Abstract
Arabidopsis plants were transformed with acyl carrier protein (ACP)-4 in antisense conformation driven by the cauliflower mosaic virus 35S promoter. It was hypothesized that reduction of ACP4 in leaf tissue would result in a reduction in lipid biosynthesis and, in addition, affect fatty acid composition and leaf physiology. Several transgenic lines have been generated with reduced ACP4 protein in leaf tissue. Dramatic reductions in ACP4 resulted in a reduction of leaf lipid content (22%–60%) based on fresh leaf weight and a bleached appearance and reduced photosynthetic efficiency. In addition, a decrease in 16:3 as a percentage of the total fatty acid composition was noted. There were no changes in leaf lipid class distribution; however, there was a decrease in the relative amount of 16:3 in monogalactosyldiacylglycerol. These results suggest that ACP4 plays a major role in the biosynthesis of fatty acids for chloroplast membrane development. Alterations in the ACP isoform profile of Arabidopsis leaf also appear to alter the flow of fatty acids between the prokaryotic and eukaryotic pathways for assembly of galactolipids. However, it has not yet been determined if the changes in fatty acid composition are due to changes in the profile of ACP isoforms, or if they are actually a reaction to a reduction in fatty acid precursors.
In higher plants, fatty acids have many diverse and important functions. They are critical components of membrane lipids and cuticular waxes in addition to being precursors to several signaling and defense compounds such as jasmonate (Somerville et al., 2000). In many plants, especially oilseeds, fatty acids are also stored in triacylglycerides as a concentrated energy source for use during seed germination. De novo synthesis of fatty acids in plants takes place primarily in plastids (Ohlrogge et al., 1979). The pathways for plant fatty acid and lipid biosynthesis are well elucidated (for review, see Harwood, 1996). However, little is known about how these pathways are regulated.
Acyl carrier protein (ACP) is a critical cofactor for fatty acid biosynthesis in higher plants. This small (9 kD) acidic protein carries growing acyl chains through the various enzymatic steps in fatty acid biosynthesis. All higher plants that have been studied contain several isoforms of ACP, some of which are expressed constitutively (in all tissues) and others that are expressed in a tissue-specific manner (Battey and Ohlrogge, 1990). Although ACP isoforms have been characterized in various plants, little is known about the functional role of these isoforms in plant lipid and fatty acid biosynthesis or why all plants have more than one isoform. One of the goals of the current research is to further understand the role of ACP isoforms in fatty acid biosynthesis, particularly their role in determining fatty acid content and composition of plant lipids. To reach this goal, in vivo manipulations of Arabidopsis ACP isoforms are being conducted through sense and antisense expression.
Arabidopsis has five isoforms of plastidial ACP (Mekhedov et al., 2000) and at least one mitochondrial form (Shintani and Ohlrogge, 1994). ACP1 (product of gene Acl1.1, GenBank accession no. X13708) was first described by Post-Beittenmiller et al. (1989a). It is expressed in leaf, root, and seed tissue (Hloušek-Radojčić et al., 1992), but it is more highly expressed in seed tissue than leaf tissue. Mature ACP2 and ACP3 (Acl1.2 and Acl1.3, GenBank accession nos. X57698 and X57699) differ only by one amino acid (Lamppa and Jacks, 1991) and are also expressed constitutively (Hloušek-Radojčić et al., 1992). The most prominent ACP isoform in leaf tissue, which has also been referred to as the leaf major isoform (Shintani, 1996), is ACP4 (Acl1.4, GenBank accession no. AY084704). The fifth isoform has not been characterized but appears to be a seed-specific isoform (Hloušek-Radojčić et al., 1992).
In a study of the regulation of ACP isoforms in Arabidopsis, Bonaventure and Ohlrogge (2002) found that ACP4 appeared to be regulated in a similar manner to genes involved in photosynthesis, such as ferredoxin A and Rubisco, whereas ACP2 and ACP3 seemed to be more regulated by factors influencing growth. ACP4 mRNA levels in leaf tissue were increased by light, whereas ACP2 and ACP3 mRNA levels remained at a steady state after leaves were illuminated. In addition, mRNA levels for both ACP4 and Rubisco1A small subunit decreased in cells kept in the dark on Suc-containing media, whereas these conditions resulted in a 2.5-fold increase in ACP2 and ACP3 mRNA levels. Their results suggest that ACP isoforms are differentially regulated to meet the demand for fatty acid biosynthesis for different processes and that perhaps ACP4 could play a major role in the synthesis of fatty acids for photosynthetic membrane lipids in growing tissue. In the present study, reduction of ACP4 in leaf tissue through antisense expression resulted in a decrease in total leaf lipid content and an alteration in the fatty acid composition of leaf lipids. In addition, reduction in lipid content and/or alterations in fatty acid composition compromised plant health and photosynthesis.
RESULTS
Generation and Phenotype of Antisense ACP4 Transgenic Plants
Thirty-two independent kanamycin-resistant plants were selected by screening T1 seeds generated from transformation of Arabidopsis with antisense ACP4. These plants will henceforth be referred to as leaf major isoform antisense. The general appearance of the plants was compared with that of wild-type plants grown side by side at 4 weeks after germination. Of the 32 T1 transgenic plants, 20 exhibited a bleached appearance (Fig. 1). This phenotype varied in severity from plant to plant. In some plants, only the outer edges of leaves were bleached, whereas others had a few leaves that were bleached throughout along with green leaves. Certain plants were bleached in appearance throughout all green tissues; the severity of bleaching ranged from slightly less green than the wild-type plants to very yellow. Many of the bleached transgenic plants were also smaller in size compared with wild-type and nonbleached transgenic plants. In addition, characteristics such as delayed bolting and shorter bolts were noted in the bleached transgenic plants. All of the transgenic plants produced seeds. However, some severely bleached plants were very small and only produced approximately 10% of the normal number of seeds.
