Abstract
Phytoene synthase catalyzes the dimerization of two molecules of geranylgeranyl pyrophosphate to phytoene and has been shown to be rate limiting for the synthesis of carotenoids. To elucidate if the capacity to produce phytoene is limiting also in the seed of Arabidopsis (Wassilewskija), a gene coding for an endogenous phytoene synthase was cloned and coupled to a seed-specific promoter, and the effects of the overexpression were examined. The resulting transgenic plants produced darker seeds, and extracts from the seed of five overexpressing plants had a 43-fold average increase of β-carotene and a total average amount of β-carotene of approximately 260 μg g–1 fresh weight. Lutein, violaxanthin, and chlorophyll were significantly increased, whereas the levels of zeaxanthin only increased by a factor 1.1. In addition, substantial levels of lycopene and α-carotene were produced in the seeds, whereas only trace amounts were found in the control plants. Seeds from the transgenic plants exhibited delayed germination, and the degree of delay was positively correlated with the increased levels of carotenoids. The abscisic acid levels followed the increase of the carotenoids, and plants having the highest carotenoid levels also had the highest abscisic acid content. Addition of gibberellic acid to the growth medium only partly restored germination of the transgenic seeds.
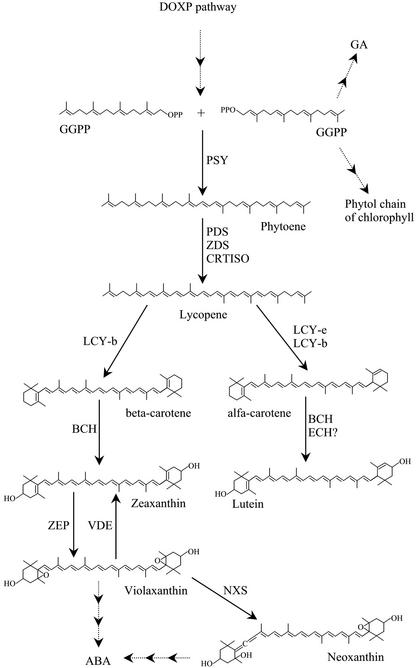
In higher plants, carotenoids are synthesized in the plastid via the 1-deoxy-d-xylulose-5-phosphate (DOXP) isoprenoid biosynthetic pathway (Lichtenthaler et al., 1997). These pigments can act as visual attractants, function as structural components in the photosystems, and trigger biochemical reactions. Phytoene synthases dimerize two geranylgeranyl pyrophosphate molecules to prephytoene diphosphate and the following conversion to phytoene, a noncolored hydrophobic C40 carbon molecule (Dogbo et al., 1988). Consecutive desaturation, isomerization, cyclization, and oxygenation result in a number of different carotenoids (Fig. 1). Phytoene synthases are regulated both at the transcriptional and posttranscriptional levels and are a rate-limiting key enzyme in the biosynthetic pathway of carotenoid synthesis (Burkhardt et al., 1997; Hirschberg, 2001). In white mustard (Sinapis alba), the enzyme is inactive in dark-grown plantlets and localized to the prolamellar body of the plastid. White light results in relocalization, formation of thylakoids, and activation of the enzyme (Welsch et al., 2000). Purified phytoene synthase requires Mn2+ and ATP for its activity, and β-carotene was shown to inhibit the reaction (Fraser et al., 2000).
Figure 1.
Overview of the biosynthesis of isoprenoids in plastids. Several steps have been omitted for simplification. PSY, Phytoene synthase; PDS, phytoene desaturase; ZDS, zeta-carotene desaturase; CRTISO, carotene isomerase; LCY-b, lycopene β-cyclase; LCY-e, lycopene ε-cyclase; BCH, β-carotene hydroxylase; ECH, ε-carotene hydroxylase; ZEP, zeaxanthin epoxidase; VDE, violaxanthin de-epoxidase; NXS, neoxanthin synthase.
