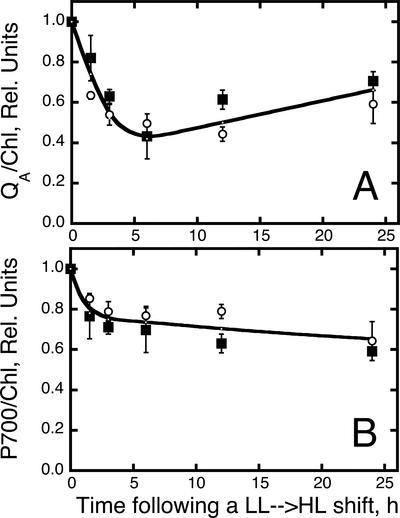
In addition to the analysis on D1 processing after photodamage in the presence of lincomycin (Fig. 5), changes in photochemical activity of WT and zea1 were measured in situ after an LL → HL shift of the cultures in the absence of this inhibitor. Figure 6A shows that functional PSII centers (QA/Chl) are lowered by about 60% within 6 h after the LL → HL shift (absence of lincomycin). At longer incubation times under HL, the QA/Chl ratio was stabilized or gradually increased because of a lowering in the Chl content of the cells. This is consistent with earlier results from this laboratory (Kim et al., 1993). The effect of an LL → HL shift on the PSI photochemical activity is also shown (Fig. 6B). Loss in P700 activity also occurs as a result of the sudden change in the level of irradiance. The amplitude of this adverse effect, however, is less than that for PSII. It is important to note that no significant difference was observed between WT and zea1 mutant in this experimentation.
Figure 6.
Time course of PS concentration (QA and P700) after an LL → HL shift of WT (white circles) and zea1 mutant (squares) of D. salina. A, Photochemically competent PSII measured from the amplitude of QA photoreduction. B, Photochemically competent PSI measured from the amplitude of P700 photoreduction.
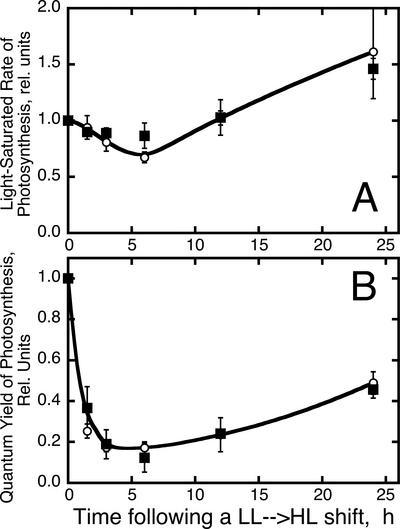
Photodamage and photo-inhibition in WT and zea1 mutant were investigated further after an LL → HL shift from the Pmax and Φ of photosynthesis. Figure 7A shows a transient loss in Pmax, reaching a low after about 6 h in HL. Subsequently, Pmax gradually increased, partly because of the establishment of a steady state between photodamage and repair, and in part due to the lowering in the Chl content (Fig. 1A) as cells acclimate to HL. Figure 7B shows a substantial loss in Φ, reaching a low of only 15% of the control after about 6 h incubation under HL. This precipitous loss in Φ most likely reflects the ensuing dissociation of the LHC-II antenna from the photodamaged PSII core complex (Melis, 1991). A dissociated LHC-II antenna would absorb light but would not contribute to the Φ. Once again, this characteristic photodamage and photo-inhibition phenomenology was invariant between WT and zea1 mutant.
Figure 7.
Changes in the Pmax and Φ of photosynthesis after an LL → HL shift of the cultures. A, Measurement of the light-saturated rate of photosynthesis in WT (white circles) and zea1 mutant (squares). B, Measurement of the Φ in WT (white circles) and zea1 mutant (squares).
PSII Repair and Recovery from Photo-Inhibition
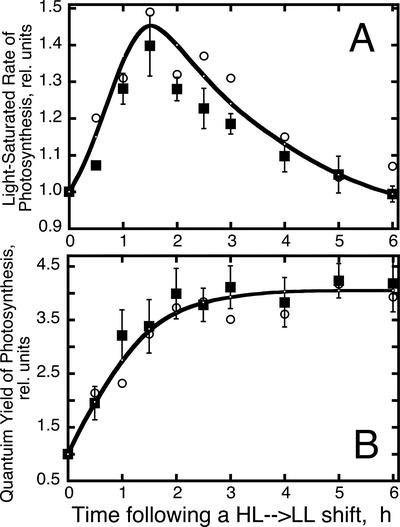
The preceding results suggested that constitutive accumulation of Z in the zea1 strain did not enhance protection of PSII from photodamage. We investigated whether constitutive accumulation of Z may affect the repair of PSII from photodamage and/or the recovery of photosynthesis from photo-inhibition. In such experiments, both WT and zea1 mutant were grown under continuous irradiance stress conditions (2,500 μmol photons m−2 s−1). Under these conditions, growth occurs while the yellowish cells exist in steady-state photo-inhibition, with up to 80% of all PSII centers being photochemically inert at any given point in time (Vasilikiotis and Melis, 1994). Such HL-grown cells were shifted to LL growth conditions (HL → LL). Samples were collected at different time intervals after the HL → LL shift for analysis. Two main photosynthesis parameters, Pmax and Φ, were measured as a way by which to monitor the recovery of photosynthesis from photo-inhibition. Figure 8A shows the adjustment of the Pmax in cells after an HL → LL transition. It is evident that Pmax increased promptly as a function of time upon the LL → HL transition, reaching a 50% greater value within approximately 2 h under the LL. This change reflects the repair of photodamaged PSII centers, which results in a greater capacity for photosynthesis (Neidhardt et al., 1998). Incubation of the cultures for more than approximately 2 h under LL conditions caused a gradual decline in the value of Pmax (Fig. 8A), reflecting the accumulation of Chl in the chloroplasts (Fig. 1A), and increase in the light-harvesting Chl antenna size, which resulted in a lower per Chl Pmax value.
