Abstract
A 3,300-bp DNA fragment encoding the carboxyl-transferase domain of the multidomain, chloroplastic acetyl-coenzyme A carboxylase (ACCase) was sequenced in aryloxyphenoxypropionate (APP)-resistant and -sensitive Alopecurus myosuroides (Huds.). No resistant plant contained an Ile-1,781-Leu substitution, previously shown to confer resistance to APPs and cyclohexanediones (CHDs). Instead, an Ile-2,041-Asn substitution was found in resistant plants. Phylogenetic analysis of the sequences revealed that Asn-2,041 ACCase alleles derived from several distinct origins. Allele-specific polymerase chain reaction associated the presence of Asn-2,041 with seedling resistance to APPs but not to CHDs. ACCase enzyme assays confirmed that Asn-2,041 ACCase activity was moderately resistant to CHDs but highly resistant to APPs. Thus, the Ile-2,041-Asn substitution, which is located outside a domain previously shown to control sensitivity to APPs and CHDs in wheat (Triticum aestivum), is a direct cause of resistance to APPs only. In known multidomain ACCases, the position corresponding to the Ile/Asn-2,041 residue in A. myosuroides is occupied by an Ile or a Val residue. In Lolium rigidum (Gaud.), we found Ile-Asn and Ile-Val substitutions. The Ile-Val change did not confer resistance to the APP clodinafop, whereas the Ile-Asn change did. The position and the particular substitution at this position are of importance for sensitivity to APPs.
Acetyl-CoA carboxylase (ACCase; EC 6.4.1.2) is a key enzyme in fatty acid biosynthesis in eukaryotes and prokaryotes (Harwood, 1988). ACCase is a biotinylated enzyme that catalyzes the carboxylation of acetyl-CoA to produce malonyl-CoA. This reaction is a two-step process, consisting of the ATP-dependent carboxylation of the biotin group on the carboxyl carrier domain by the biotin-carboxylase activity, followed by the transfer of the carboxyl group from biotin to acetyl-CoA by the carboxyl-transferase (CT) activity. In plants, two ACCase isoforms are found in the cytosol and in the chloroplast, respectively (Sasaki et al., 1995; Konishi et al., 1996). The cytosolic ACCase isoform in all plants studied so far is a multidomain enzyme. It provides malonyl-CoA for the synthesis of very long-chain fatty acids and flavonoids and for malonylation (Sasaki et al., 1995). The chloroplastic ACCase isoform catalyzes the first committed step in fatty acid biosynthesis. In most plant species, chloroplastic ACCase is a multisubunit enzyme, the subunits of which are encoded in the nDNA, except the β-subunit of CT that is encoded by a chloroplastic gene (Konishi et al., 1996). However, in Poaceae (grasses), the chloroplastic ACCase is a multidomain enzyme (Konishi et al., 1996) encoded by a nuclear gene distinct from that coding for the cytosolic ACCase isoform (Gornicki et al., 1994, 1997; Podkowinski et al., 1996).
The chloroplastic, multidomain form of ACCase in Poaceae is the target of two chemically distinct classes of inhibitors, aryloxyphenoxypropionates (APPs) and cyclohexanediones (CHDs). These chemicals inhibit the CT activity, thus blocking the transfer of the carboxyl group to acetyl-CoA (Rendina et al., 1990; Burton et al., 1991). Multisubunit-type ACCases and cytosolic, multidomain-type ACCases are insensitive and significantly less sensitive, respectively, to CHDs and APPs than chloroplastic, multidomain-type ACCase (Egli et al., 1993; Alban et al., 1994). Thus, most plant species other than Poaceae are insensitive to these herbicides, as are most other eukaryotes and prokaryotes. This makes APPs and CHDs effective graminicide herbicides.
APP and CHD herbicides, introduced to world agriculture in the 1980s, have become widely used. As a consequence, resistant biotypes have appeared in many grass weeds (for review, see Devine and Shukla, 2000; see also the International Survey of Herbicide Resistant Weeds Web site at http://www.weedscience.com). Many studies have established that resistance to these herbicides is often due to acquired resistance of chloroplastic ACCase. Various patterns of resistance across and within the APPs and the CHDs have been characterized in particular resistant biotypes, indicating that several different mutations of ACCase may be involved. However, the molecular basis of resistance or sensitivity of ACCase to APPs and CHDs is still largely unknown. Recent work showed that a 412-amino acid fragment of wheat (Triticum aestivum) chloroplastic ACCase, encompassing a part of the CT domain, contained a major determinant for herbicide sensitivity (Nikolskaya et al., 1999). An Ile residue contained within this 412-amino acid fragment and located inside chloroplastic ACCase CT domain was shown to be critical for sensitivity to APP and CHD inhibitors in resistant biotypes of Lolium rigidum (Gaud.) (Zagnitko et al., 2001), Setaria viridis L. Beauv. (Zhang and Devine, 2000; Délye et al., 2002c), Alopecurus myosuroides (Huds.) (Délye et al., 2002a), and Avena fatua (Christoffers et al., 2002). In A. myosuroides, this Ile/Leu residue is located at position 1,781 within the chloroplastic ACCase (Délye et al., 2002a). The replacement of Ile with Leu was shown to confer a high level of resistance to some, but not all, APPs and CHDs in the major crop weeds L. rigidum (Zagnitko et al., 2001) and A. myosuroides (Délye et al., 2002b). Here, we demonstrate that another Ile residue, located at position 2,041 within the A. myosuroides ACCase protein sequence, is critical for sensitivity to APP inhibitors but not to CHD inhibitors in multidomain ACCases. This residue is situated outside the 412-amino acid fragment but within the CT domain of chloroplastic ACCase.