Constitutive expressions of phytoene synthases in tomato (Lycopersicon esculentum) and tobacco (Nicotiana tabacum) resulted in dwarfism, chlorosis, and differential coloring of the plants (Fray et al., 1995; Busch et al., 2002). In the tomato plants, the levels of GA were decreased, and in some instances, a small decrease of the levels of abscisic acid (ABA) was also found (Fray et al., 1995). By using tissue-specific promoters for expression of phytoene synthases, many of the problems associated with expression in vegetative parts of the plant that causes dwarfism and chlorosis can be circumvented. For example, expression of a bacterial phytoene synthase in tomato in a fruit-specific manner resulted in elevated levels of phytoene, lycopene, lutein, and β-carotene (Fraser et al., 2002). In canola (Brassica napus) seed-specific expression of a bacterial phytoene synthase resulted in a 50-fold increase of the total carotenoid content. The main increase was due to elevated levels of α- and β-carotene, whereas the level of lutein remained unaltered, and it was noticed that the seed germinated 1 to 2 d later than the control plant (Shewmaker et al., 1999). Mature wild-type seeds of Nicotiana plumbaginifolia exhibiting seed dormancy have been found to contain higher amounts of ABA than non-dormant seeds (Grappin et al., 2000).
Many genes that affect seed dormancy and germination have been shown to be involved in the synthesis of carotenoids. Seed dormancy and germination have recently been reviewed, and a complex set of genes has emerged affecting these features (Koornneef et al., 2002). Genes acting early in the pathway of carotenoid synthesis like DOXP synthase and later in the pathway exemplified by zeaxanthin epoxidase and 9-cis-epoxy-carotenoid dioxygenase (NCED), have been shown to affect germination and synthesis of ABA (Frey et al., 1999; Thompson et al., 2000; Estévez et al., 2001). Dioxygenases are enzymes that cleave 9-cis-epoxycarotenoids to xanthoxin and C25 metabolites. Inducible overexpression of NCED genes has proven its importance for the synthesis of ABA in relation to seed germination (Thompson et al., 2000; Qin and Zeevaart, 2002). In this study, we use a seed-specific promoter coupled to an Arabidopsis phytoene synthase gene to show that spatial and temporal specific expression of a phytoene synthase gene results in accumulation of carotenoids, ABA, and chlorophyll in the seed. The transgenic plants produced seed with delayed germination, and the carotenoid level was negatively correlated with germination and positively correlated with the ABA level.
RESULTS
More Pigmented Seed and Changed Carotenoid Composition
To better understand the regulation and synthesis of carotenoids in the seed, a phytoene synthase gene from Arabidopsis was cloned and expressed under control of a seed-specific promoter. The seed-specific expression was used instead of constitutive expression to limit the effects of increased levels of carotenoids to the embryo, thereby avoiding effects of these metabolites in the vegetative parts of the plant. The napA promoter from oilseed rape is known to induce a strong seed-specific expression during embryo development (Stålberg et al., 1996). The entire phytoene synthase-coding sequence, including the signal sequence for chloroplast import, was fused to the napA promoter. The junction between the promoter and the coding sequence was modified to better fit consensus sequences for efficient initiation and expression.
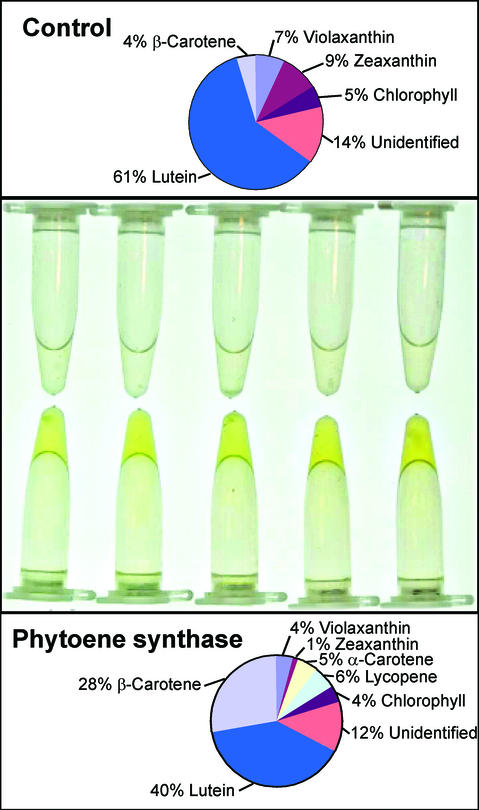
Approximately 50 spectinomycin-resistant plants were selected and analyzed. A slightly delayed germination was observed when screening for these resistant plantlets, compared with other transgenic plantlets produced at the same time. By direct visual examination of mature seeds obtained from various transgenic plants, it was possible to see differences in intensity of color, some being of darker color than others. When 5 mg of seeds were extracted in acetone, it became obvious that extracts from transgenic plants had a deep yellow color, whereas wild-type extracts did not (Fig. 2). The intensity in color was later shown to correspond to the amount of carotenoids in the seed. A calculation of the peak area distribution in percentage of the total peak areas of HPLC chromatograms showed that lutein was the major carotenoid in the control seeds, contributing 61% of the peak areas, whereas the peak containing β-carotene contributed only 4% of the total (Fig. 2). In the transgenic plants, the peak area distribution was different with 40% lutein and 28% β-carotene, which might indicate that hydroxylation limited the formation of lutein (Fig. 2). The formation of small amounts of lycopene in the transformants can similarly be explained by incomplete formation of cyclic carotenoids.
