Abstract
Background
Infection with group B streptococcus (GBS) is a major cause of neonatal illness and death. We examined the antenatal and perinatal risk factors for early-onset GBS disease among neonates.
Methods
We identified cases by population-based surveillance in all microbiology laboratories serving Alberta. A case was defined as any instance of a positive sterile-site GBS culture in an infant born between 1993 and 1997 who was either less than 7 days old or stillborn after 20 weeks' gestation. We randomly selected controls from a computer-compiled list of all hospital births, including stillbirths after 20 weeks' gestation, in Alberta during the study period. To increase power, we chose 5 or 6 control infants born in the same year as each case infant. We reviewed hospital, prenatal clinic and physician health records and, between 1997 and 1999, conducted maternal interviews by telephone.
Results
There were no differences between the 90 cases and 489 controls in sociodemographic variables or in many reproductive and behavioural variables. Case infants were more likely than control infants to be of low birth weight (odds ratio [OR] 3.60, 95% confidence interval [CI] 1.68–7.65), to have been delivered preterm (OR 3.89, 95% CI 2.08–7.27), or to have a mother with amnionitis (OR 15.03, 95% CI 5.58–41.89), intrapartum fever (OR 4.65, 95% CI 2.48–8.69) or premature rupture of the membranes (OR 2.39, 95% CI 1.38–4.14). After adjustment for potential confounders, intrauterine fetal monitoring was associated with a more than 2-fold increase in the risk of neonatal GBS disease (OR 2.24, 95% CI 1.22–4.13).
Interpretation
Intrauterine fetal monitoring should be added to the list of risk factors in risk-based screening. Since many of the cases had no identifiable maternal risk factors, universal screening for GBS may be appropriate.
Over the last 3 decades, infection with group B streptococcus (GBS) has emerged as a major cause of neonatal mortality and morbidity.1,2,3 Before the implementation of preventive guidelines in 1994,4 Canadian rates of GBS infection ranged from 0.44 to 2.1 per 1000 live births,5 but they declined to 0.25 per 1000 by 1999.6,7 Risk factors for GBS infection identified in the guidelines included preterm delivery, previous infant with GBS infection, GBS bacteriuria, intrapartum fever and premature rupture of the membranes (more than 18 hours before delivery).
Most studies identifying risk factors for neonatal GBS infection have lacked a comparison group, have been institution-based rather than population-based or have not included maternal interviews. A population-based study in the United States that used maternal interviews to identify risk factors did not use health record information and was limited by a high refusal rate (76%).2,8,9,10,11 Finally, overmatching may have masked some risk factors.12
The magnitude of risk associated with identified risk factors has varied considerably.9,10,11,13,14,15 For example, studies in Australia11 and the United States15 found that 79% and 28%, respectively, of GBS-infected infants were preterm. Furthermore, many factors that may contribute to risk, such as sexual practices, use of prenatal medication, prenatal visits, vaginal examinations and intrauterine fetal monitoring, have not been thoroughly examined. A multistate US study16 suggested that almost 50% of all cases of GBS infection had none of the currently identified risk factors.
We examined known and new risk factors for GBS disease in all neonates in Alberta, using a population-based case–control study with multiple information sources.
Methods
We defined a case as any instance of a positive sterile-site GBS culture (e.g., of blood or cerebrospinal fluid) in an infant born between 1993 and 1997 who was either less than 7 days old or stillborn after 20 weeks' gestation.7 During this period, many obstetric care providers were following either the Canadian consensus guidelines17 or the American Academy of Pediatrics guidelines.18 We identified cases by population-based surveillance in all microbiology laboratories serving Alberta (1995 population 2.69 million and average annual birth rate 38 00019). The province is a mixed urban–rural region with defined geographic boundaries that are easily identified by the first character of the postal code (T). During the study period, there were 262 398 births (live and still). Laboratory audits were carried out 6 and 18 months after the start of the study and at completion of the study to confirm complete case ascertainment.7
We randomly selected controls from a computer-compiled list of all hospital births, including stillbirths after 20 weeks' gestation, in Alberta during the study period. To increase power, we chose 5 or 6 control infants born in the same year as each case infant.