Figure 1.
Appearance of the T1 generation antisense ACP4 (LMIA) transgenic plants.
Seeds (T2) from 12 of the 20 bleached transgenic plants were screened on Murashige and Skoog agar with kanamycin. Four resistant seedlings from each line were transferred to soil-less mix and put in a growth chamber with regulated temperature, light, and humidity. Surprisingly, the bleached phenotype seen in the T1 plants did not carry through to all of the T2 plants. In some cases, the bleached phenotype was noted in one or two of the four T2 plants. However, in some cases, none of the four progeny from a bleached parent had a bleached appearance. Only in a few of the lines was a bleached appearance noted in all four T2 progeny. Because only approximately 25 seeds were plated per line, segregation analysis could not be determined. However, our observation with the small number of seeds that were plated was that most of the lines did not segregate in a Mendelian fashion expected for a single insert. Based on further western and lipid analysis of leaf tissue from T2 progeny, the bleached phenotype seemed to segregate together with reduced ACP4 protein and reduced leaf lipid content. Therefore, it is suspected that in at least some of the plants, multiple copies of the transgene exist and that all of the copies must be present to achieve reduced ACP4 and the associated phenotype. However, the loss of phenotype in some plants could also be due to differences in environmental conditions (mainly light intensity) during growth of T1 versus T2 plants or to transgene instability.
Western Analysis of ACP Proteins
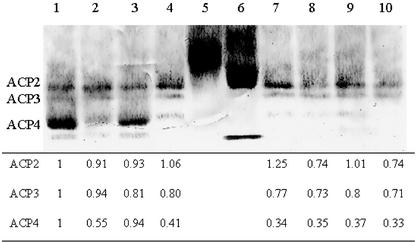
Western-blot analysis of protein from LMIA leaf tissue indicates that 0.5- to 4-fold reductions in ACP4 were achieved (Fig. 2). The ACP4 band was barely visible in extracts with 3- to 4-fold reductions as determined by measuring the mean intensity level. Levels of the other leaf ACP isoforms, ACP2 and ACP3, were not altered except in those transgenic plants where there was a 3- to 4-fold reduction in ACP4. In those plants, ACP2 and ACP3 levels were reduced by 25% to 50%. It is possible that these reductions are due to the antisense ACP4 mRNA; ACP4 is 50% similar to ACP2 and ACP3 at the cDNA level. Otherwise, these reductions may be a response to the reduction in ACP4 or a secondary response to low lipid levels. In addition, because the transgenic plants were chlorotic in many cases, and chloroplast levels are often correlated with chlorophyll content, the idea that the apparent reduction in ACP2 and ACP3 levels may actually be reflecting lower chloroplast levels cannot be discounted. There was a visual correlation between the severity of the bleached phenotype and the decrease in ACP4 protein. Even leaves within the same plant showed variability in the reduction of ACP4. This was confirmed by western-blot analysis; when protein extracts from a bleached leaf were run on a gel next to protein extracts from a less bleached leaf from the same plant, there was a difference in the reduction of ACP4 between the two leaf protein extracts (data not shown).
Figure 2.
Western blot of ACP proteins in Arabidopsis whole-leaf tissue. Lane 1, Wild type; lanes 2 to 4 and 7 to 10, T1 generation LMIA transgenic plants; lane 5, ACP-1 standard; lane 6, ACP-2 standard. The table underneath the blot displays the levels of ACP2, ACP3, and ACP4 relative to levels in wild type. Forty microliters of leaf protein extract was loaded in each lane of a native PAGE gel. Electrophoresis and blotting conditions are as described in “Materials and Methods.”
Lipid Analysis of LMIA Leaf Tissue
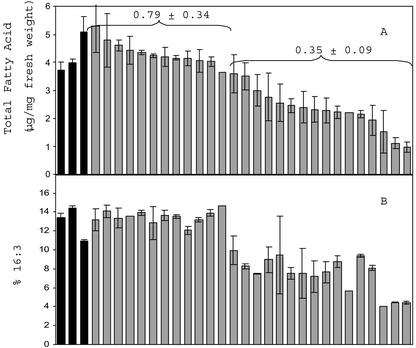
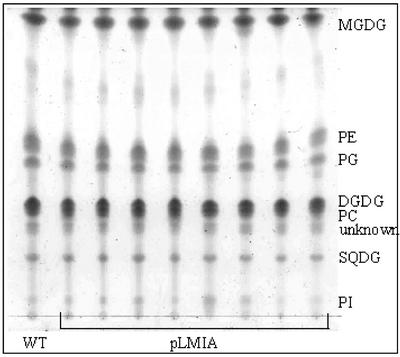
Transgenic plants with bleached phenotype and reduced ACP4 also had lower lipid content (22%–68% lower lipid by fresh weight) compared with wild-type plants (Fig. 3A). Again, the plants that had leaves with the low lipid content were also bleached in appearance. This suggests that the reduction in leaf lipid content is due to reductions in ACP levels. Those transgenic plants with reduced lipid content also had lower 16:3 as a percentage of the total fatty acids (Fig. 3B) compared with wild-type leaves. Because 16:3 was lower in the transgenic plants with reduced lipid, we hypothesized that there may be a decrease in monogalactosyldiacylglycerol (MGDG) in lipids from these plants. However, when an equal amount of total lipid was spotted and run on a thin-layer chromatography (TLC) plate, there were no dramatically visible alterations in polar leaf lipid classes of transgenic plants compared