Figure 8.
Changes in Pmax and Φ of photosynthesis after an HL → LL shift of the cultures. A, Measurement of the light-saturated rate of photosynthesis in WT (white circles) and zea1 mutant (squares). B, Measurement of the Φ in WT (white circles) and zea1 mutant (squares).
Figure 8B shows the adjustment of the Φ in D. salina WT and zea1 mutant after an HL → LL transition. Φ increased exponentially from a low relative value (Φ = 1) in HL to a high relative value (Φ = 4) after about 2 to 3 h in LL, i.e. a change by a factor of about 4, which underscores the difference between control and photo-inhibited samples. This HL → LL-dependent transition in the value of Φ is also consistent with the repair of photodamaged PSII centers, which now contribute to useful photochemistry, thereby resulting into a greater Φ. (Note that the Φ is independent of the Chl antenna size and remains at the 4 “relative units” level, even though the antenna size of the PSs continues to expand in the 2–6-h interval after the HL → LL shift.) The kinetics of this adjustment in Φ showed a half time of approximately 1 h, consistent with earlier findings on the half time of the PSII repair from photodamage (Sundby et al., 1993; Vasilikiotis and Melis, 1994; Baroli and Melis, 1996; Neidhardt et al., 1998).
The above characteristics and related phenomenology pertaining to the recovery of photosynthesis from photo-inhibition were invariant between WT and the zea1 mutant, suggesting that a constitutive accumulation of Z instead of A, V, and N does not in any substantial way affect repair and recovery of PSII from photo-inhibition.
Kinetics of PSII Photodamage/Recovery and Z Accumulation/Decay
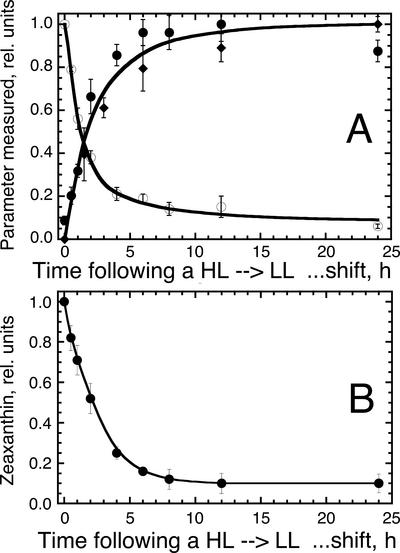
The above results suggested that constitutive accumulation of Z in the thylakoid membrane of the zea1 strain, occurring in the place of A, V, and N, did not bring about an effect on the properties of photo-acclimation, photodamage, or repair of the photosynthetic apparatus from photo-inhibition. The first part of this work, however, suggested parity between relative amount of Z, as measured from the steady-state epoxidation level (Figs. 1C, 2A, and 4B) and the fraction of photodamaged PSII centers in the WT thylakoids (Figs. 2, B and D, and 3A). These results suggested that Z accumulates in direct proportion to the photodamaged PSII reaction centers in the chloroplast thylakoids. To explore this notion with a different experimental approach, light shift experiments were conducted, and the kinetics of V loss and Z accumulation were noted in relation to the kinetics of PSII photodamage measured upon an LL → HL shift of WT D. salina cultures. Figure 9A shows identical kinetics of PSII photodamage (1 − QA), Z accumulation, and V loss occurring in vivo with a half time of about 100 min upon an LL → HL shift of D. salina cultures. These results suggest that photodamage and the prompt disassembly of the PSII holocomplex were accompanied by a V de-epoxidation to Z.
Figure 9.
A, Comparative kinetic analysis of the accumulation of Z (solid circles), accumulation of photodamaged PSII reaction centers (solid diamonds), and loss of V (white circles) after an LL → HL shift of D. salina WT cultures. B, Decay kinetics of Z to V conversion after an HL → LL shift of the D. salina WT cultures.
A quantitative analysis of Car pool levels after such an LL → HL shift was undertaken. After about 8 h following the LL → HL shift, when nearly 90% of the PSII reaction centers had accumulated in a photodamaged state (Fig. 9A), the pool of Z had increased by about 5.2 × 10−16 mol cell−1. Concomitantly, the pool of V decreased by about 4.0 × 10−16 mol cell−1. Thus, there was a 1.2 × 10−16 mol cell−1 Z formed that could not be accounted for by the corresponding loss in V. This persistent lack of quantitative parity between Z accumulation and V loss after an LL → HL shift could be explained by a conversion of β-carotene to Z, occurring upon the disassembly of the photodamaged PSII reaction centers, as proposed by Depka et al. (1998). Consistent with this interpretation, we found a corresponding lowering of the β-carotene pool size, by about 1.4 × 10−16 mol cell−1, after an LL → HL shift (not shown).