Figure 2.
Integration analysis of HPLC chromatograms represented as average peak areas of five individual control plants and five transgenic plants. The peak areas of respective carotenoid are the percentage of total peak areas. The wavelength was set at 450 nm, and the extracts were prepared according to Fraser et al. (2000). Top, Control plants upper part; middle, acetone extracts from the corresponding samples is shown; and bottom, phytoene synthase-overexpressing plants.
A 43-Fold Increase of β-Carotene and Significant Increase of Other Carotenoids and Chlorophyll
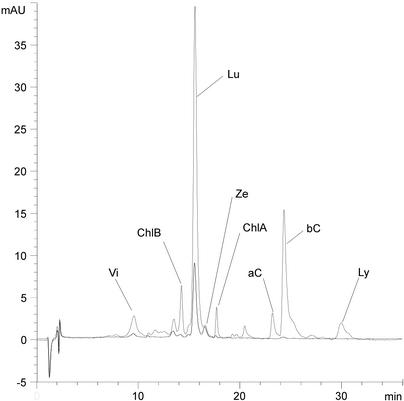
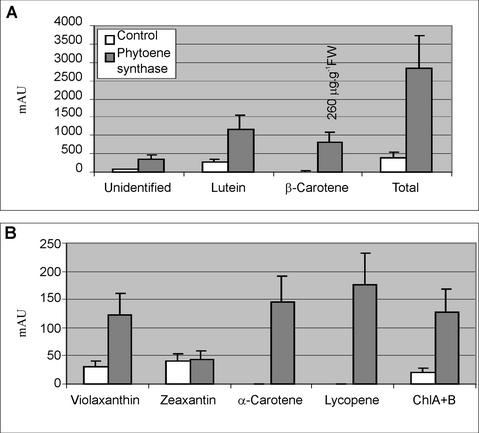
HPLC analysis of seed extracts from phytoene-overexpressing plants and control plants showed an overall increase of the carotenoid levels of the transgenic material. Several known and unknown carotenoids appeared in the transgenic plants that were not present in the control seeds (Fig. 3). Comparison of individual peak areas having similar spectra and retention times from seed extracts of five independent T1 plants and five control plants revealed an overall increase of the levels of carotenoids (Fig. 4, A and B). A significant increase of violaxanthin, lutein, and β-carotene was detected. A comparison of the zeaxanthin levels showed that this carotenoid was remarkably constant and only increased by a factor 1.1 (Fig. 4B). A similar result with stabilized zeaxanthin levels has also been obtained for Arabidopsis seeds overexpressing an endogenous hydroxylase enzyme under control of the napA promoter (K. Stålberg, O. Lindgren, and A.-S. Hoglund, unpublished data).
Figure 3.
HPLC chromatogram from a control plant (black line) and a transgenic plant (gray line) overexpressing a phytoene synthase gene (Psy). Peaks indicated are as follows: Vi, violaxanthin; ChlB, chlorophyll-B; Lu, lutein; Ze, zeaxanthin; ChlA, chlorophyll-A; aC, α-carotene; bC, β-carotene; and Ly, lycopene.
Figure 4.
Comparison of HPLC average peak areas (mAUs at 450 nm) from seed extracts of five wild-type (white bar) and five transgenic plants (gray bar). Calculated Student's t test (two-tailed equal or unequal variance, tested by F-test) significances comparing wild type to phytoene transformant peak areas: violaxanthin, P > 0.001; zeaxanthin, P > 0.1; lutein, P > 0.001; β-carotene, P > 0.001; chlorophyll A, P = 0.0017; and chlorophyll B, P > 0.001. Total peak areas, P > 0.001.