with wild-type plants when visualized with iodine vapor (Fig. 4). To further investigate the reason for the lower 16:3 content in leaf lipids of transgenic plants, lipids were again run on TLC plates. Portions of the silica in each lane corresponding to known lipid standards were scraped from the plates, and the fatty acid composition of each lipid was analyzed. Because most of the 16:3 in Arabidopsis leaf tissue is found on the sn-2 position of MGDG, it was of particular interest to determine the amount of 16:3 in MGDG from LMIA plants (Table I). MGDG content was, on average, calculated to be lower in transgenic plants compared with wild-type plants, but it was not found to be statistically lower due to the high variation of measurement. The variability is directly related to the high variability in recovery of the lipid classes from the silica. To confirm that the ratios of polar leaf lipids were not different between wild-type and LMIA plants, lipid extracts from wild-type and LMIA-25 leaf tissue were analyzed by HPLC. The HPLC method that was used sufficiently separated MGDG, digalactosyldiacylglycerol (DGDG), sulfaquinovosyldiacylglycerol, phosphatidylethanolamine, and phosphatidylglycerol (PG). However, PI could not be separated from PG; therefore, it is reported as part of PG. The variation in analysis by HPLC was much lower (Table II) than the TLC analysis. There was very little difference in the polar lipid profile between wild-type and LMIA-25 leaf lipids even though lipid content and chlorophyll content of LMIA-25 leaf tissue was lower. This confirms visual results from TLC plates (Fig. 4) and the results from TLC analysis of polar leaf lipids. Although there was not a significant decrease in the relative percentage of MGDG or any of the other polar lipids from LMIA plants, there was a reduction of the percentage of 16:3 in MGDG with a concomitant increase in 18:3 in MGDG from LMIA leaf tissue. In addition, there was an overall increase in the C18:C16 ratio in LMIA plants.
Figure 3.
Total fatty acid content (A) and percentage 16:3 (B) in wild-type (black bars) and T1 generation LMIA (gray bars) leaf tissue. The value displayed above the bars represents the average ± sd of ACP4 levels (relative to wild-type levels) for plants within each group (separated by parentheses) that were analyzed by western blot. From the first group, all 12 were analyzed by western blot; from the second group, 13 of 15 of the plants were analyzed by western blot. Leaf lipids were extracted and quantitated by gas chromatography (GC) as described in “Materials and Methods.” Bars represent the average ± sd of two leaves sampled from each plant. Plants were grown in a single 36-well flat and were sampled at the same time.
Figure 4.
TLC of polar leaf lipids from wild type (WT) and T2 generation LMIA transgenic plants. Each lane contains 100 μg of total lipid. Solvent system consisted of chloroform:methanol:acetic acid:water (170:30:20:7 [v/v]). PE, Phosphatidylethanolamine; PC, phosphatidylcholine; SQDG, sulfaquinovosyldiacylglycerol; PI, phosphatidylinositol.
Table I.
Analysis of total leaf lipids and MGDG in wild-type and T2 generation LMIA leaf tissue
Lipids were extracted from 100 mg of leaf tissue, and a portion was removed and derivatized for analysis of total lipid content as described in “Materials and Methods.” Lipids were spotted on NH4SO4-impregnated silica gel plates and developed in acetone:toluene:water (91:30:8 [v/v]). Spots corresponding to known lipid standards were scraped from the plates and derivatized after addition of appropriate amount of standard. Fatty acids were analyzed by GC as described in the text.
| Plant Line | Wild Type (n = 4)a | LMIA-3 (n = 3) | LMIA-16 (n = 3) | LMIA-17 (n = 3) | LMIA-18 (n = 2) | LMIA-25 (n = 3) | Average LMIA |
|---|---|---|---|---|---|---|---|
| Leaf lipid content (μg mg fresh wt-1) | 5.1 ± 0.8 | 3.7 ± 0.5b | 3.7 ± 0.9b | 3.2 ± 0.1b | 3.3 ± 0.8b | 4.0 ± 0.6 | 3.6 ± 0.6b |
| % 16:3 (mol % of total) | 12.0 ± 0.7 | 7.7 ± 1.2b | 8.4 ± 2.8b | 7.0 ± 2.2b | 7.9 ± 2.6b | 6.5 ± 1.4b | 7.5 ± 1.9b |
| C18:C16c | 2.9 ± 0.1 | 3.5 ± 0.2b | 3.6 ± 0.5b | 3.9 ± 0.3b | 3.8 ± 0.5b | 4.1 ± 0.1b | 3.8 ± 0.4b |
| MGDG (% total leaf lipids) | 53.7 ± 10.1 | 53.3 ± 9.5 | 41.4 ± 3.3 | 49.3 ± 19.5 | 30.2 ± 0.3b | 36.9 ± 10.5 | 43.1 ± 12.6 |
| % 16:3 in MGDG | 27.4 ± 1.3 | 18.1 ± 4.3b | 19.4 ± 3.3b | 14.6 ± 1.7b | 18.1 ± 5.1b | 16.6 ± 1.9b | 17.3 ± 3.2b |
n = No. of plants sampled from each line. b Significantly different from wild-type values (P < 0.05). c Ratio of C18 to C16 fatty acids in total leaf lipid extract.
Table II.