If Z is a component of the PSII repair process, as the above results would strongly suggest, then decay of Z (a reversible epoxidation to V) should follow a chloroplast recovery from photo-inhibition. Figure 9B shows the decay kinetics of Z (conversion to V) upon an HL → LL shift of D. salina. The half time for the decay of Z was measured to be about 2 h under these conditions. The repair of the photodamaged PSII was measured to occur with a half time of about 1 h (Fig. 8B), whereas growth of the light-harvesting Chl antenna size after the HL → LL shift took more than 6 h (Fig. 8A; Neidhardt et al., 1998). Therefore, it is likely that a temporal sequence of events, after an HL → LL shift, is first repair of PSII reaction centers followed by Z epoxidation to V. The latter apparently occurs soon after the repair and functional recovery of the photodamaged PSII reaction centers and precedes the build-up of the light-harvesting Chl antenna size.
DISCUSSION
A xanthophyll aberrant mutant (zea1) of D. salina was unable to synthesize any of the epoxy-xanthophylls A, V, and N but constitutively accumulated Z instead. The zea1 mutant did not show any discernible difference from the WT in terms of growth either under LL or HL conditions (Jin et al., 2003). This work provided evidence that WT and zea1 could not be distinguished on the basis of rate or Φ of photosynthesis, efficiency of PSII charge separation, or photo-acclimation characteristics. Constitutive accumulation of Z in zea1 occurred without any changes in total cellular Chl or Car content and without affecting the Chl a/b or PSII/PSI ratio in the thylakoid membrane. WT and zea1 could not be distinguished on the basis of susceptibility to photodamage or recovery from photo-inhibition. However, it was noted that Z in the WT accumulated in parallel with the accumulation of photodamaged PSII centers in the chloroplast thylakoids and decayed in tandem with the chloroplast recovery from photo-inhibition. There was a clear correlation between the reversible xanthophyll cycle and the PSII repair cycle and a lack of correlation between xanthophyll cycle and Chl antenna size in the thylakoid membrane. These results would suggest, therefore, that Z is a component of the PSII repair process (Jahns et al., 2000; Jin et al., 2001). It is proposed that the “photoprotective” mechanism of Z in the chloroplast thylakoids operates after PSII photodamage and disassembly has occurred and before functional recovery and reconstitution of the PSII holocomplex.
Our working hypothesis is that at least a fraction of the Z pool accumulating in the WT confers photoprotection to the disassembled PSII from further and possibly irreversible photobleaching (Jin et al., 2001). This hypothesis is consistent with the observation that V de-epoxidation occurs immediately upon photodamage, and Z accumulation occurs in parallel with the accumulation of photodamaged PSII reaction centers in the chloroplast thylakoids. A prompt disassembly of the PSII holocomplex (Melis, 1991; Aro et al., 1993) and formation of a PSII repair intermediate (Melis, 1999; Yokthongwattana et al., 2001) are known to follow photodamage. Highly photo-active tetrapyrrole pigments are released by the PSII D1/D2 reaction center in the course of reaction center disassembly and D1 degradation and replacement. It could be argued that quenching of tetrapyrrole excitation by Z (Wentworth et al., 2000) is needed at this stage because this repair intermediate stage renders PSII most vulnerable to massive and irreparable photooxidative bleaching. According to this hypothesis, Z might play a role in the photoprotection of the photodamaged and disassembled PSII core, including the D2, CP47, and CP43 Chl proteins. This hypothesis is also consistent with the effect of high photon flux densities on mutants that are deficient in the V de-epoxidase enzyme. Upon photooxidative stress, the npq1 mutant of Arabidopsis was subject to a loss of bulk Chl, bleaching, and/or pronounced lipid peroxidation (Havaux and Niyogi, 1999; Havaux et al., 2000). Similarly (Verhoeven et al., 2001), transgenic tobacco (Nicotiana tabacum) with suppressed Z formation was found to be susceptible to stress-induced photo-inhibition, consistent with the notion of Z being a photoprotective pigment functioning in the PSII repair process. Our proposed hypothesis is also consistent with the role of the Cbr protein, which appears upon photooxidative damage (Fig. 4B) and was reported to involve stabilization of assemblies highly enriched in Z and possibly containing other unbound pigments (Banet et al., 2000).
Thus, Cbr and Z may participate in the PSII repair process and may be critical for the protection of PSII because the latter is in the process of degrading and replacing nonfunctional D1 reaction center proteins (Jin et al., 2001). A return of Z to V in the WT, and a removal of the Cbr protein from the thylakoid membrane, would logically follow the repair of PSII from photodamage and the recovery of the chloroplast from photo-inhibition. Such a mechanism must also operate in the zea1 strain, evidenced by the onset of photodamage upon an LL → HL shift (Fig. 5), accumulation of photo-inhibited PSII centers (Fig. 3A), and parallel accumulation of the Cbr protein (Fig. 4B) in HL. The converse is observed during the repair and recovery of the photosynthetic apparatus upon a HL → LL shift. The only difference (without a functional consequence) in this respect was the constitutive presence of Z and the absence of an irradiance-induced V de-epoxidation in the zea1 strain.