The chlorophyll levels were increased in the transgenic plants and correlated well with the increases in peak areas of carotenoids (Fig. 4B). The phytol tail of chlorophyll is synthesized from geranylgeranyl phosphate directly upstream of phytoene (Fig. 1), and an increased level of downstream carotenoids was anticipated to withdraw geranylgeranyl phosphate and to compete with the synthesis of chlorophyll, and therefore we found this increase somewhat unexpected. Extracts from 5 mg of seed of the control plants were not enough to detect any lycopene and α-carotene, whereas these compounds were easily detected in extracts from the transgenic plants (Fig. 3). Several unidentified carotenoids were present in control and transgenic plants, and these carotenoids constituted 12% to 14% of the total peak areas of the carotenoids. Among these unidentified carotenoids, peaks resembling neoxanthin (9-cis-neoxanthin and all-trans-neoxanthin) spectra were present in higher amounts in the transgenic plant compared with the non-transformed plant, although the levels were too low to obtain spectra for their definitive identification. The amount of β-carotene was estimated by comparing an external β-carotene standard to the levels of β-carotene in the extract. Approximately 260 μg g–1 fresh weight was found in the transgenic seed and 6 μg g–1 fresh weight in the control material. The relative increase of the carotenoids is shown in Figure 4A and was the result of integration of peaks obtained from analysis of five overexpressing plants and five control plants. The sum of all peak areas of the carotenoids of the transgenic seed divided by the sum of the peak areas from the control material showed a 7-fold increase of the total peak areas. The ratio of peak areas of transgenic to control seed expressed as -fold increase was the following: violaxanthin (3.9); zeaxanthin (1.1); lutein (4.5); β-carotene (43.4); chlorophyll A+B (6.0); and unidentified (6.1; Fig. 4).
Delayed Germination Was Coupled to Increased Levels of Carotenoids, ABA, and Chlorophyll Levels
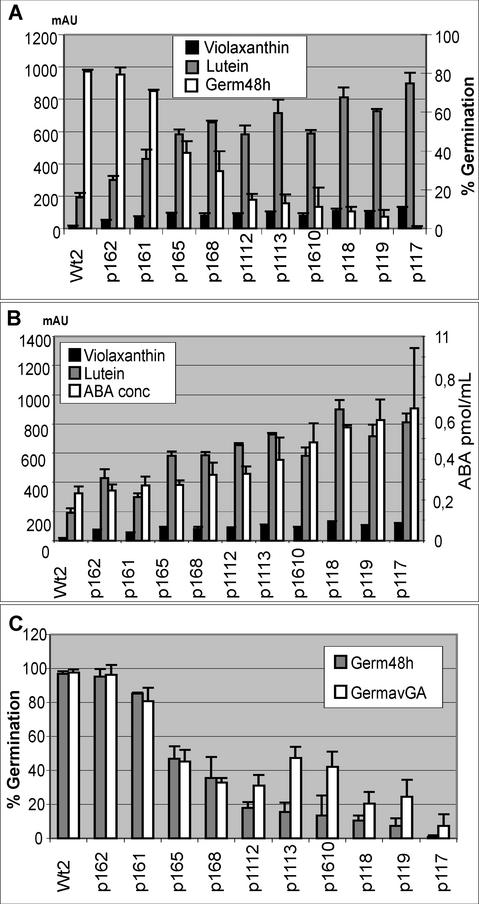
The amounts of ABA, the relative amounts of carotenoids, and percentage germination were analyzed for seeds from 10 T3 lines of phytoene synthase-overexpressing plants and a wild-type plant. The selected transgenic seed batches had lutein levels varying from low to high. Germination, ABA contents, and the levels of several carotenoids were changed in parallel. Germination frequency could be manipulated and stabilized by stratification for 3 d in darkness at 4°C, a treatment normally considered to break seed dormancy. A negative correlation between the levels of xanthophyll (lutein and violaxanthin) and germination frequency was found (Fig. 5A). The correlation coefficients were –0.88 for violaxanthin and –0.92 for lutein when compared with the corresponding germination frequencies of the seed batches (Fig. 5A).