Analysis of polar leaf lipids by HPLCa
| Lipid Class | Wild Type (n = 3)b | LMIA-25 (n = 3) |
|---|---|---|
| MGDG | 42.3 ± 0.4 | 41.0 ± 0.5 |
| DGDG | 12.6 ± 0.2 | 12.5 ± 0.8 |
| PG | 12.3 ± 0.3 | 12.8 ± 0.8 |
| Phosphatidylethanolamine | 11.6 ± 0.1 | 11.4 ± 0.2 |
| Phosphatidylcholine | 18.5 ± 0.3 | 19.2 ± 0.5 |
| Sulfaquinovosyldiacylglycerol | 2.5 ± 0.1 | 3.1 ± 0.3 |
| Lipid content (μg mg-1 fresh leaf tissue) | 2.9 ± 0.1 | 1.7 ± 0.3 |
| Total chlorophyll (μg mg-1 leaf tissue) | 1.53 ± 0.2 | 1.06 ± 0.28 |
Values for polar leaf lipids are reported as the average (±SD) percentage of the total lipids. b n = No. of plants. Lipids were extracted from 100 mg of leaf tissue from 4-week-old plants that were selected on Murashige and Skoog agar and transferred to soil after 10 d. The transgenic plants were from the second generation (T2). Portions were removed for determination of lipid content by GC as described in “Materials and Methods.” Approximately 10 μg of lipids was injected onto the HPLC column. The conditions for separation are also described in “Materials and Methods.” Each sample was injected twice. Chlorophyll analysis was run separately on leaf samples from the same plants using the method of Lichtenthaler (1987).
PSII Maximum Quantum Yield (Fv/Fm) Measurements
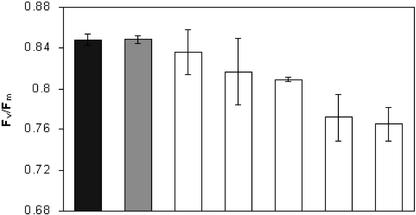
Because plants with reduced ACP4 and lower lipid content were bleached, we hypothesized that photosynthesis might be affected. The Fv/Fm of dark-adapted leaves from wild-type and LMIA plants was measured. Wild-type Arabidopsis leaves had an average dark-adapted Fv/Fm value of 0.850, as did the LMIA plants that were green and did not have dramatically reduced ACP4 or lipid content. However, for those plants with a bleached phenotype, the Fv/Fm average was 0.796 (significantly lower, P = 0.0003), indicating that the potential quantum efficiency of PSII was disrupted (Fig. 5).
Figure 5.
Fv /Fm of wild-type (black bars) and T2 generation LMIA (gray and white bars) dark-adapted leaf tissue. Gray bar, Average Fv /Fm value for a representative non-bleached LMIA plant; white bar, average values from bleached LMIA plants. Bars represent the average ± sd of two measurements (two leaves) taken from two different plants from each line.
DISCUSSION
There are three ACP isoforms (ACP2, ACP3, and ACP4) present in Arabidopsis leaf tissue at levels sufficient for detection by western-blot analysis. Of these isoforms, ACP4 is the most abundant; thus, a reduction in ACP4 constitutes a major reduction in total ACP available as a cofactor for fatty acid biosynthesis. Plants with reduced ACP4 had reduced lipid content. Therefore, the reduction in leaf lipid content is likely due to the reduction in total ACP. This is consistent with in vitro studies demonstrating that immunoprecipitation of ACP from spinach (Spinacia oleracea) leaf homogenates inhibited fatty acid synthesis (Ohlrogge et al., 1979). The levels of ACP necessary to support fatty acid biosynthesis in leaf tissue have not been determined. However, analysis of free versus acylated ACP levels in spinach revealed that approximately 60% of the ACPs are present in a nonacylated form (Post-Beittenmiller et al., 1991), indicating that the normal pool of ACPs is sufficient to support fatty acid synthesis. In addition, increases in ACP protein levels in tobacco (Nicotiana tabacum) and Arabidopsis leaf tissue did not increase leaf lipid content (Post-Beittenmiller et al., 1989b; Branen et al., 2001).
Because ACP is a critical cofactor for fatty acid biosynthesis, it might be expected that reduction of one isoform in a particular tissue might result in compensatory increases in the other ACP isoforms. However, levels of ACP2 and ACP3 in transgenic leaf tissue with reduced ACP4 were not increased in comparison with wild-type levels of these proteins. As previously mentioned, our analysis was based on equal loading of tissue extracts based on leaf fresh weight; we did not control for chlorophyll or chloroplast content. Thus, it is possible that although overall levels of ACP2 and ACP3 did not increase, levels of these isoforms in the chloroplast were actually increased. However, a lack of response of ACP2 and ACP3 to decreased ACP4 would be consistent with evidence that these isoforms are differentially regulated. For example, ACP4 mRNA levels are increased by light (Bonaventure and Ohlrogge, 2002), as is fatty acid biosynthesis in young leaves (Browse et al., 1981; Bao et al., 2000). In contrast, ACP2 and ACP3 mRNA levels remained constant when plants were transferred from dark to light conditions. In addition, ACP2 and ACP3 mRNA levels responded to factors influencing growth, whereas ACP4 mRNA levels were more reflective of photosynthesis-related genes. For example, starvation of Arabidopsis cells in the light reduced ACP2 and ACP3 mRNA levels, whereas ACP4 mRNA levels remained unchanged (Bonaventure and Ohlrogge, 2002). Furthermore, although Suc supplementation of Arabidopsis cells in the dark increased transcript levels of ACP2 and ACP3, ACP4 mRNA levels decreased under these conditions.
In addition to a decrease in total lipid content, transgenic plants with reduced ACP4 also had a lower 16:3 content. In the initial screening of T1 plants, lower 16:3 levels seemed to be compensated for by higher 16:0 levels. However, in subsequent analysis of T2 plants, the lower 16:3 actually seemed to result in an increase in C18 fatty acids, with an overall increase in the C18:C16 ratio (Table I). The lower 16:3 content would be indicative of lower MGDG levels, which might be expected based on the chlorotic appearance of the transgenic plants. However, it was found that the levels of chloroplast lipids, including MGDG, as a fraction of the total lipid were actually similar to levels in wild-type plants. The lower 16:3 is attributed to lower 16:3 on the MGDG moiety. This leads us to conclude that there is a decrease in the proportion of MGDG synthesized through the prokaryotic pathway.