We estimated the adequacy of prenatal care with the Kessner index,20 which categorizes prenatal care as inadequate, intermediate or adequate on the basis of the timing of the initiation of prenatal care, gestational age at delivery and the number of visits for prenatal care. For the multivariable models, we used 2 categories: adequate (including intermediate) and inadequate.21 Amnionitis or chorioamnionitis was noted if diagnosed by the attending physician and mentioned in the health record. Our classification of chronic diseases was similar to that used in the Ontario Health Survey.22
We developed the interview questionnaire using items from tested or standardized questionnaires, including the Behavioral Risk Factor Questionnaire,23 the Pregnancy, Infection, and Nutrition study,24 the National Alcohol and Drug Survey,25 the Drug Use Screening Inventory-Revised26 and the Canadian census. The questionnaire was translated into Chinese, French, Punjabi, Spanish, Urdu and Vietnamese. Techniques known to improve the validity of the responses and to minimize tendencies to provide socially desirable responses were used in questionnaire construction and the interviews.27,28,29,30,31
All telephone interviews occurred between 1997 and 1999. We tried contact telephone numbers as many times as required to determine whether the mother could be reached. Verbal and then mailed written consent was obtained for the interview and for access to hospital and prenatal clinic records. Interviews were scheduled and forms coded and given to 2 interviewers trained in cognitive interviewing techniques and blind to disease status.32,33,34
Using pretested, standard data collection forms, 2 trained nurses reviewed hospital and prenatal clinic charts, mailed questionnaires to physicians' offices to collect prenatal information and verify some chart information, and made reminder telephone calls to encourage completion of the questionnaires.
All data were entered into a database and analyzed with statistical software. For univariate analyses and differences between cases and controls in categorical variables, we used the Z test or the χ2 test for differences in proportions. For bivariate associations we used the χ2 test (or Fisher's exact test when the expected values were less than 5) and calculated odds ratios (ORs) and Cornfield 95% confidence intervals (CIs).35 We performed preliminary stratified analyses to inform variable selection for multivariate modelling and developed unconditional logistic regression models to examine important risk associations while adjusting for possible confounders, applying the Pearson χ2 to evaluate the fit of the models.36 Variables were included in the final model on the basis of their relations with the outcome in the bivariate and stratified analyses and after careful consideration of probable causal pathways. We examined the impact of colinearity by separately entering suspected colinear variables (e.g., duration of labour and premature rupture of the membranes, intrapartum fever and amnionitis) into the regression model. We based the power calculation on the prevalence of the exposure variable (the risk of preterm birth among GBS-positive infants versus all other Alberta infants): 6.8% in 1994 to 7.4% in 1996.37 The study had an 80% or greater power to show a relative risk of 2.30 or more when the risk exposure rate in controls was 10% with α = 0.05 and a relative risk of 3.00 or more when the risk exposure rate in controls was 5%.
The Conjoint Medical Research Ethics Board of the University of Calgary reviewed and approved the study protocol.
Results
Of the 92 cases of early-onset GBS disease identified between 1993 and 1997, a chart was not located for 1 case, and 1 mother refused to participate. Another 16 mothers (17.8% of the remaining 90 cases) consented to chart review but refused an interview or consented to an interview but could not be contacted for it; therefore, the chart was the only source of information in these cases. Of the 570 controls randomly selected, 11 were excluded by nonresidence. Of the remaining 559, 70 (12.5%) refused to participate and 6 (1.1%) refused only the interview; as well, 13 (2.7%) of those who consented to the interview could not be reached. Thus, the health records of 489 controls were reviewed, and 470 control interviews were conducted. All physician questionnaires were returned. Of the early-onset cases, excluding the 9 stillbirths, 11 (13.6%) resulted in death.
There were no differences between case and control infants in sociodemographic variables, including year of birth, sex, maternal residence, mother's marital status, socioeconomic status and year of interview (Table 1). Nor were there important differences in maternal reproductive history, including parity, previous spontaneous or therapeutic abortions, previous pregnancies or previous stillbirths, or in prevalence of maternal chronic disease (Table 2); notably, diabetes mellitus was present in 3 of 90 case mothers and 9 of the 489 control mothers, not a significant difference. However, significantly more case infants than control infants weighed less than 2500 g at birth, were born before 37 weeks' gestation or had a mother less than 20 years of age at the time of delivery (Table 2). In only 5 families (1 of 90 case families and 4 of 489 control families) had there previously been early-onset GBS infection in an infant. Although black race has been shown to be a risk factor for GBS infection in the United States, there were too few black patients (3) in this study to permit meaningful analysis.