Figure 5.
A, Lutein and violaxanthin levels of seeds from 10 transformed plants and one control plant (primary y axis) and germination percentage after 48 h (secondary y axis). B, Lutein and violaxanthin levels in seed extract from 10 transgenic and one control plant (primary y axis) and ABA levels (secondary y axis). C, Comparison of germination percentage after 48 h between seeds treated with 0.1 μg mL–1 GA3 (GermavGA) and untreated seeds (Germ48h).
Lutein and violaxanthin levels were positively correlated with ABA levels (Fig. 5B). In the mature seed, the correlation coefficients were 0.75 for violaxanthin and ABA and 0.79 for lutein and ABA. Zeaxanthin did not correlate well with either germination or ABA levels. A comparison of ABA measurements of plants with low levels of carotenoids to plants with high levels of carotenoids showed that the plants with high carotenoid contents also had significantly (P > 0.009) higher amounts of ABA. The role of GA was studied by adding GA3 to the growth medium to see whether GA could counteract the delay of germination. The GA concentration was optimized by testing a range of concentrations, and the most promoting concentration was chosen. As shown in Figure 5C, addition of GA3 did not restore germination of the transgenic seeds.
DISCUSSION
In the present paper, we show that seed-specific overexpression of an endogenous Arabidopsis phytoene synthase gene results in significant increases of the levels of β-carotene, luteins, and violaxanthin. Significant amounts of lycopene and α-carotene were also produced in the transgenic plants. Some of the results are in sharp contrast to results obtained from similar expression studies in canola. For example, overexpression of a bacterial phytoene synthase resulted in an equal increase of α- and β-carotene amounts, whereas the levels of violaxanthin and lutein remained unaltered (Shewmaker et al., 1999). The increase in β-carotene accumulation is similar to the result obtained in this study. However, in the seeds of the Arabidopsis transformants, a substantial increase in the levels of lutein and violaxanthin was found. Moreover, a smaller increase of lycopene was detected in canola compared with the relative levels in Arabidopsis. A clear difference in the accumulation of α-carotene was also found because the Arabidopsis plants did not produce relative levels of α-carotene as high as the canola seed. Even more intriguing is the difference between the levels of chlorophyll. In Arabidopsis, the levels of chlorophyll were significantly increased, and in canola the levels were decreased. We have no explanation for this difference. The differences in the levels of α-carotene might be explained by differences in hydroxylation capacity because lutein is derived from α-carotene. Differences in the origin of the phytoene synthases, bacterial and Arabidopsis, respectively, could explain the different results as well. It is possible that the bacterial phytoene synthase does not exhibit domains for protein/protein interaction, so that the formation of competent complexes for channeling the synthesis of downstream carotenoids could be partly impaired.
In Arabidopsis, the relative amount of zeaxanthin remained constant in the transgenic seeds compared with the wild-type seeds, although the levels of violaxanthin and β-carotene increased dramatically in the transgenic seeds. The reason for maintaining a constant level of zeaxanthin in the seed is currently unknown, but we speculate that a protein/lipophilic structure specifically binds zeaxanthin, and could keep these molecules in a nonreactive condition, so that they are unavailable for epoxidation. Proteins that bind specifically to zeaxanthin have been found and isolated in the resurrection plant Craterostigma plantagineum (Alamillo and Bartels, 2001). This protein, dsp 22, is homologous to early light-induced proteins (ELIP) and was shown to be colocalized to zeaxanthin after fractionation of pigment protein complexes. Furthermore, inhibition of the synthesis of zeaxanthin decreased the levels of the protein, and both dsp 22 protein and zeaxanthin accumulated during desiccation stresses, whereas the levels of violaxanthin were decreased (Alamillo and Bartels, 2001). When the seed matures, it also becomes desiccation tolerant, a situation similar to the process in C. plantagineum. Whether an ELIP like protein that is regulated by the zeaxanthin levels exists in the seed of Arabidopsis remains to be elucidated. However, Arabidopsis ELIP proteins highly homologous to the dsp 22 protein can be found in the Arabidopsis genome database.