The quantitative replacement of A, V, and N by Z in the zea1 strain is consistent with findings by Bishop et al. (1998) pertaining to the properties of S. obliquus mutants with deletions in Car biosynthesis. They are also consistent with results from Z-accumulating mutants of C. reinhardtii (Polle et al., 2001) and Arabidopsis (Lokstein et al., 2002), demonstrating replacement of at least V and A in the respective LHC-binding sites by Z. The quantitative replacement of A, V, and N by Z would suggest that Z occupies positions that are normally occupied by A, V, and N in the Lhcb and Lhca gene products. However, this remains a hypothesis, and the results in the present work cannot exclude the possibility of vacancy in the former A, V, or N positions and a placement of at least part of the Z pool in a different domain, e.g. the lipid bilayer of the zea1 chloroplast thylakoids. Previous studies have shown that xanthophylls cycle Car are localized within the minor LHC (Bassi et al., 1993; Verhoeven et al., 1999) and/or may be found as free pigment in the lipid matrix of the thylakoid membrane (Tardy and Havaux, 1996; Bassi and Caffarri, 2000). Relevant in this respect are the findings of Havaux and Niyogi (1999) and Havaux et al. (2000), who showed that the npq1 mutant of Arabidopsis, which cannot perform a de-epoxidation of V to Z, is subject to enhanced lipid peroxidation upon photooxidative stress. It is also possible that Z, forming upon de-epoxidation of V during irradiance stress, occupies a thylakoid membrane domain that is different from that occupied by V (Jahns et al., 2001) under physiological conditions. The question of translocation of pigments and of different domain localization for V and Z, dynamically occurring as part of the reversible xanthophyll cycle, has not been thoroughly considered. In this respect, Z forming upon de-epoxidation of V at HL may have substantially different properties from Z that constitutively accumulates in the zea1 strain under LL conditions. Clearly, more research is needed to delineate between these alternatives and to fully elucidate the role of the reversible xanthophyll cycle and the photoprotective role of Z in the thylakoid membrane of photosynthesis.
Why possess a xanthophyll cycle and not simply constitutively retain Z in the pigment bed of photosynthesis if it makes no apparent difference in the performance of the organism? Obviously, the reasons are not totally clear. It is possible that subtle quenching effects do occur under physiological conditions when Z is the only xanthophyll present, e.g. the npq2 lor1 strain of C. reinhardtii (Polle et al., 2001). It could be argued that even subtle quenching effects would tend to give a slight but significant competitive advantage to organisms that possess the xanthophyll cycle because these would benefit from the photoprotection afforded by Z while minimizing the quenching upon Z epoxidation to V under physiological conditions. It is also possible that a constitutive expression of Z slows down specific steps of the PSII repair process, e.g. Figure 5, which might become limiting under certain conditions. Alternatively, one cannot exclude the possibility that, under conditions of irradiance stress combined with additional and fluctuating environmental stresses, an active xanthophyll cycle would confer advantage over a constitutive expression of Z (Niyogi et al., 1998).
In summary, Z accumulation and absence of β,β-epoxy xanthophylls in the zea1 mutant of D. salina did not affect rates of photodamage or cell recovery from photo-inhibition. The acclimation of the photosynthetic apparatus to the level of irradiance was not affected by the constitutive accumulation of Z in the zea1 strain either, as evidenced upon the irradiance-depended adjustment in the amount of the LHCII in WT and zea1 thylakoids. However, results strongly support the notion that Z is a component of the PSII repair process. Z forms in situ upon photodamage and stays in association with the disassembled and photochemically inert PSII-core, until such time when the repair of the affected PSII center permits the return of individual units into the pool of functional PSII. In the WT strain, Z returns to V at the end of the PSII damage and repair cycle. This and other related possibilities on the functional role of Z in chloroplast thylakoids are currently under investigation.
MATERIALS AND METHODS
Algal Strains and Growth Conditions
The unicellular green alga Dunaliella salina Teod. strain 1644 was obtained from the UTEX culture collection (Starr, 1978). The zea1 mutant strain of D. salina was isolated in this laboratory after chemical mutagenesis and screening (Jin et al., 2003). Strains were grown photoautotrophically in hypersaline medium (Pick et al., 1986) in the presence of 25 mm NaHCO3 as a supplemental inorganic carbon source in 1-L Roux Bottles at a light intensity range of 100 to 2,000 μmol photons m−2 s−1. Irradiance was measured with a model LI-185B radiometer (LI-COR, Lincoln, NE). The cultures were shaken to ensure uniform illumination of the cells. Cells were harvested at a density of 2 to 2.5 × 106 cells mL−1. Cell density was monitored via a Neubauer ultraplane hemacytometer (Reichert, Buffalo, NY). To block translation of the chloroplast-encoded D1 protein, lincomycin, an inhibitor of plastidic protein biosynthesis, was added to the D. salina cultures as recently described (Baroli and Melis, 1996).
Pigment Analyses
For pigment determination, cells or thylakoid membranes were extracted in 80% (v/v) acetone, and debris was removed by centrifugation at 10,000g for 3 min. The absorbance of the supernatant was measured with a UV-160U spectrophotometer (Shimadzu, Columbia, MD). The Chl (a and b) concentration of the samples was determined according to Arnon (1949), with equations corrected as in Melis et al. (1987). HPLC analysis was done as recently described (Jin et al., 2001).