Table 1
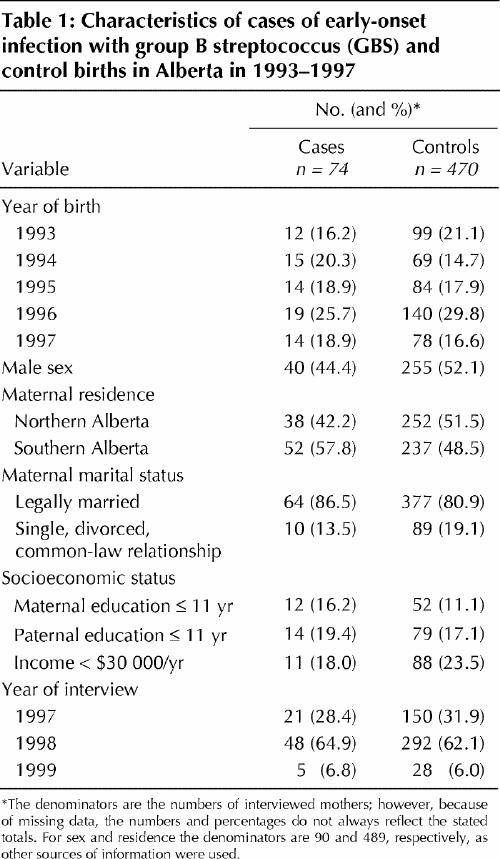
Table 2
From the interviews, neither oral contraceptive use nor intrauterine device (IUD) use was found to be associated with infant disease, whether measured as “ever used” or “duration of use” (data not shown). Sexual practices in the 6 months before pregnancy as well as during pregnancy, including frequency of intercourse and number of partners, were also not associated. Despite a high risk (OR = 6.41) associated with reported prostitution, this response was too rare for reliable estimation. Among substance use variables, including environmental exposure to tobacco smoke and use of tobacco and alcohol, no significant associations were found, but among those reporting extreme levels of substance use there were suggestive trends toward elevated risk. Neither over-the-counter nor prescription medication use was associated with GBS status (data not shown).
Rates of refusal to answer sensitive questions were generally very low. Among case families, there were no refusals. Among control families, the refusal rates were as follows: household income, 1.33%; oral contraceptive and IUD use, 0%; sexual practices, 0.2%–0.7%; smoking, alcohol and substance abuse, 0%.
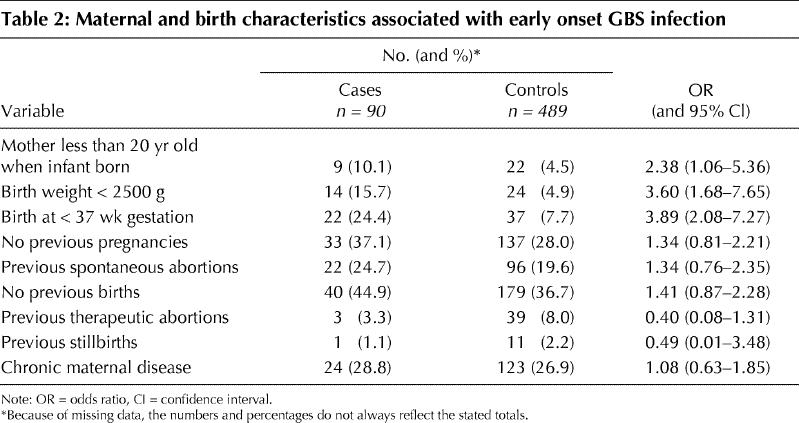
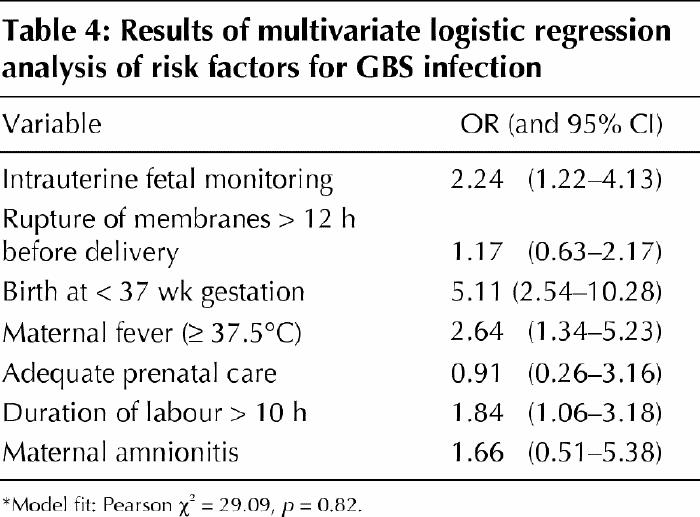
Distinct patterns of risk were evident for certain antepartum and intrapartum variables (Table 3). Risk was not associated with adequacy of prenatal care, attendance at prenatal classes, antenatal screening for GBS, antibiotic use (for either prophylaxis or therapy) either antenatally or during labour, or duration of labour. However, case status was strongly associated with indicators of active disease that were manifest during labour (premature rupture of the membranes [OR 2.39, 95% CI 1.38–4.14], maternal fever [OR 4.65, 95% CI 2.48–8.69] and amnionitis [OR 15.03, 95% CI 5.58–41.89]) as well as factors that represented consequences of disease (neonatal intubation [OR 3.42, 95% CI 1.65–7.06] and emergency cesarean section [OR 2.51, 95% CI 1.29–4.83]). The number of vaginal examinations and artificial rupture of the membranes showed no association with infant disease. However, the use of intrauterine fetal monitoring doubled the risk (OR 1.94, CI 1.09–3.42) in univariate analysis, and the magnitude of the association did not change (OR 2.24, 95% CI 1.22–4.13) after adjustment for premature rupture of the membranes, gestational age, maternal fever, number of prenatal visits, duration of labour and adequacy of prenatal care (Table 4). Of the 90 mothers of infants with GBS infection 42 had no identified risk factors.