RESULTS
Polymorphism within ACCase CT Domain and Sensitivity to APPs
In the following, the reference sequence for A. myosuroides chloroplastic ACCase is EMBL accession AJ310767 (Délye et al., 2002a). All nucleotide and amino acid positions referred to in this paper correspond to those in this sequence. The 34 A. myosuroides seedlings used for sequencing experiments consisted of 18 resistant and 16 seedlings sensitive to APP herbicides. Eleven seedlings, of which seven were resistant, contained two identical ACCase alleles. Thus, a total of 57 sequences were obtained for analysis. Their alignment was 3,339 bp long, and included four short introns. The positions of these introns, located between nucleotide positions 4,532 and 4,533, 4,746 and 4,747, 4,926 and 4,927, and 7,062 and 7,063, respectively, corresponded to those of the four last introns in wheat cytosolic ACCase sequence (GenBank accession no. U39321; Podkowinski et al., 1996). Here, we only considered A. myosuroides ACCase coding sequence for analysis. Within this sequence, a total of 35 single-nucleotide polymorphisms (SNPs), consisting of 28 synonymous and seven non-synonymous changes and including 14 singleton SNPs, were identified. The 57 sequences comprised a total of 29 haplotypes, 17 of which contained non-synonymous SNPs. Alignment of the nucleotide sequences of the 29 haplotypes with the reference sequence has been deposited in the EMBL database (accession no. ALIGN_000483).
Among the seven non-synonymous SNPs recorded, only one was found in ACCase haplotypes exclusively present in resistant seedlings (Table I). This change was a T6,278A transversion (second position in codon 2,041) causing an Ile-2,041-Asn substitution in eight haplotypes. These haplotypes were present in 16 of the 18 seedlings resistant to APPs. Two fenoxaprop-resistant seedlings and all sensitive seedlings contained an Ile residue at position 2,041.
Table I.
Non-synonymous mutations found in A. myosuroides ACCase sequences
| Nucleotide Substitution | Amino Acid Change | No. of Haplotypes | Plant Phenotype
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Homozygous
plantsa
|
Heterozygous
plantsb
|
|||||||||||||
| Fenoxaprop
|
Clodinafop
|
Haloxyfop
|
Fenoxaprop
|
Clodinafop
|
Haloxyfop
|
|||||||||
| Rc | Sc | R | S | R | S | R | S | R | S | R | S | |||
| T6,278A | Ile-2,041-Asn | 8 | 0 | 0 | 1 | 0 | 4 | 0 | 3 | 0 | 2 | 0 | 6 | 0 |
| A6,947G | Lys-2,264-Arg | 4 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 4 | 2d | 0 | 0 | 4 |
| (A6,947G + A4,976G) | (Lys-2,264-Arg + Asn-1,607-Ser) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1d | 0 |
| A4,535G | Asn-1,460-Ser | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| A6,389G | Asp-2,078-Gly | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1d | 0 |
| G6,443C | Gly-2,096-Ala | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1d | 1 | 0 | 0 | 0 | 0 |
| G6,877A | Ala-2,241-Thr | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
Plants containing two ACCase haplotypes with the same non-synonymous change. b Plants containing two ACCase haplotypes, one of which contains a given non-synonymous change. c S, Sensitive, R, resistant. d Plants containing a second ACCase haplotype with an Ile-2,041-Asn mutation.
To check whether this SNP was consistently associated with resistance to herbicides, a bidirectional allele-specific PCR assay simultaneously detecting the Ile-2,041 and Asn-2,041 ACCase alleles was used to genotype a total of 2,000 A. myosuroides seedling from populations from the field that did not contain Leu-1,781 ACCase alleles. There was no association between the presence of Asn-2,041 ACCase alleles and resistance to the CHD herbicides clethodim and cycloxydim (Table II). In contrast, all 592 seedlings containing at least one Asn-2,041 ACCase allele were resistant to one of the three APPs studied. No APP-sensitive seedling contained the Asn-2,041 ACCase allele. Results from the “purified” population 02-F1, consisting of 100% of seedlings each containing two Asn-2,041 ACCase alleles, supported these findings, with 100% of the seedlings being sensitive to CHDs or resistant to APPs (Table II).