Thylakoid Membrane Isolation
Cells were harvested by centrifugation at 1,000g for 3 min at 4°C. Samples were diluted with sonication buffer containing 100 mm Tris-HCl (pH 6.8), 10 mm NaCl, 5 mm MgCl2, 0.2% (w/v) polyvinylpyrrolidone 40, 0.2% (w/v) sodium ascorbate, 1 mm aminocaproic acid, 1 mm aminobenzamidine, and 100 μm phenylmethylsulfonylfluoride. Cells were broken by sonication in a Branson 200 Cell Disruptor (Branson Ultrasonics Corporation, Danbury, CT) operated at 4°C for 30 s (pulse mode, 50% duty cycle, output power 5). Unbroken cells and starch grains were removed by centrifugation at 3,000g for 4 min at 4°C. The thylakoid membranes were collected by centrifugation of the supernatant at 75,000g for 30 min at 4°C. The thylakoid membrane pellet was resuspended in a buffer containing 250 mm Tris-HCl (pH 6.8), 20% (w/v) glycerol, 7% (w/v) SDS, and 2 m urea. Solubilization of thylakoid proteins was carried out for 30 min at room temperature. Samples were centrifuged in a microfuge for 5 min to remove unsolubilized material, β-mercaptoethanol was added to yield a final concentration of 10% (v/v), and the samples were stored at −80°C.
SDS-PAGE and Western-Blot Analysis
Samples were brought to room temperature before loading for electrophoresis and diluted accordingly to yield equal Chl concentrations. Gel lanes were loaded with an equal amount of Chl per lane. SDS-PAGE analysis was carried out according to Laemmli (1970). Gels were stained with 0.1% (w/v) Coomassie Brilliant Blue R for protein visualization. Identification of thylakoid membrane proteins was accomplished with specific polyclonal antibodies raised in rabbit in this laboratory against the isolated reaction center D1 protein and the LHC-II apoproteins (Kim et al., 1993). Anti-Cbr antibody was kindly provided by Dr. Ada Zamir (Weizmann Institute of Science, Rehovot, Israel). Immunoreactive bands were detected either by enhanced chemiluminesence employing horseradish peroxidase-conjugated secondary antibodies (Amersham Pharmacia Biotech, Piscataway, NJ) or by cross-reaction with the antibodies was detected by a chromogenic reaction with anti-IgG secondary antibodies conjugated with alkaline phosphatase (Bio-Rad, Hercules, CA). Immunoblots were scanned with an HP Scan Jet 5300C optical scanner (Hewlett-Packard, Palo Alto, CA) connected to a MacIntosh/G3 computer (Apple Computer, Cupertino, CA). The NIH Image version 1.6 program (National Institutes of Health, Bethesda, MD) was used for the deconvolution and quantitation of the bands.
Spectrophotometric Analyses
For spectrophotometric measurements, the thylakoid membrane pellet was resuspended in a buffer containing 50 mm Tricine (pH 7.8), 10 mm NaCl, and 5 mm MgCl2. The amount of functional PSI and PSII reaction centers was estimated from the light-minus-dark absorbance difference measurements of P700 photooxidation and QA photoreduction, respectively (Melis, 1989).
Oxygen Evolution Measurements
Oxygen evolution of the cultures was measured at 26°C with a Clark-type oxygen electrode illuminated with a slide projector lamp. Yellow actinic excitation was provided by a CS 3–69 cut-off filter (Corning, Corning, NY) in combination with an Ealing 35–5453 VIQ5–8 filter (Ealing, Inc., Rocklin, CA). An aliquot of 5 mL of cell suspension (2 μm Chl) was transferred to the oxygen electrode chamber. To ensure that oxygen evolution was not limited by the carbon source available to the cells, 100 μL of 0.5 m sodium bicarbonate solution (pH 7.4) was added to the suspension before the oxygen evolution measurements. The light saturation curve of photosynthesis was obtained with the oxygen electrode, beginning with the registration of dark respiration in the cell suspension, and followed by measurements of the rate of oxygen evolution at sequentially increasing irradiance levels. Registration and the rate (slope) of oxygen evolution at each light intensity step were recorded for about 2 min. The photon use efficiency of the cells was calculated from the initial slope of the light saturation curves of photosynthesis.
Statistical Analyses
Results shown are the average of three to five independent experiments ± se.
ACKNOWLEDGMENTS
We wish to thank Dr. Kris Niyogi for helpful suggestions and for making available HPLC equipment and Dr. Ada Zamir for providing antibodies against the Cbr protein.
Footnotes
This work was supported by the U.S. Department of Agriculture-National Research Initiative (grant no. FD–2002–35100–12278–MELI–08/04).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.019620.