A shift in the flux of fatty acid from the prokaryotic to the eukaryotic pathways has been noted in several Arabidopsis mutants. A common theme observed among these plants is that although the prokaryotic pathway was interrupted, the balance of chloroplast lipids normally produced through the prokaryotic pathway was maintained through increased synthesis of these lipids via the eukaryotic pathway. The act1 mutant, deficient in chloroplast glycerol-3-phosphate acyltransferase (G-3-PAT) activity, had dramatically reduced flux through the prokaryotic pathway (Kunst et al., 1988). There was reduced 16:3 on MGDG and DGDG moieties and a general reduction of C16 fatty acids in all of the leaf lipids, with the exception of PG, yet the proportion of chloroplast lipid was maintained. An alteration in flux of fatty acids from the prokaryotic pathway to the eukaryotic pathway was also noted in the Arabidopsis mutant deficient in chloroplast 16:1/18:1 desaturase (Browse et al., 1989), and it was speculated that this shift took place to maintain a balance of desaturated fatty acids in the chloroplast membrane lipids. In these plants, there was also little alteration in the relative amounts of polar lipids. Although the results seen in the LMIA plants with reduced lipid and ACP4 are less dramatic than seen in the act1 mutant, the reduction of 16:3 in MGDG and the increase in the C18:C16 ratio in the T2 LMIA plants indicates that fatty acids are also being redirected to the eukaryotic pathway in these plants. These data support a hypothesis that lipid metabolism in the two pathways of leaf lipid biosynthesis is somehow regulated by the demand for the correct balance of lipids and perhaps even the correct balance of saturated versus unsaturated fatty acids, necessary for chloroplast membrane biogenesis (Kunst et al., 1988; Browse et al., 1989).
Although it is becoming clear that there is close coordination between the prokaryotic and eukaryotic pathways, the mechanisms mediating partitioning of fatty acids between the two pathways are still not understood. There are several possible explanations for the shift in flux between the two pathways in LMIA plants with reduced lipid. One possibility may be that activity or expression of chloroplast lipid biosynthetic enzymes such as G-3-PAT, the first enzyme to direct lipids to the prokaryotic pathway, is lowered in response to the reduction in fatty acid biosynthesis. Because plastid G-3-PAT acts at the branch point of fatty acid incorporation between the prokaryotic and eukaryotic pathways, lower G-3-PAT activity could result in higher flux through the eukaryotic pathway. This would be consistent with the results seen with the act1 mutant.
Another possibility still being investigated is that alterations in the ACP isoform profile may influence the balance between the prokaryotic and eukaryotic pathways because of possible differential activity of enzymes toward acyl-ACP isoforms or by another unknown mechanism. In vitro specificity for acyl-ACP isoforms has been demonstrated by spinach oleoyl-ACP thioesterase and acyl-ACP-G-3-PAT (Guerra et al., 1986) and has also been demonstrated by acyl-ACP desaturases in coriander (Coriandrum sativum) and Thunbergia alata (Suh et al., 1999). In a previous study, overexpression of ACP1 in Arabidopsis leaf tissue to levels similar to typical wild-type levels of ACP4 resulted in similar, but less dramatic, alterations in the fatty acid composition than seen in LMIA transgenic plants (Branen et al., 2001). One possible explanation that would fit well with both models is that ACP4 is more highly favored by chloroplast G-3-PAT and that when this isoform is out-competed by another ACP isoform, or when it is not present in high enough quantities, G-3-PAT activity is lowered and fatty acids are directed through the eukaryotic pathway. Specificity of the ACP4 isoform to the prokaryotic pathway for synthesis of chloroplast lipids would also be consistent with its high expression in leaf tissue and its similarity to photosynthesis-related genes in transcriptional and posttranscriptional regulation (Bonaventure and Ohlrogge, 2002).
Transgenic plants with lower ACP4 levels and reduced leaf lipid content displayed varying degrees of a bleached phenotype, smaller size, and shorter bolts. Measurement of leaf Fv/Fm revealed that photosynthesis is affected in the bleached transgenic plants. Studies of Arabidopsis lipid mutants have demonstrated that fatty acid composition and total lipid content play an important role in chloroplast membrane structure and photosynthetic capability. Previous reports demonstrate that chloroplast structure is altered but overall photosynthesis, growth, and development of plants is not severely affected by altering fatty acid composition when polyunsaturated fatty acids and the relative proportion of chloroplast lipids are maintained at near-normal levels (Browse et al., 1989; Kunst et al., 1989; Miquel and Browse, 1992). However, it is apparent that photosynthesis, and in turn growth and development, are severely affected by significantly increased saturates/decreased polyunsaturates and by decreases in chloroplast lipids such as MGDG and DGDG (Lightner et al., 1994a, 1994a; Dörmann et al., 1995; McConn and Browse, 1998; Jarvis et al., 2000).