Table II.
Herbicide sensitivity of and codon present at position 2,041 on each copy of the gene encoding chloroplastic ACCase in A. myosuroides and L. rigidum seedlings from field populations
| A. myosuroides | Genotypesa | CHD herbicides
|
APP herbicides
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cycloxydim | Clethodim | Fenoxaprop | Clodinafop | Haloxyfop | |||||||
| Rb | Sb | R | S | R | S | R | S | R | S | ||
| 00-017c | Ile/Ile | 0 | 50 | 0 | 50 | 0 | 50 | 0 | 50 | 0 | 50 |
| 02-F1c, d | Asn/Asn | 0 | 50 | 0 | 50 | 50 | 0 | 50 | 0 | 50 | 0 |
| Total of seven populations from the fielde | Ile/Ile | 7 | 196 | 5 | 197 | 81 | 123 | 25 | 179 | 10 | 190 |
| Ile/Asn | 4 | 69 | 11 | 72 | 76 | 0 | 76 | 0 | 71 | 0 | |
| Asn/Asn | 4
|
70
|
4
|
61
|
70
|
0
|
70
|
0
|
79
|
0
|
|
| L. rigidum | Cycloxydim | Diclofop | Clodinafop | Haloxyfop | |||||||
| Rb | Sb | R | S | R | S | R | S | ||||
| Total of two populations from the fielde | Ile/Ile | 0 | 45 | 0 | 65 | 0 | 52 | 0 | 53 | ||
| Ile/Asn | 0 | 14 | 7 | 0 | 2 | 0 | 6 | 0 | |||
| Asn/Asn | 0 | 6 | 3 | 0 | 11 | 0 | 8 | 0 | |||
| Ile/Val | 0 | 5 | 0 | 0 | 1 | 4 | 3 | 0 | |||
X/Z, plants containing chloroplastic ACCase copies with an X and a Z codon at position 2,041. b S, Sensitive; R, resistant. c Populations used for ACCase enzyme assay. d “Purified” population (see “Materials and Methods”). e The results from several populations from the field were pooled together.
We found that 116 seedlings resistant to APPs contained Ile-2,041 ACCase alleles only (Table II). In our sequencing experiments, we found that two fenoxaprop-resistant seedlings did not contain Asn-2,041 ACCase alleles (Table I). Besides, 35 seedlings containing Asn-2,041 and/or Ile-2,041 ACCase alleles were resistant to CHDs (Table II). This was consistent with previous demonstrations that resistance to ACCase inhibitors in A. myosuroides may be due to the presence of altered target enzyme and/or to enhanced herbicide metabolization (Cocker et al., 1999; Délye et al., 2002a).
L. rigidum ACCase Study
To determine whether the results obtained with A. myosuroides could be extended to another grass weed species in which extensive resistance to APPs and CHDs has been reported, we cloned and sequenced a 1,022-bp DNA fragment from one homozygous APP-sensitive L. rigidum seedling using primers ACVII11 and ACVII11R. The sequence has been deposited in the EMBL database (accession no. AJ519781). We found that A. myosuroides and L. rigidum sequences were not similar enough to use our allele-specific PCR assay to detect Asn ACCase alleles in L. rigidum. The mutation causing an Ile-Asn substitution in L. rigidum would delete an EcoRI restriction site. Thus, we used PCR with primers ACVII11 and ACVII11R followed by EcoRI digestion to genotype L. rigidum seedlings assayed for herbicide sensitivity. The PCR fragment was not digested in three clodinafop-sensitive seedlings, which seemed conflicting with A. myosuroides data. Sequencing in these three L. rigidum seedlings revealed that they did not contain a T-to-A transversion at the second position of the critical Ile codon. Instead, they contained an A-to-G transition at the first position of this codon, causing an Ile-Val substitution. This substitution would delete the EcoRI restriction site also. Therefore, we used a combination of EcoRI and XmnI digestions to discriminate Ile, Asn, and Val ACCase alleles in L. rigidum. We geno-typed a total of 280 L. rigidum seedlings from two populations that were tested using herbicide bioassay (Table II). Asn and Val ACCase alleles were detected in 57 and 13 seedlings, respectively. Both Asn and Val ACCase alleles were present in the two L. rigidum populations investigated. As in A. myosuroides, association was found between the presence of Asn ACCase alleles and resistance to APPs but not to CHDs (Table II). The low number of L. rigidum seedlings containing Val ACCase alleles did not enable us to determine the cross resistance pattern associated with this mutation. However, the presence of Val ACCase alleles in four clodinafop-sensitive seedlings (Table II) suggested that Val ACCase alleles do not confer a significant level of resistance to clodinafop.