The constant level of zeaxanthin could also be maintained by the reverse reaction of a violaxanthin de-epoxidase. In leaves, the levels of violaxanthin and zeaxanthin can be dramatically changed and depend on the light/dark cycle that regulates the violaxanthin cycle. At night, the level of violaxanthin is increased, and upon high light, the level of zeaxanthin is increased. Overexpression of zeaxanthin epoxidase has been shown to result in increased amounts of ABA and delayed germination (Frey et al., 1999). Similarly, overexpression of NCED resulted in increased levels of ABA and delayed germination, indicating that these enzymes are rate limiting for the synthesis of ABA (Thompson et al., 2000; Qin and Zeevaart, 2002). It is intriguing to find that several enzymatic steps in the biosynthesis of carotenoids influence the synthesis of ABA, at least in seeds. Several studies indicate a complex regulation of carotenoids and the synthesis of the phytohormone ABA. For example, it was found that inhibition of the synthesis of plastidic isopentenyl diphosphate by use of fosmidomycin resulted in reduced seed dormancy in tomato and loss of carotenoid accumulation (Rodríguez-Concepción et al., 2001). Moreover, plants overexpressing a gene coding for DOXP synthase gave rise to increased levels of carotenoids and ABA (Estévez et al., 2001). Adding to these results, it can be suggested that increased production of phytoene in embryonic tissues is rate limiting for the synthesis of ABA, because overexpression of an endogenous phytoene synthase resulted in delayed germination and increased amounts of ABA. It can also be hypothesized that the dormancy effect in the phytoene-overexpressing plants depends on increased levels of ABA and its precursors in the embryo before maturation, because the napA promoter activates transcription only in the seed during maturation and not after imbibition. By using the more generally functioning cauliflower mosaic virus 35S promoter, this distinction cannot be made. A more general model of how this complex pathway is regulated remains to be determined, but a precise analysis of the different carotenoids that accumulate might answer the question of which steps in the synthesis are rate limiting for the synthesis of ABA.
In conclusion, overexpression of a phytoene synthase in seeds result in increased amounts of carotenoids, chlorophyll, and ABA. Accumulation of lycopene and β-carotene indicates that cyclization and hydroxylation limit the formation of xanthophyll. ABA produced in the embryo is one component that determines germination, and the synthesis of ABA is correlated with the synthesis of upstream carotenoids.
MATERIALS AND METHODS
Constructs and Cloning
An endogenous phytoene synthase was sequenced and cloned by RT-PCR from Arabidopsis homologous to the gene cloned by Scolnik and Bartley (1994). The oligonucleotide sequence was used to amplify and clone a cDNA from Arabidopsis. A 5′-BamHI site was introduced, and two nucleotides adjacent to the ATG codon were changed to better match a consensus for efficient initiation of transcription. The 3′ oligonucleotide was synthesized with a KpnI site using the following primer CTCAGGATCCTGAAAATGTCTTCTTCTGTAGCAG. The SacI site of the construct –1,101 (Stålberg et al., 1993) was changed to a KpnI site, because the phytoene synthase had an internal SacI site. The HindIII/EcoRI fragment consisting of the napA promoter coupled to the phytoene synthase-coding region and the Nos-termination sequence was inserted into the corresponding sites of a binary plasmid pBP1. The binary plasmid was kindly provided by Dr. Bo Pontoppidan (Genetic Center, Uppsala) and had the spectinomycin AADA resistant marker gene for plant selection and a chloramphenicol resistant marker for bacteria selection.
Transformation and Growth Condition
Arabidopsis (Wassilewskija) plants were transformed by a vacuum infiltration protocol according to Bariola et al. (1999). Green spectinomycin resistant Arabidopsis plantlets were selected, planted on soil, and grown at 16 h of light, 22°C and 8 h of dark, 20°C. Seeds were collected at maturity and stored at –20°C.
Selection of Plants for ABA Determination and Germination Test
Seeds of two high-yielding T2 plants were selected on sterile medium containing 4.3 g L–1 Murashige and Skoog media (Duchefa, Haarlem, The Netherlands), 0.5 g L–1 MES, 3 g L–1 Gelrite (Duchefa), 1% (w/v) Suc, and 10 μg L–1 of spectinomycin. Forty resistant seedlings were transferred to soil and grown together with wild type at 16 h of light, 25°C and 8 h of dark, 16°C. Seeds from all plants were immediately harvested upon maturation and stored at –20°C.