An 80% decrease in biotin carboxylase protein level was noted in an antisense transgenic tobacco plant (Shintani et al., 1997). Under low-light conditions, this plant was severely stunted in growth and slightly chlorotic. Under both high- and low-light conditions, total lipid levels were lower in the transgenic plant leaf tissue compared with wild-type leaf samples, but the difference was more apparent (26% decrease) under low light compared with high light (8% decrease). The lower lipid content of transgenic leaf tissue under low-light conditions may explain the chlorotic appearance and stunted growth. A severe phenotype of curly leaves, chlorotic patches, early senescence, distorted siliques, reduced fertility, premature cell death, and semidwarfism were noted in mod1 mutants deficient in enoyl-ACP reductase activity (Mou et al., 2000). Although enoyl-ACP reductase activity was almost undetectable in the mutant plants, leaf lipid content was only 9% to 12% lower, demonstrating that even slight reductions in leaf fatty acid synthesis can have pleiotropic effects on plant growth and development. A report that describes major reductions in total leaf lipid content comparable with that seen in the LMIA transgenic plants is in the act1/dgd1 double mutant described by Klaus et al. (2002). The leaf lipids in the act1/dgd1 double mutant were reduced by 50% compared with wild-type plants and with the individual parental mutants. This was attributed to a block in both the prokaryotic and eukaryotic pathways in the leaf tissue. In addition, this double mutant was more severely affected in photosynthesis, growth, and development than could be attributed to the individual parental mutants. It was concluded that the severe growth defects in the act1/dgd1 double mutant were primarily due to an overall decrease in thylakoid membrane lipid biosynthesis. Because the amount of polyunsaturates in lipids of LMIA plants was not significantly reduced, it is likely that the bleached appearance and other morphological characteristics are also due primarily to an overall decrease in lipid biosynthesis in the leaf tissue.
In conclusion, reduction of ACP4, the major ACP isoform in Arabidopsis leaf tissue, led to a decrease in total leaf lipids. In addition, there appeared to be an increase in the proportion of lipids packaged via the eukaryotic pathway in plants with reduced leaf lipids, but the mechanism for this alteration is unknown. Size, appearance, and photosynthetic capability were compromised in transgenic plants with reduced ACP4, probably due to the decrease in total lipid biosynthesis. These results indicate that ACP4 plays a major role in the biosynthesis of fatty acids for chloroplast membrane lipids. Transgenic plant development is currently under way to generate plants with overexpressed ACP1 and reduced ACP4 in leaf tissue to answer the question of whether the LMIA phenotype is due simply to an overall reduction in the ACP pool or if it may be a specific response to reduction in the ACP4 isoform.
MATERIALS AND METHODS
Plant Growth Conditions
To grow Arabidopsis ecotype Columbia plants for transformation, seeds were directly germinated in a soil-less mix (Sunshine Mix LC1) and grown in a temperature-controlled room at 22°C ± 1°C with a 16-h-light (55 μmol m–2 s–1) photoperiod. After transformation, T1 seeds were surface sterilized and screened on agar plates consisting of 4.3 g L–1 Murashige and Skoog basal salt mixture, 0.5 g L–1 MES (pH 5.7), 9.6 g L–1 agar (Becton-Dickinson, Sparks, MD), and 50 mg L–1 kanamycin. Resistant seedlings were transferred to soil, and T1 plants were grown in the same temperature-controlled room. Seeds from T1 plants were also screened on Murashige and Skoog agar with kanamycin. Resistant T2 plants were transferred to soil after 10 d and were placed in a growth chamber (Percival Scientific, Boone, IA) at 22°C and 60% humidity with a 16-h-light (128 μmol m–2 s–1) photoperiod. Western analysis of ACP protein levels and total lipid content and composition analysis was performed on T1 plants. All further analyses, including further western analysis and lipid analysis, were performed using tissue from T2 plants.
Antisense ACP4 Construct and Plant Transformation
The Arabidopsis ACP4 cDNA, including some of the 5′-untranslated region, was cloned in antisense conformation into the vector p1079 behind the cauliflower mosaic virus 35S promoter. The resulting construct was designated p0424A. The 1.6-kb NotI fragment containing 35S-ACP4 (antisense) was subcloned into the binary vector pART27 (Gleave, 1992) to generate the vector pLMIA. Agrobacterium tumefaciens strain C58C1 (pMP90) was transformed by electroporation. Arabidopsis plants were transformed using the dip infiltration method (Clough and Bent, 1998).
Determination of Alterations in ACP4 Levels
Tissue from transgenic plants was analyzed for alterations in ACP protein levels by western blot as described by Battey and Ohlrogge (1990). Leaf tissue (10–20 mg) was ground in 5× volumes of MOPS buffer (50 mm MOPS [pH 6.8], FisherBiotech, Fairlawn, NJ; and 10 mm dithiothreitol, added fresh). The suspension was centrifuged, and the supernatant was mixed with an appropriate volume of native sample buffer. Equal volumes were separated by native polyacrylamide (13% [v/v] 40:1 acylamide:bisacrylamide) gel electrophoresis as described by Rock and Cronan (1981). Sample buffer consisted of (for 4×): 0.25 m Tris (pH 6.8; Research Organics Inc., Cleveland), 40% (v/v) glycerol, 0.05% (w/v) bromphenol blue, and 20 mm dithiothreitol (added fresh). Gels were transferred to 0.2-μm nitrocellulose membranes (Schleicher & Schull, Keene, NH) using a Trans-Blot SD semidry transfer cell (Bio-Rad, Hercules, CA). ACP protein was detected with rabbit anti-spinach (Spinacia oleracea) ACP polyclonal antibodies and developed with goat anti-rabbit IgG-alkaline phosphatase conjugate (Kirkegaard and Perry Lab., Gaithersburg, MD). The developed western blots were scanned, and Kodak 1D Image Analysis Software (Eastman Kodak, Rochester, NY) was used to calculate the intensity of bands. These data were transferred to Excel (Microsoft, Redmond, WA), which was used for determining levels of various ACP isoforms relative to levels in wild-type extracts from the same blot.