LITERATURE CITED
- Anderson JM. Photoregulation of the composition, function and structure of thylakoid membranes. Annu Rev Plant Physiol. 1986;37:93–136. [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts: polyphenol-oxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro EM, Virgin I, Andersson B. Photoinhibition of photosystem II: Inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- Banet G, Pick U, Zamir A. Light-harvesting complex II pigments and proteins in association with Cbr, a homologue of higher-plant early light-inducible proteins in the unicellular green alga Dunaliella. Planta. 2000;210:947–955. doi: 10.1007/s004250050702. [DOI] [PubMed] [Google Scholar]
- Baroli I, Melis A. Photoinhibition and repair in Dunaliella salina acclimated to different growth irradiances. Planta. 1996;198:640–646. doi: 10.1007/BF00262653. [DOI] [PubMed] [Google Scholar]
- Bassi R, Caffarri S. Lhc proteins and the regulation of photosynthetic light harvesting function by xanthophylls. Photosynth Res. 2000;64:243–256. doi: 10.1023/A:1006409506272. [DOI] [PubMed] [Google Scholar]
- Bassi R, Pineau B, Dainese P, Marquardt J. Carotenoid-binding proteins of photosystem II. Eur J Biochem. 1993;212:297–303. doi: 10.1111/j.1432-1033.1993.tb17662.x. [DOI] [PubMed] [Google Scholar]
- Bishop NI, Bulga B, Senger H. Photosynthetic capacity and quantum requirement of three secondary mutants of Scenedesmus obliquus with deletions in carotenoid biosynthesis. Bot Acta. 1998;111:231–235. [Google Scholar]
- Bugos RC, Yamamoto HY. Molecular cloning of violaxanthin de-epoxidase from romaine lettuce and expression in Escherichia coli. Proc Natl Acad Sci USA. 1996;93:6320–6325. doi: 10.1073/pnas.93.13.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig B, Winter K, Krüger A, Czygan FC. Photoinhibition and zeaxanthin formation in intact leaves: a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol. 1987;84:218–224. doi: 10.1104/pp.84.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B. Carotenoids and photoprotection in plants: a role for the xanthophyll zeaxanthin. Biochim Biophys Acta. 1990;1020:1–24. [Google Scholar]
- Demmig-Adams B, Adams WWIII. Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:599–626. [Google Scholar]
- Demmig-Adams B, Gilmore AM, Adams WW. Carotenoids: III. In vivo function of carotenoids in higher plants. FASEB J. 1996;10:403–412. doi: 10.1096/fasebj.10.4.8647339. [DOI] [PubMed] [Google Scholar]
- Depka B, Jahns P, Trebst A. beta-carotene to zeaxanthin conversion in the rapid turnover of the D1 protein of photosystem II. FEBS Lett. 1998;424:267–270. doi: 10.1016/s0014-5793(98)00188-4. [DOI] [PubMed] [Google Scholar]
- Gilmore AM. Mechanistic aspects of xanthophyll cycle-dependent photoprotection in higher plant chloroplasts and leaves. Physiol Plant. 1997;99:197–209. [Google Scholar]
- Gilmore AM, Hazlett TL, Govindjee Xanthophyll cycle–dependent quenching of photosystem II chlorophyll a fluorescence: formation of a quenching complex with a short fluorescence lifetime. Proc Natl Acad Sci USA. 1995;92:2273–2277. doi: 10.1073/pnas.92.6.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AM, Yamamoto HY. Linear models relating xanthophylls and lumen acidity to non-photochemical fluorescence quenching: evidence that antheraxanthin explains zeaxanthin-independent quenching. Photosynth Res. 1993;35:67–78. doi: 10.1007/BF02185412. [DOI] [PubMed] [Google Scholar]
- Goss R, Böhme K, Wilhelm C. The xanthophyll cycle of Mantoniella squamata converts violaxanthin into antheraxanthin but not to zeaxanthin: consequences for the mechanism of enhanced non-photochemical energy dissipation. Planta. 1998;205:613–621. [Google Scholar]
- Green BR, Kühlbrandt W. Sequence conservation of light-harvesting and stress-response proteins in relation to the three-dimensional molecular structure of LHCII. Photosynth Res. 1995;44:139–148. doi: 10.1007/BF00018304. [DOI] [PubMed] [Google Scholar]
- Guenther JE, Melis A. The physiological significance of photosystem II heterogeneity in chloroplasts. Photosynth Res. 1990;23:105–110. doi: 10.1007/BF00030070. [DOI] [PubMed] [Google Scholar]
- Hager A. The reversible, light-induced conversions of xanthophylls in the chloroplast. In: Czygan FC, editor. Pigments in Plants. Stuttgart: Fischer; 1980. pp. 57–79. [Google Scholar]
- Havaux M, Bonfils JP, Lutz C, Niyogi KK. Photodamage of the photosynthetic apparatus and its dependence on the leaf developmental stage in the npq1 Arabidopsis mutant deficient in the xanthophyll cycle enzyme violaxanthin de-epoxidase. Plant Physiol. 2000;124:273–284. doi: 10.1104/pp.124.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M, Niyogi KK. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Natl Acad Sci USA. 1999;96:8762–8767. doi: 10.1073/pnas.96.15.8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- Huner NPA, Oquist G, Sarhan F. Energy balance and acclimation to light and cold. Trends Plant Sci. 1998;3:224–230. [Google Scholar]