ACCase Inhibition by Herbicides
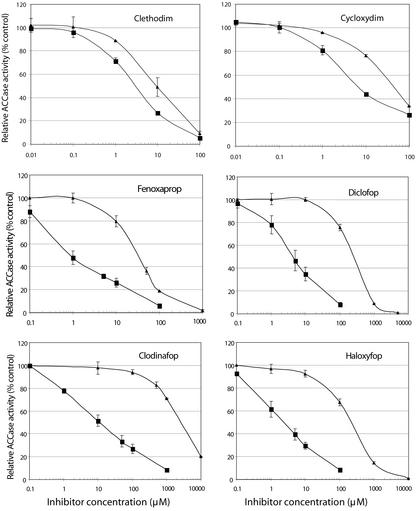
ACCase-specific activity measured without the presence of inhibitors was always lower in extracts from the resistant A. myosuroides population 02-F1 than in extracts from the sensitive population 00-017 (not shown). The action of four APP and two CHD inhibitors upon enzymatic activity of Ile-2,041 (population 00-017) and Asn-2,041 (population 02-F1) ACCase alleles is shown in Figure 1. The inhibition patterns of the two ACCase alleles were similar for the CHD inhibitors clethodim and cycloxydim, although concentrations inhibiting 50% of ACCase activity (I50) values were slightly higher for Asn-2,041 ACCase than for Ile-2,041 ACCase, respectively. In contrast, Asn-2,041 ACCase displayed a high level of resistance to all four APPs assayed (Table III). These findings fully supported the association of the presence of the Ile-2,041-Asn substitution with resistance to APPs found using allele-specific PCR in A. myosuroides and in L. rigidum (Table II). We concluded that this substitution is a direct cause of resistance to APPs but not CHDs.
Figure 1.
Inhibition of ACCase activity in sensitive (00-017, ▪) and resistant (02-F1, ▴) A. myosuroides population by clethodim and cycloxydim (CHDs) and by fenoxaprop, diclofop, clodinafop, and haloxyfop (APPs). ACCase from sensitive plants have Ile at positions 1,781 and 2,041, whereas ACCase from resistant plants have Ile and Asn at positions 1,781 and 2,041, respectively. Averages of two independent experiments are shown with error bars. ACCase activity is expressed as a percentage of ACCase activity without inhibitor for each population.
Table III.
I50 values obtained for Ile-2,041 (population 00-017) and Asn-2,041 (population 02-F1) ACCases
Values are mean ± se of two independent experiments.
| Inhibitors | 00-017 (S) | 02-F1 (R) | R:S I50 ratio |
|---|---|---|---|
| μM | μM | ||
| Clethodim (CHD) | 3.9 ± 0.2 | 9.6 ± 1.2 | 2.5 |
| Cycloxydim (CHD) | 6.7 ± 0.2 | 30.3 ± 0.3 | 4.5 |
| Fenoxaprop (APP) | 0.98 ± 0.1 | 36.2 ± 2.0 | 37.0 |
| Diclofop (APP) | 5.2 ± 0.8 | 263.5 ± 8.8 | 50.5 |
| Clodinafop (APP) | 10.9 ± 1.7 | 2,205.5 ± 106.4 | 202.5 |
| Haloxyfop (APP) | 2.7 ± 1.1 | 209.6 ± 25.9 | 77.5 |
DISCUSSION
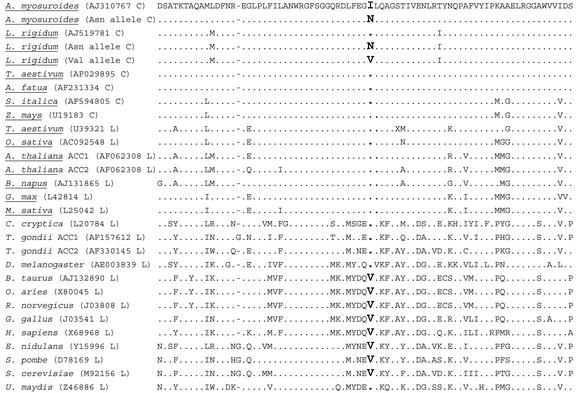
Although it has been known since the early 1990s that APPs and CHDs inhibit ACCase by interfering at the CT level, few data are still available concerning the molecular basis of this interaction. Recent studies have established that an Ile-Leu substitution, located at position 1,781 in the A. myosuroides sequence, conferred a high level of resistance to the CHDs sethoxydim and cycloxydim and to the APPs diclofop and fenoxaprop. This substitution also conferred a moderate level of resistance to the APPs haloxyfop and clodinafop and to the CHD cethoxydim (Joachimiak et al., 1997; Zagnitko et al., 2001; Délye et al., 2002a, 2002b). In this paper, we demonstrated that an Ile residue located at position 2,041 in A. myosuroides chloroplastic ACCase sequence is critical for sensitivity to the same four APPs but not to CHDs. This Ile residue is conserved in all known cytosolic and chloroplastic multidomain ACCases from plants (Fig. 2). It is also conserved in sequences from the protozoan Toxoplasma gondii and the fungus Ustilago maydis (Fig. 2). In all other known ACCase sequences, this position is occupied by a Val residue, as it is in chloroplastic ACCase from some APP-resistant L. rigidum seedlings. None of the known ACCase sequences contain an Asn residue at this position. In the following, an “X-&-Z ACCase” will refer to an ACCase enzyme with residues X and Z at positions corresponding to positions 1,781 and 2,041, respectively, in A. myosuroides chloroplastic ACCase.