About 50 seeds from each transformant and wild-type were sown in triplicates on agar plates with exactly 25 mL of medium containing 4.3 g L–1 Murashige and Skoog medium (Duchefa), 0.5 g L–1 MES, and 3 g L–1 Gelrite (Duchefa). Seeds were then put in a 4°C cold-room and stored for 3 d in darkness. The growth conditions were 16 h of light, 25°C and 8 h of dark, 16°C. Germination was scored as positive when the radicle had visibly protruded the seed coat. Test of GA-induction was performed using the concentration 10–4 mg mL–1 (0.29 μm) GA3 (Sigma-Aldrich, St. Louis). This concentration was earlier found to be optimal for promoting germination of the transformants.
HPLC and Extraction
Carotenoids from 5 mg of seeds of T1 and T3 transformants were extracted according to Fraser et al. (2000). After evaporation under nitrogen gas, the samples were dissolved in 25 or 30 μL of methanol and 25 or 30 μL of chloroform. The injection volume was 25 μL. The column and HPLC-conditions were according to MacCrehan and Schonberger (1987), except that the pH was set to pH 4 instead of 3.5. Homogenization and HPLC analysis of seed extracts from the different T1and T3 plants were made in triplicate, except for the initial screen of the transgenic plants. The HPLC chromatograms were analyzed by HP G2710AA ChemStation software (Hewlett Packard, Palo Alto, CA) using automated integration and when necessary, manual integration, for determination of the peak areas.
ABA Measurements
Measurements of ABA content were performed in triplicates of extracts from each of the 10 selected T3 plants and seed from triplicates from a wild-type plant. All plant material was cultivated and sampled at the same time. The homogenates consisted of 1 mL of 80% (v/v) acetone and 40 mg of homogenized seeds, which were incubated overnight according to Artsaenko et al. (1995). ABA was quantified with a Phytodetek-ABA-kit (Agdia, Elkhart, IN), using extracts diluted 1:10 in Tris-buffered saline.
Acknowledgments
We acknowledge Kenneth Backström for technical assistance.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.017053.
This work was supported by Stiftelsen för Lantbruksforskning, Ulla och Curt Nicolins Stipendiefond, and AstaCaroteneAB.
References
- Alamillo JM, Bartels D (2001) Effects of desiccation on photosynthesis pigments and the ELIP-like dsp 22 protein complexes in the resurrection plant Craterostigma plantagineum. Plant Sci 160: 1161–1170 [DOI] [PubMed] [Google Scholar]
- Artsaenko O, Peisker M, Nieden U, Fiedler U, Weiler EW, Müntz K, Conrad U (1995) Expression of a single-chain Fv antibody against abscisic acid creates a wilty phenotype in transgenic tobacco. Plant J 8: 745–750 [DOI] [PubMed] [Google Scholar]
- Bariola PA, MacIntosh GC, Green PJ (1999) Regulation of S-like ribonuclease levels in Arabidopsis: antisense inhibition of RNS1 or RNS2 elevates anthocyanin accumulation. Plant Physiol 119: 331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt PK, Beyer P, Wunn J, Kloti A, Armstrong GA, Schledz M, von Lintig J, Potrykus I (1997) Transgenic rice (Oryza sativa) endosperm expressing daffodil (Narcissus pseudonarcissus) phytoene synthase accumulates phytoene, a key intermediate of provitamin A biosynthesis. Plant J 11: 1071–1078 [DOI] [PubMed] [Google Scholar]
- Busch M, Seuter A, Hain R (2002) Functional analysis of the early steps of carotenoid biosynthesis in tobacco. Plant Physiol 128: 439–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogbo O, Laferriere A, Dharlingue A, Camara B (1988) Carotenoid biosynthesis ENDASH isolation and characterization of a bifunctional enzyme catalyzing the synthesis of phytoene. Proc Natl Acad Sci USA 85: 7054–7058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estévez JM, Cantero A, Reindl A, Reichler S, León P (2001) 1-Deoxy-d-xylulose-5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. J Biol Chem 276: 22901–22909 [DOI] [PubMed] [Google Scholar]