- Hurry V, Andersson JM, Chow WS, Osmond CB. Accumulation of zeaxanthin in abscicic acid-deficient mutants of Arabidopsis does not affect chlorophyll quenching or sensitivity to photoinhibition in vivo. Plant Physiol. 1997;113:639–648. doi: 10.1104/pp.113.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahns P, Depka B, Trebst A. Xanthophyll cycle mutants from Chlamydomonas reinhardtii indicate a role for zeaxanthin in the D1 protein turnover. Plant Physiol Biochem. 2000;38:373–376. [Google Scholar]
- Jahns P, Wehner A, Paulsen H, Hobe S. De-epoxidation of violaxanthin after reconstitution into different carotenoid binding sites of light-harvesting complex II. J Biol Chem. 2001;276:22154–22159. doi: 10.1074/jbc.M102147200. [DOI] [PubMed] [Google Scholar]
- Jin ES, Feth B, Melis A. A mutant of the green alga Dunaliella salina constitutively accumulates zeaxanthin under all growth conditions. Biotechnol Bioeng. 2003;81:115–124. doi: 10.1002/bit.10459. [DOI] [PubMed] [Google Scholar]
- Jin ES, Polle JWE, Melis A. Involvement of zeaxanthin and of the Cbr protein in the repair of photosystem-II from photoinhibition in the green alga Dunaliella salina. Biochim Biophys Acta. 2001;1506:244–259. doi: 10.1016/s0005-2728(01)00223-7. [DOI] [PubMed] [Google Scholar]
- Kim JH, Nemson JA, Melis A. Photosystem II reaction center damage and repair in D. salina green alga: analysis under physiological and irradiance-stress conditions. Plant Physiol. 1993;103:181–189. doi: 10.1104/pp.103.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lers A, Levy H, Zamir A. Co-regulation of a gene homologous to early light-induced genes in higher plants and β-carotene biosynthesis in the alga Dunaliella baradawil. J Biol Chem. 1991;266:13698–13705. [PubMed] [Google Scholar]
- Levy H, Gokhman I, Zamir A. Regulation and light harvesting complex II association of Dunaliella protein homologous to early light-induced proteins in higher plants. J Biol Chem. 1992;267:18831–18836. [PubMed] [Google Scholar]
- Levy H, Tal T, Shaish A, Zamir A. Cbr, an algal homoloug of plant early light-induced proteins, is a putative zeaxanthin binding protein. J Biol Chem. 1993;268:20892–20896. [PubMed] [Google Scholar]
- Lokstein H, Tian L, Polle JEW, DellaPenna D. Xanthophyll biosynthetic mutants of Arabidopsis thaliana: altered nonphotochemical quenching of chlorophyll fluorescence is due to changes in photosystem II antenna size and stability. Biochim Biophys Acta. 2002;1553:309–319. doi: 10.1016/s0005-2728(02)00184-6. [DOI] [PubMed] [Google Scholar]
- Marin E, Nussaume L, Quesada A, Gonneau M, Sotta B, Hugueney P, Frey A, Marion-Poll A. Molecular identification of zeaxanthin epoxidase of Nicotiana plumbaginifolia, a gene involved in absicic acid biosynthesis and corresponding to ABA locus of Arabidopsis thaliana. EMBO J. 1996;15:2331–2342. [PMC free article] [PubMed] [Google Scholar]
- Mattoo AK, Edelman M. Intramembrane translocation and posttranslational palmitoylation of the chloroplast 32-kDa herbicide-binding protein. Proc Natl Acad Sci USA. 1987;84:1497–1501. doi: 10.1073/pnas.84.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A. Spectroscopic methods in photosynthesis: photosystem stoichiometry and chlorophyll antenna size. Phil Trans R Soc Lond B. 1989;323:397–409. [Google Scholar]
- Melis A. Dynamics of photosynthetic membrane composition and function. Biochim Biophys Acta. 1991;1058:87–106. [Google Scholar]
- Melis A. Photosystem II damage and repair cycle in chloroplast: what modulates the rate of photodamage in vivo? Trends Plant Sci. 1999;4:130–135. doi: 10.1016/s1360-1385(99)01387-4. [DOI] [PubMed] [Google Scholar]
- Melis A, Nemson JA. Characterization of a 160 kD photosystem II reaction center complex isolated from photoinhibited Dunaliella salina thylakoids. Photosynth Res. 1995;46:207–211. doi: 10.1007/BF00020432. [DOI] [PubMed] [Google Scholar]
- Melis A, Spangfort M, Andersson B. Light-absorption and electron-transport balance between photosystem II and photosystem I in spinach chloroplasts. Photochem Photobiol. 1987;45:129–136. [Google Scholar]
- Neidhardt J, Benemann JR, Zhang L, Melis A. Photosystem-II repair and chloroplast recovery from irradiance stress: relationship between chronic photoinhibition, light-harvesting chlorophyll antenna size and photosynthetic productivity in Dunaliella salina (green algae) Photosynth Res. 1998;56:175–184. [Google Scholar]
- Niyogi KK. Photoprotection revisited: Genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:333–359. doi: 10.1146/annurev.arplant.50.1.333. [DOI] [PubMed] [Google Scholar]