Figure 2.
Alignment of amino acid sequences of multidomain ACCases around the site of the Ile residue critical for APP sensitivity (in bold). Higher plant ACCases are underlined. C, Chloroplastic ACCases. L, ACCase isoforms containing a Leu residue at position 1,780 in sequence AJ310767. Dots, Residues identical to those in sequence AJ310767. Dashes, Gaps. The fragment shown extends from Asp 2,002 to Ser 2,079 in sequence AJ310767.
The evidence of the role played by the Ile/Asn/Val residue in sensitivity to APP inhibitors is supported by consistent biological and enzyme data. We demonstrated that an Ile-2,041-Asn substitution in A. myosuroides chloroplastic ACCase is sufficient to confer resistance to all APPs tested (Fig. 1; Tables II and III). Additional evidence also supports this conclusion. First, CT domains of plant cytosolic Leu-&-Ile ACCases are highly similar to those of chloroplastic Ile-&-Ile ACCases (75% identity and 88% similarity on average). Ile-&-Ile and Leu-&-Ile ACCases are sensitive and moderately resistant to the APP haloxyfop, respectively (Joachimiak et al., 1997; Zagnitko et al., 2001). In contrast, we showed that Ile-&-Asn ACCase is far more resistant to haloxyfop. The comparison can be extended to the CT domain of Leu-&-Ile ACC1 gene from T. gondii (50% identity and 67% similarity on average with multidomain, chloroplastic ACCases) that is highly resistant to the CHDs sethoxydim and cethoxydim and moderately resistant to the APPs haloxyfop and clodinafop (Jelenska et al., 2002). Second, the human (Homo sapiens) Leu-&-Val ACCase is not sensitive to APPs or to CHDs (Zuther et al., 1999). The Leu-&-Val ACCase from yeast (Saccharomyces cerevisiae) is also highly resistant to the CHDs sethoxydim and cethoxydim and to the APP haloxyfop (Joachimiak et al., 1997). CT domains from these genes are 53% and 52% identical to that of chloroplastic multidomain ACCases, respectively. In our work, mutant Ile-&-Val chloroplastic ACCases were found in a few L. rigidum seedlings only. Our data suggested that Ile-&-Val ACCases are likely not highly resistant to clodinafop (Table II). This suggests that the Ile-Val substitution does not confer resistance to all APPs investigated, in contrast with the Ile-Asn substitution. Third, the grass Vulpia bromoides, which is naturally insensitive to APPs and CHDs, contains a chloroplastic Leu-&-Asn ACCase (X.-Q. Zhang, unpublished data).
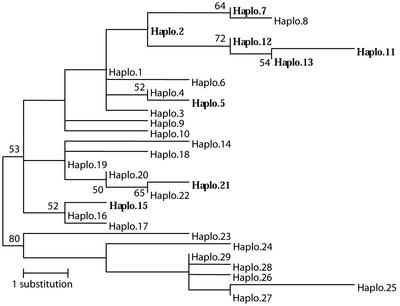
Phylogenetic analysis conducted upon the 29 ACCase haplotypes using the maximum parsimony method revealed that the evolution of the different haplotypes containing an Ile-2,041-Asn substitution very likely required independent sources of Asn-2,041 ACCase alleles (Fig. 3). This analysis enabled us to distinguish at least four distinct apparitions of Asn-2,041 ACCase alleles (Fig. 3), suggesting that Ile-&-Asn ACCases have appeared independently in geographically distant A. myosuroides populations. Here, we investigated A. myosuroides populations that were selected by the APP herbicide clodinafop. All of them contained Ile-&-Asn ACCase mutants, which are very highly resistant to clodinafop (Table III). None of them contained Leu-&-Ile or Ile-&-Val ACCase mutants, which is not surprising considering that such mutants will be moderately target site resistant to this molecule. In previous works, we studied A. myosuroides populations mostly selected by exposure to fenoxaprop (Délye et al., 2002a, 2002b). In most of the resistant populations, we found mutant Leu-&-Ile ACCases. Genotyping these seedlings with the allele-specific PCR assay described here, we did not find Ile-&-Asn mutant ACCases. Given that both Ile-&-Asn and Leu-&-Ile ACCases are resistant to fenoxaprop, this suggests that Leu-1,781 ACCase alleles may be more easily selected in A. myosuroides than Asn-2,041 alleles, perhaps because of a lower fitness cost. The two L. rigidum populations studied here were selected with diclofop and clodinafop. Both contained Ile-&-Val and Ile-&-Asn ACCases. The predominance of the latter form may be explained by Ile-&-Asn ACCases being resistant to both selecting APPs, whereas Ile-&-Val alleles are not highly resistant to clodinafop.