Lipid Analysis
Total lipid was extracted from leaves by the method of Bligh and Dyer (1959) using chloroform:methanol (1:2 [v/v]) and 0.15 n acetic acid. An aliquot of the total lipid extract was spiked with pentadecanoic acid as an internal standard, dried under nitrogen, and derivatized by heating at 95°C in 3 n methanolic HCl (Supelco, Bellefonte, PA) for 40 min. After cooling to room temperature, fatty acid methyl esters were extracted with hexane. Samples were injected onto a 5890 series II gas chromatograph (Hewlett-Packard, Palo Alto, CA) equipped with a flame ionization detector and a 30-m DB-225 0.25-μm i.d. capillary column (J&W Scientific, Folsom, CA). Chromatography conditions consisted of an initial temperature of 180°C for 3 min, ramping to 230°C at 3°C min–1, and holding at 230°C for 5 min. Peaksimple software (SRI Instruments, Torrance, CA) was used for data collection and integration. Fatty acid data were analyzed using Statistical Analysis System (SAS Inc., Cary, NC).
Leaf polar lipid classes were separated by TLC on silica gel plates (60 Å, 250 μm, Whatman, Maidstone, UK) with either chloroform:methanol:acetic acid:water (170:30:20:7 [v/v/v/v]) as a solvent system or on NH4SO4-impregnated silica gel plates with acetone:toluene:water (91:30:8 [v/v]; Kahn and Williams, 1977) with toluene replacing benzene as described by Härtel et al. (2000). Bands were visualized with iodine vapor and were identified by comparing with known standards. Fatty acid composition of individual lipid classes was determined by first separating lipids by TLC as described above, covering the plates (except for marker lanes) with aluminum foil, exposing to iodine, scraping bands corresponding to marker standards, and preparing fatty acid methyl esters directly from silica as described above.
For HPLC separation of polar leaf lipids, lipid extracts were dried, resuspended in chloroform, and filtered. Lipids were separated on a 3.9× 300-mm silicic acid column (10 μm Porasil, Supelco). A Hewlett-Packard 1050 autosampler combined with an HP 1050 pump were utilized for injection and gradient control. The solvent system, adapted from Demandre et al. (1985), consisted of a linear gradient from 100% (w/v) solvent A (isopropanol:hexane [4:3 {v/v}] with 2 mm ammonium acetate) to 100% solvent B (isopropanol:hexane:water [8:6:1.5 {v/v}] with 2 mm ammonium acetate) for 15 min, isocratic flow of 100% solvent B for 10 min, a linear gradient returning to 100% (w/v) solvent A in 10 min, followed by recovery of the column in 100% (w/v) solvent A for 5 min for a total run time of 40 min. The flow rate was 1 mL min–1 during the entire analysis. Lipids were detected using a Sedex55 evaporative light scattering detector (S.E.D.E.R.E., Alfortville, cedex, France) operated with a nebulizer gas (N2) pressure of 2.4 Bar and a temperature of 42°C; gain was set to 7. Quantification was achieved by developing standard curves using known standards. Data were collected and analyzed using Hewlett-Packard HPLC 2D Chemstations.
Leaf Fluorescence Measurements
Fluorescence measurements were taken from leaves of 6-week-old T2 plants using a fluorescence monitoring system (FMS2, Hansatech Instruments, Norfolk, UK). Leaves were dark adapted for 2 min, then placed under a modulating measuring beam with a photon flux density of 100 μmol m–2 s–1 to determine F0, the initial fluorescence. The leaf was then pulsed with a saturating light pulse to measure the maximum fluorescence, Fm. The ratio of Fv/Fm was calculated where Fv = Fm – F0.
Acknowledgments
Our thanks to Dr. John Ohlrogge for providing the ACP antibody. We would also like to thank Lisa Ainsworth and Dr. Steven Long for assistance with leaf fluorescence measurements and for use of the fluorometer.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.018622.
This work was supported in part by the State of Illinois Competitive Funding for Food and Agricultural Research.
References
- Bao X, Focke M, Pollard M, Ohlrogge JB (2000) Understanding in vivo carbon precursor supply for fatty acid synthesis in leaf tissue. Plant J 22: 39–50 [DOI] [PubMed] [Google Scholar]
- Battey JF, Ohlrogge JB (1990) Evolutionary and tissue-specific control of expression of multiple acyl-carrier protein isoforms in plants and bacteria. Planta 180: 352–360 [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917 [DOI] [PubMed] [Google Scholar]
- Bonaventure G, Ohlrogge JB (2002) Differential regulation of mRNA levels of acyl carrier protein isoforms in Arabidopsis. Plant Physiol 128: 223–235 [PMC free article] [PubMed] [Google Scholar]
- Branen JK, Chiou T-J, Engeseth NJ (2001) Overexpression of acyl carrier protein-1 alters fatty acid composition of leaf tissue in Arabidopsis. Plant Physiol 127: 222–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J, Kunst L, Anderson S, Hugly S, Somerville C (1989) A mutant of Arabidopsis deficient in the chloroplast 16: 1/18:1 desaturase. Plant Physiol 90: 522–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J, Roughan PG, Slack CR (1981) Light control of fatty acid synthesis and diurnal fluctuations of fatty acid composition in leaves. Biochem J 196: 347–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Demandre C, Tremolieres A, Justin A, Mazliak P (1987) Analysis of molecular species of plant polar lipids by high-performance and gas liquid chromatography. Phytochemistry 24: 481–485 [Google Scholar]
- Dörmann P, Hoffman-Benning S, Balbo I, Benning C (1995) Isolation and characterization of an Arabidopsis mutant deficient in the thylakoid lipid digalactosyl diacylglycerol. Plant Cell 7: 1801–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleave AP (1992) A versatile binary vector system with a T-DNA organizational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol 20: 1203–1207 [DOI] [PubMed] [Google Scholar]