- Niyogi KK, Björkman O, Grossman AR. Chlamydomonas xanthophyll cycle mutants identified by video imaging of chlorophyll fluorescence quenching. Plant Cell. 1997a;9:1369–1380. doi: 10.1105/tpc.9.8.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Björkman O, Grossman AR. The roles of specific xanthophylls in photoprotection. Proc Natl Acad Sci USA. 1997b;94:14162–14167. doi: 10.1073/pnas.94.25.14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Grossman AR, Bjorkman O. Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell. 1998;10:1121–1134. doi: 10.1105/tpc.10.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Shih C, Chow WS, Pogson BJ, DellaPenna D, Björkman O. Photoprotection in a zeaxanthin- and lutein-deficient double mutant of Arabidopsis. Photosynth Res. 2001;67:139–145. doi: 10.1023/A:1010661102365. [DOI] [PubMed] [Google Scholar]
- Pick U, Karni L, Avron M. Determination of ion content and ion fluxes in the halotolerant alga Dunaliella salina. Plant Physiol. 1986;81:92–96. doi: 10.1104/pp.81.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polle JWE, Niyogi KK, Melis A. Absence of lutein, violaxanthin and neoxanthin affects the functional chlorophyll antenna size of photosystem-II but not that of photosystem-I in the green alga Chlamydomonas reinhardtii. Plant Cell Physiol. 2001;42:482–491. doi: 10.1093/pcp/pce058. [DOI] [PubMed] [Google Scholar]
- Powles SB. Photoinhibition of photosynthesis induced by visible light. Annu Rev Plant Physiol. 1984;35:15–44. [Google Scholar]
- Powles SB, Critchley C. Effect of light intensity during the growth on photoinhibition of intact attached bean leaflets. Plant Physiol. 1980;65:1181–1187. doi: 10.1104/pp.65.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock CD, Bowlby NR, Hoffmann-Benning S, Zeevaart JAD. The aba mutant of Arabidopsis thaliana (L.) Heynh. has reduced chlorophyll fluorescence yields and reduced thylakoid stacking. Plant Physiol. 1992;100:1796–1801. doi: 10.1104/pp.100.4.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock CD, Zeevaart JAD. The aba mutant of Arabidopsis thaliana is impaired in epoxy-carotenoid biosynthesis. Proc Natl Acad Sci USA. 1991;88:7496–7499. doi: 10.1073/pnas.88.17.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BM, Morrissey PJ, Guenther JE, Nemson JA, Harrison MA, Allen JF, Melis A. Response of the photosynthetic apparatus in Dunaliella salina (green algae) to irradiance stress. Plant Physiol. 1990;93:1433–1440. doi: 10.1104/pp.93.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr RC. The culture collection of algae at the University of Texas, Austin. J Phycol. 1978;14:47–100. [Google Scholar]
- Sundby C, McCaffery S, Anderson JM. Turnover of the photosystem II D1 protein in higher plants under photoinhibitory and nonphotoinhibitory irradiance. J Biol Chem. 1993;268:25476–25482. [PubMed] [Google Scholar]
- Tanaka A, Melis A. Irradiance-dependent changes in the size and composition of the chlorophyll a-b light-harvesting complex in the green alga Dunaliella salina. Plant Cell Physiol. 1997;38:17–24. [Google Scholar]
- Tardy F, Havaux M. Photosynthesis, chlorophyll fluorescence, light-harvesting system and photoinhibition resistance of a zeaxanthin-accumulating mutant of Arabidopsis thaliana. J Photochem Photobiol B. 1996;34:87–94. doi: 10.1016/1011-1344(95)07272-1. [DOI] [PubMed] [Google Scholar]
- Telfer A, Dhami S, Bishop SM, Phillips D, Barber J. Beta-carotene quenches singlet oxygen formed by isolated photosystem II reaction centers. Biochemistry. 1994;33:14469–14474. doi: 10.1021/bi00252a013. [DOI] [PubMed] [Google Scholar]
- Vass I, Styring S, Hundal T, Koivuniemi A, Aro A-M, Andersson B. Reversible and irreversible intermediates during photoinhibition of photosystem II: stable reduced QA species promote chlorophyll triplet formation. Proc Natl Acad Sci USA. 1992;89:1408–1412. doi: 10.1073/pnas.89.4.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilikiotis C, Melis A. Photosystem-II reaction center damage and repair cycle: chloroplast acclimation strategy to irradiance stress. Proc Natl Acad Sci USA. 1994;91:7222–7226. doi: 10.1073/pnas.91.15.7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven AS, Adams IIIWW, Demmig-Adams B, Croce R, Bassi R. Xanthophyll cycle pigment localization and dynamics during exposure to low temperatures and light stress in Vinca major. Plant Physiol. 1999;120:727–737. doi: 10.1104/pp.120.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven AS, Bugos RC, Yamamoto HY. Transgenic tobacco with suppressed zeaxanthin formation is susceptible to stress-induced photoinhibition. Photosynth Res. 2001;67:27–39. doi: 10.1023/A:1010684327864. [DOI] [PubMed] [Google Scholar]
- Wentworth M, Ruban A, Horton P. Chlorophyll fluorescence quenching in isolated light harvesting complexes induced by zeaxanthin. FEBS Lett. 2000;471:741–745. doi: 10.1016/s0014-5793(00)01369-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto HY. Biochemistry of the violaxanthin cycle in higher plants. Pure Appl Chem. 1979;51:639–648. [Google Scholar]
- Yokthongwattana K, Chrost B, Behrman S, Casper-Lindley C, Melis A. Photosystem II damage and repair cycle in the green alga Dunaliella salina: involvement of a chloroplast-localized HSP70. Plant Cell Physiol. 2001;42:1389–1397. doi: 10.1093/pcp/pce179. [DOI] [PubMed] [Google Scholar]