Figure 3.
Phylogenetic tree of the 29 A. myosuroides ACCase haplotypes calculated by the maximum parsimony method. The haplotype names are the same as in EMBL accession number ALIGN_000483. Bootstrap values ≥ 50% are shown. Haplotypes containing an Ile-2,041-Asn mutation are in bold.
APPs and CHDs are mutually exclusive inhibitors (for review, see Gronwald, 1991). They may either bind to a common or to two overlapping sites, or each class of compounds may induce allosteric changes preventing the binding of the other. Although the Ile/Leu residue at position 1,781 in A. myosuroides is a determinant of sensitivity to both CHD and APP inhibitors, the Ile/Asn/Val at position 2,041 in A. myosuroides is a determinant of sensitivity to APPs only. This argues in favor of two partially overlapping binding sites for APPs and CHDs. Whether the Ile/Leu and the Ile/Asn/Val residues are adjacent within CT active site remains to be elucidated. Clearly, not only the position but the particular amino acid substitution at a given site is of importance, as illustrated by the Ile/Asn substitution conferring resistance to clodinafop in L. rigidum, whereas the Ile/Val does not. Similar conclusions have been drawn with studies on other enzymes, such as the extensively investigated acetolactate synthase (for review, see Boutsalis et al., 1999). Thus, a tridimensional model for ACCase CT domain is definitely needed to design new inhibitors able to block the CT activity of mutant, resistant ACCase alleles.
Previous work identified a 412-amino acid domain in wheat chloroplastic ACCase that is crucial for herbicide sensitivity (Nikolskaya et al., 1999). In A. myosuroides, this domain is 417 amino acid long and includes the first 252 of the 566 amino acids constituting the CT domain. It encompasses the highly conserved β-CT subdomain but not the highly conserved α-CT subdomain that is adjacent to the Ile/Asn/Val residue. Thus, we conclude that other determinants for APP and CHD sensibility should be searched within the entire CT domain, and not exclusively within a region corresponding to the wheat 412-amino acid domain.
MATERIALS AND METHODS
Plant Material and Chloroplastic ACCase CT Domain Sequencing
We used eight Alopecurus myosuroides (Huds.) populations (Table II) originating from French fields where APP resistance was suspected. Resistance to three APP and two CHD herbicides was assessed using 50 seedlings per population and per herbicide as described elsewhere (Letouzé and Gasquez, 1999). APP herbicides used were fenoxaprop, clodinafop, and haloxyfop, and CHD herbicides used were cycloxydim and clethodim. The concentrations discriminating resistant from sensitive seedlings were 6 μm for clethodim and as described for other herbicides (Délye et al., 2002b). Seedlings were collected, and DNA was extracted for PCR-based experiments as before (Délye et al., 2002a, 2002b).
We first confirmed using allele-specific PCR (Délye et al., 2002b) that the eight A. myosuroides populations did not contain the Leu-1,781 chloroplastic ACCase alleles. A total of 34 seedlings were selected for sequencing experiments. They consisted of 10 haloxyfop-resistant and eight haloxyfop-sensitive seedlings, five fenoxaprop-resistant and six fenoxaprop-sensitive seedlings, and three clodinafop-resistant and two clodinafop-sensitive seedlings. Two pairs of nested primers were designed to PCR clone a DNA fragment including nucleotide positions 4,368 to 7,329 in A. myosuroides chloroplastic ACCase coding sequence (EMBL accession no. AJ310767; Délye et al., 2002a). This fragment encompassed the entire CT domain of A. myosuroides ACCase, plus the N-terminal end of the 412-amino acid fragment involved in herbicide sensitivity (Nikolskaya et al., 1999) that is not included within the CT domain. PCR with a proofreading polymerase was as described (Délye et al., 2002c). A first round of PCR with primers ACVII16 (CTTGTCAGACAACCCAGTGCAGGCAAC) and ACVII14R (GTTCTTGCCAACAGGAGGCAAAACCCG; 0.2 μm each) was followed by a second round PCR using primers ACVII8 (AGGACACGCAGAGGAACCTCTTTCATTTAC) and ACVRT1 (CATCCAGTTACACTCATCATCAACCAGCC; 0.05 μm each). The amplicon was purified using a Nucleospin Extract kit (Macherey-Nagel GmbH, Düren, Germany) and directly sequenced on both strands using gene-specific primers. When seedlings contained two different ACCase alleles, fragment ACVII8/ACVRT1 was cloned in plasmid pGEM-T (Promega, Madison, WI), and three different DNA inserts were sequenced for each ACCase allele. Sequence analysis and alignments were performed using the BioEdit software (Hall, 1999) and the Multalin software (Corpet, 1988), respectively. The Mega software (Sudhir Kumar, Koichiro Tamura, Ingrid B. Jakobsen, and Masatoshi Nei, Arizona State University, Tempe) was used to calculate phylogenetic trees.