Table 3
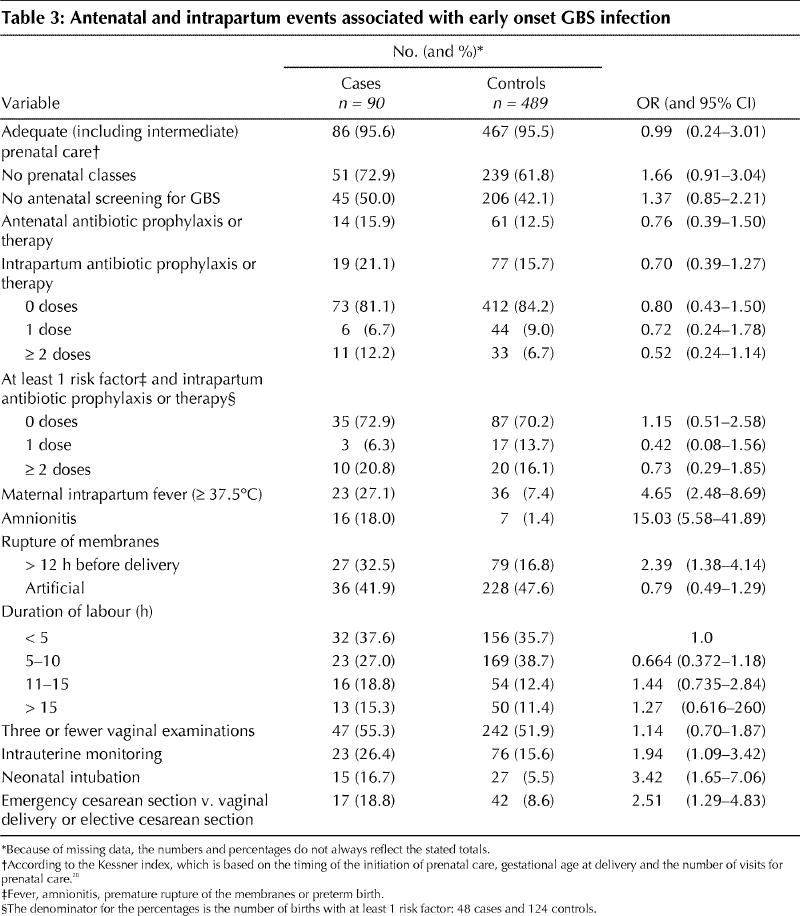
Table 4
Interpretation
Our study of neonatal GBS infection quantified risk factors in a population on the basis of multiple sources of information, including maternal interviews, caregiver questionnaires and chart reviews. We looked for both known and previously unknown factors. Intrauterine monitoring emerged as an independent risk factor. The hypothesis that monitoring increases the risk of GBS disease has biologic plausibility because there is a disruption of the skin barrier, which could allow GBS into the vascular system, as with herpetic infections.38,39,40,41,42,43,44,45
An association between intrauterine monitoring and amnionitis has been reported,46 but an association with early-onset GBS infection had not been confirmed owing to inadequacies of study design or sample size. In a multistate case–control study of 99 cases of early-onset GBS infection, internal monitor use was found to be associated with the disease by univariate but not multivariate analysis.2 The study may have been affected by selection bias, since the families of 76% of identified cases did not participate in the study. Adams and colleagues9 also found that, after adjustment, use of an intrauterine pressure catheter did not appear to be a risk factor for early-onset GBS disease. However, this study investigated an outbreak of 23 cases over an 8-month period. Because such outbreaks are unusual, it is not known whether these cases differ from those not part of an outbreak. Bramer and coworkers8 identified internal monitoring as a risk factor for GBS disease. However, owing to the small number of GBS-positive cultures (19), the case definition was extended to positive cultures from nonsterile sites. Yancey and associates47 found an association between internal monitoring for more than 12 hours and neonatal sepsis; 10 of 15 cases of culture-proven sepsis were due to GBS.
So far, ours is the largest and most complete study to examine this association. Our positive finding suggests that intrauterine monitoring be added to the list of risk factors for neonatal GBS disease. The association was stable after adjustment for potential confounding factors — those that might have led to a higher likelihood of intrauterine monitoring (inadequate prenatal care, maternal fever or amnionitis, prolonged labour and preterm delivery) — and thus reinforced our conclusion that intrauterine monitoring is an independent risk factor. However, universal screening for GBS may be more appropriate than using risk factors, given the absence of risk factors in nearly half the cases.