- Fraser PD, Pinto MES, Holloway DE, Bramley PM (2000) Technical advance: application of high-performance liquid chromatography with photodiode array detection to the metabolic profiling of plant isoprenoids. Plant J 24: 551–558 [DOI] [PubMed] [Google Scholar]
- Fraser PD, Romer S, Shipton CA, Mills PB, Kiano JW, Misawa N, Drake RG, Schuch W, Bramley PM (2002) Evaluation of transgenic tomato plants expressing an additional phytoene synthase in a fruit-specific manner. Proc Natl Acad Sci USA 99: 1092–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray RG, Wallace A, Fraser PD, Valero D, Hedden P, Bramley PM, Grierson D (1995) Constitutive expression of a fruit phytoene synthase gene in transgenic tomatoes causes dwarfism by redirecting metabolites from the gibberellin pathway. Plant J 8: 693–701 [Google Scholar]
- Frey A, Audran C, Marin E, Sotta B, Marion-Poll A (1999) Engineering seed dormancy by the modification of zeaxanthin epoxidase gene expression. Plant Mol Biol 39: 1267–1274 [DOI] [PubMed] [Google Scholar]
- Grappin P, Bouinot D, Sotta B, Miginiac E, Jullien M (2000) Control of seed dormancy in Nicotiana plumbaginifolia: post-imbibition abscisic acid synthesis imposes dormacy maintenance. Planta 210: 279–285 [DOI] [PubMed] [Google Scholar]
- Hirschberg J (2001) Carotenoid biosynthesis in flowering plants. Curr Opin Plant Biol 4: 210–218 [DOI] [PubMed] [Google Scholar]
- Koornneef M, Bentsink L, Hilhorst H (2002) Seed dormancy and germination. Curr Opin Plant Biol 5: 33–36 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Schwender J, Disch A, Rohmer M (1997) Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway. FEBS Lett 400: 271–274 [DOI] [PubMed] [Google Scholar]
- MacCrehan WA, Schonberger E (1987) Determination of retinol, alpha-tocopherol, and beta-carotene in serum by liquid-chromatography with absorbency and electrochemical detection. Clin Chem 33: 1585–1592 [PubMed] [Google Scholar]
- Qin X, Zeevaart JAD (2002) Overexpression of a 9-cis-epoxycarotenoid dioxygenase gene in Nicotiana plumbaginifolia increases abscisic acid and phaseic acid levels and enhances drought tolerance. Plant Physiol 128: 544–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Concepción M, Ahumada I, Diez-Juez E, Sauret-Güeto S, Lois LM, Gallego F, Carretero-Paulet L, Campos N, Boronat A (2001) 1-Deoxy-d-xylulose 5-phosphate reductoisomerase and plastid isoprenoid biosynthesis during tomato fruit ripening. Plant J 27: 213–222 [DOI] [PubMed] [Google Scholar]
- Scolnik PA, Bartley GE (1994) Nucleotide sequence of an Arabidopsis cDNA for phytoene synthase. Plant Physiol 104: 1471–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewmaker CK, Sheehy JA, Daley M, Colburn S, Ke DY (1999) Seed-specific overexpression of phytoene synthase: increase in carotenoids and other metabolic effects. Plant J 20: 401–412 [DOI] [PubMed] [Google Scholar]
- Stålberg K, Ellerstrom M, Ezcurra I, Ablov S, Rask L (1996) Disruption of an overlapping E-box/ABRE motif abolished high transcription of the napA storage-protein promoter in transgenic Brassica napus seeds. Planta 199: 515–519 [DOI] [PubMed] [Google Scholar]
- Stålberg K, Ellerstrom M, Josefsson L-G, Rask L (1993) Deletion analysis of a 2S seed storage protein promoter of Brassica napus in transgenic tobacco. Plant Mol Biol 23: 671–683 [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Jackson AC, Symonds RC, Mulholland BJ, Dadswell AR, Blake PS, Burbidhge A, Taylor IB (2000) Ectopic expression of a tomato 9-cis-epoxycarotenoid dioxygenase gene causes over-production of abscisic acid. Plant J 23: 363–374 [DOI] [PubMed] [Google Scholar]
- Welsch R, Beyer P, Hugueney P, Kleinig H, von Lintig J (2000) Regulation and activation of phytoene synthase, a key enzyme in carotenoid biosynthesis, during photomorphogenesis. Planta 211: 846–854 [DOI] [PubMed] [Google Scholar]