Bidirectional Allele-Specific PCR
Allele-specific PCR (Sommer et al., 1992) primers were designed to detect Asn-2,041 ACCase alleles resulting from an A6,278T transversion. Primers VSEl1 (GCAAAGAGATCTTTTTGAAGGAAT) and VREl1R (TTGACCCAGCCTGCAGAT) were designed to specifically prime A. myosuroides ACCase sequences containing T or A at nucleotide position 6,278, respectively. They were used together with primers ACVII11 (CTGCAAACATTGGTGGACCTCTTCCTATTAC) and ACVII11R (CAGTCGGTGCTTCCTGCTGCAGCTG) at a final concentration of 0.2 μm for each of the four primers. PCR mixes were as described (Délye et al., 2002a). The cycling program consisted of one denaturation step of 30 s at 95°C, followed by 37 cycles of 10 s at 95°C, 15 s at 64°C, and 30 s at 72°C. Primers were designed to generate up to three distinct sizes of amplicons depending on the ACCase alleles present within one plant. Primers ACVII11 and ACVII11R yielded a 1,082-bp fragment. Primers ACVII11 and VREL1R yielded a 481-bp fragment. Primers ACVII11R and primer VSEl1 yielded a 642-bp fragment.
Inhibition of A. myosuroides ACCase Activity by APPs and CHDs
Populations 00-017 and 02-F1 (Table II) were used for ACCase assay. Population 00-017 is a field-sensitive population where no Asn-2,041 and no Leu-1,781 ACCase alleles could be detected. Population 02-F1 is a “purified” population obtained by enabling free pollination between five A. myosuroides plants that survived haloxyfop treatment in the field. Sequencing fragment ACVII8/ACVRT1 in these five plants showed that each of them contained two identical Asn-2,041 ACCase alleles. No other non-synonymous mutation was revealed in the sequences when compared with sequences from sensitive plants.
ACCase extraction, enzyme assay, and determination of APP and CHD inhibitory action were performed as described (Shukla et al., 1997) from three to four leaf seedlings. Assays were performed twice for each inhibitor concentration. The data was fitted to the nonlinear curve model (exponential decay) of the Sigmaplot software (SPSS Science, Chicago), and the I50 values were computed accordingly for each inhibitor.
Acknowledgments
The authors are grateful to Annick Matéjicek for performing some of the herbicide bioassays.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.021139.
This work was supported in part by the Département Santé des Plantes et Environnement of the Institut National de la Recherche Agronomique and by the Conseil Régional de Bourgogne (grant no. HCP 01/5112/12).
References
- Alban C, Baldet P, Douce R (1994) Localization and characterization of two structurally different forms of acetyl-CoA carboxylase in young pea leaves, of which one is sensitive to aryloxyphenoxypropionate herbicides. Biochem J 300: 557–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutsalis P, Karotam J, Powles SB (1999) Molecular basis of resistance to acetolactate synthase-inhibiting herbicides in Sisymbrium orientale and Brassica tournefortii. Pestic Sci 55: 507–516 [Google Scholar]
- Burton JD, Gronwald JW, Keith RA, Somers DA, Gegenbach BG, Wyse DL (1991) Kinetics of inhibition of acetyl-coenzyme A carboxylase by sethoxydim and haloxyfop. Pestic Biochem Physiol 39: 100–109 [DOI] [PubMed] [Google Scholar]
- Christoffers MJ, Berg ML, Messersmith CG (2002) An isoleucine to leucine mutation in acetyl-CoA carboxylase confers herbicide resistance in wild oat. Genome 45: 1049–1056 [DOI] [PubMed] [Google Scholar]
- Cocker KM, Moss SR, Coleman JOD (1999) Multiple mechanisms of resistance to fenaxoprop-P-ethyl in United Kingdom and other European populations of herbicide-resistant Alopecurus myosuroides (black-grass). Pestic Biochem Physiol 65: 169–180 [Google Scholar]
- Corpet F (1988) Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 16: 10881–10890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Délye C, Calmès É, Matéjicek A (2002a) SNP markers for black-grass (Alopecurus myosuroides Huds.) genotypes resistant to acetyl CoA-carboxylase inhibiting herbicides. Theor Appl Genet 104: 1114–1120 [DOI] [PubMed] [Google Scholar]
- Délye C, Matéjicek A, Gasquez J (2002b) PCR-based detection of resistance to acetyl-CoA carboxylase-inhibiting herbicides in black-grass (Alopecurus myosuroides Huds) and ryegrass (Lolium rigidum Gaud). Pest Manag Sci 58: 474–478 [DOI] [PubMed] [Google Scholar]