- Guerra DJ, Ohlrogge JB, Frentzen M (1986) Activity of acyl carrier protein isoforms in reactions of plant fatty acid metabolism. Plant Physiol 82: 448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtel H, Dörmann P, Benning C (2000) DGD1-independent biosynthesis of extraplastidic galactolipids after phosphate deprivation in Arabidopsis. Proc Natl Acad Sci USA 97: 10649–10654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood JL (1996) Recent advances in the biosynthesis of plant fatty acids. Biochim Biophys Acta 1301: 7–56 [DOI] [PubMed] [Google Scholar]
- Hloušek-Radojčić A, Post-Beittenmiller D, Ohlrogge JB (1992) Expression of constitutive and tissue-specific acyl carrier protein isoforms in Arabidopsis. Plant Physiol 98: 206–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis P, Dörmann P, Peto CA, Lutes L, Benning C, Chory J (2000) Galactolipid deficiency and abnormal chloroplast development in the Arabidopsis MGD synthase1 mutant. Proc Natl Acad Sci USA 97: 8175–8179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn JU, Williams JP (1977) Improved thin-layer chromatographic method for the separation of major phospholipids and glycolipids from plant lipid extracts and phosphatidyl glycerol and bis(monoacylgyceryl) phosphate from animal extracts. J Chromatogr 140: 179–185 [DOI] [PubMed] [Google Scholar]
- Klaus D, Härtel H, Fitzpatrick LM, Froehlich JE, Hubert J, Benning C, Dörmann P (2002) Digalactosyldiacylglycerol synthesis in chloroplasts of the Arabidopsis dgd1 mutant. Plant Physiol 128: 885–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst L, Browse JA, Somerville CR (1988) Altered regulation of lipid biosynthesis in a mutant of Arabidopsis deficient in chloroplast glycerol-3-phosphate acyltransferase activity. Proc Natl Acad Sci USA 85: 4143–4147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst L, Browse J, Somerville C (1989) Altered chloroplast structure and function in a mutant of Arabidopsis deficient in glycerol-3-phosphate acyltransferase activity. Plant Physiol 90: 846–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamppa G, Jacks C (1991) Analysis of two linked genes coding for the acyl carrier protein (ACP) from Arabidopsis thaliana (Columbia). Plant Mol Biol 16: 469–474 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic membranes. Methods Enzymol 148: 350–382 [Google Scholar]
- Lightner J, James DW Jr, Dooner HK, Browse J (1994a) Altered body morphology is caused by increased stearate levels in a mutant of Arabidopsis. Plant J 6: 401–412 [Google Scholar]
- Lightner J, Wu J, Browse J (1994b) A mutant of Arabidopsis with increased levels of stearic acid. Plant Physiol 106: 1443–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConn M, Browse J (1998) Polyunsaturated membranes are required for photosynthetic competence in a mutant of Arabidopsis. Plant J 154: 521–530 [DOI] [PubMed] [Google Scholar]
- Mekhedov S, Martinez de Ilarduya O, Ohlrogge J (2000) Toward a functional catalog of the plant genome: a survey of genes for lipid biosynthesis. Plant Physiol 122: 389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel M, Browse J (1992) Arabidopsis mutants deficient in polyunsaturated fatty acid synthesis. J Biol Chem 267: 1502–1509 [PubMed] [Google Scholar]
- Mou Z, He Y, Dai Y, Liu X, Li J (2000). Deficiency in fatty acid synthase leads to premature cell death and dramatic alterations in plant morphology. Plant Cell 12: 405–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge JB, Kuhn DN, Stumpf PK (1979) Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci USA 76: 1194–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post-Beittenmiller D, Jaworski JG, Ohlrogge JB (1991) In vivo pools of free and acylated acyl carrier proteins in spinach: evidence for sites of regulation of fatty acid biosynthesis. J Biol Chem 266: 1858–1865 [PubMed] [Google Scholar]
- Post-Beittenmiller MA, Hloušek-Radojèiæ A, Ohlrogge JB (1989a) DNA sequence of a genomic clone encoding an Arabidopsis acyl carrier protein (ACP). Nucleic Acids Res 17: 1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post-Beittenmiller MA, Schmid KM, Ohlrogge JB (1989b) Expression of holo and apo forms of spinach acyl carrier protein-1 in leaves of transgenic tobacco plants. Plant Cell 1: 889–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock CO, Cronan JE (1981) Acyl carrier protein from Escherichia coli. Methods Enzymol 71: 341–351 [DOI] [PubMed] [Google Scholar]
- Shintani DK (1996) How plants manage their fatty assets: a study into the organization and regulation of the plant fatty acid biosynthetic pathway. PhD thesis. Michigan State University, East Lansing
- Shintani DK, Ohlrogge JB (1994) The characterization of a mitochondrial acyl carrier protein isoform isolated from Arabidopsis thaliana. Plant Physiol 104: 1221–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani D, Roesler K, Shorrosh B, Savage L, Ohlrogge J (1997) Antisense expression and overexpression of biotin carboxylase in tobacco leaves. Plant Physiol 114: 881–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C, Browse J, Jaworski JG, Ohlrogge JB (2000) Lipids: chapter 10 In BB Buchanan, W Gruissem, RL Jones, eds, Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD, pp 456–527
- Suh MC, Schultz DJ, Ohlrogge JB (1999) Isoforms of acyl carrier protein involved in seed specific fatty acid synthesis. Plant J 17: 679–688 [DOI] [PubMed] [Google Scholar]