- Délye C, Wang T, Darmency H (2002c) An isoleucine-leucine substitution in chloroplastic acetyl-Co A carboxylase from green foxtail (Setaria viridis L. Beauv.) is responsible for resistance to the cyclohexanedione herbicide sethoxydim. Planta 214: 421–427 [DOI] [PubMed] [Google Scholar]
- Devine MD, Shukla A (2000) Altered target sites as a mechanism of herbicide resistance. Crop Prot 19: 881–889 [Google Scholar]
- Egli MA, Gegenbach BG, Gronwald JW, Somers DA, Wyse DL (1993) Characterization of maize acetyl-coenzyme A carboxylase. Plant Physiol 101: 499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gornicki P, Faris J, King I, Podkowinski J, Gill B, Haselkorn R (1997) Plastid-localised acetyl-CoA carboxylase of bread wheat is encoded by a single gene on each of the three ancestral chromosome sets. Proc Natl Acad Sci USA 94: 14179–14184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gornicki P, Podkowinski J, Scappino LA, DiMaio J, Ward E, Haselkorn R (1994) Wheat acetyl-coenzyme A carboxylase: cDNA and protein structure. Proc Natl Acad Sci USA 91: 6860–6864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronwald JW (1991) Lipid biosynthesis inhibitors. Weed Sci 39: 435–449 [Google Scholar]
- Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41: 95–98 [Google Scholar]
- Harwood JL (1988) Fatty acid metabolism. Annu Rev Plant Physiol 39: 101–138 [Google Scholar]
- Jelenska J, Sirikhachornkit A, Haselkorn R, Gornicki P (2002) The carboxyltransferase activity of the apicoplast acetyl-CoA carboxylase of Toxoplasma gondii is the target of aryloxyphenoxypropionate inhibitors. J Biol Chem 277: 23208–23215 [DOI] [PubMed] [Google Scholar]
- Joachimiak M, Tevzadze G, Podkowinski J, Haselkorn R, Gornicki P (1997) Wheat cytosolic acetyl-CoA carboxylase complements an ACC1 null mutation in yeast. Proc Natl Acad Sci USA 94: 9990–9995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi T, Shinohara K, Yamada K, Sasaki Y (1996) Acetyl-CoA carboxylase in higher plants: most plants other than gramineae have both the prokaryotic and the eukaryotic forms of this enzyme. Plant Cell Physiol 37: 117–122 [DOI] [PubMed] [Google Scholar]
- Letouzé A, Gasquez J (1999) A rapid reliable test for screening aryloxyphenoxypropionic acid resistance within Alopecurus myosuroides and Lolium spp. populations. Weed Res 39: 37–48 [Google Scholar]
- Nikolskaya T, Zagnitko O, Tevzadze G, Haselkorn R, Gornicki P (1999) Herbicide sensitivity determinant of wheat plastid acetyl-CoA carboxylase is located in a 400-amino acid fragment of the carboxyltransferase domain. Proc Natl Acad Sci USA 96: 14647–14651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podkowinski J, Sroga GE, Haselkorn R, Gornicki P (1996) Structure of a gene encoding a cytosolic acetyl-CoA carboxylase of hexaploid wheat. Proc Natl Acad Sci USA 93: 1870–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendina AR, Craig-Kennard AC, Beaudoin JD, Breen MK (1990) Inhibition of acetyl-coenzyme A carboxylase by two classes of grass-selective herbicides. J Agric Food Chem 38: 1282–1287 [Google Scholar]
- Sasaki Y, Konishi T, Nagano Y (1995) The compartimentation of acetylcoenzyme A carboxylase in plants. Plant Physiol 108: 445–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla A, Leach GE, Devine MD (1997) High-level resistance to sethoxydim conferred by an alteration in the target enzyme, acetyl-CoA carboxylase, in Setaria faberi and Setaria viridis. Plant Physiol Biochem 35: 803–807 [Google Scholar]
- Sommer SS, Groszbar AR, Bottema CDK (1992) PCR amplification of specific alleles (PASA) is a general method for rapidly detecting known single base-pair changes. Biotechniques 12: 82–87 [PubMed] [Google Scholar]
- Zagnitko O, Jelenska J, Tevzadze G, Haselkorn R, Gornicki P (2001) An isoleucine/leucine residue in the carboxyltransferase domain of acetyl-CoA carboxylase is critical for interaction with aryloxyphenoxypropionate and cyclohexanedione inhibitors. Proc Natl Acad Sci USA 98: 6617–6622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XQ, Devine MD (2000) A possible point mutation of plastidic ACCase gene conferring resistance to sethoxydim in green foxtail (Setaria viridis) (abstract no. 81). Weed Sci Soc Am 40: 33 [Google Scholar]
- Zuther E, Johnson JJ, Haselkorn R, McLeod R, Gornicki P (1999) Growth of Toxoplasma gondii is inhibited by aryloxyphenoxypropionate herbicides targeting acetyl-CoA carboxylase. Proc Natl Acad Sci USA 96: 13387–13392 [DOI] [PMC free article] [PubMed] [Google Scholar]