Frequency of vaginal examinations during labour was not associated with GBS disease, consistent with the theory that disruption of the skin or mucous membrane barrier, which occurs with intrauterine monitoring but not vaginal examination, is important in the pathogenesis of GBS disease. Univariate analyses have shown that 5 or more9 and 6 or more48 vaginal examinations, respectively, increase the risk of GBS disease. However, it is difficult to compare these studies because of differences in and lack of information about variables that may affect the risk of disease, such as the timing (before v. after rupture of the membranes) and the frequency of the exams.
Unlike previous studies in the United States,2,15 our study demonstrated no relation between socioeconomic factors and GBS disease. This is most likely due to the universal availability of health care in Canada, without concern for ability to pay. The lack of association in our study between intrapartum antibiotic prophylaxis and case status is not surprising, because there was some screening and prophylaxis during the study period, as recommended by expert bodies. However, the 81.1% of cases in which there was no intrapartum antibiotic prophylaxis represents a large group of women who may need different methods of prevention, especially since nearly half (35 of 73) had an identifiable risk factor (fever, amnionitis, premature rupture of the membranes or preterm birth). This problem reinforces the difficulty of using a risk-based approach.49,50
The random selection of controls minimized the risk of bias in this study. Refusal rates were low for both case and control groups. During the study period, the rates of low birth weight, preterm birth and neonatal death were very similar in the control group and the total neonatal population of Alberta (4.9% v. 5.9%, 7.6 v. 7.2% and 4.0 v. 4.2%, respectively).51 We minimized the risk of recall bias by conducting interviews at equal intervals from the time of birth for both case and control groups. The interviewers were blind to case status. No matching (other than frequency matching on year of birth) was used in this study. A matched case–control design has the risk of overmatching, which may hinder informative results or introduce confounding if the matching factor is correlated with exposure but not disease.12
A potential limitation of our study was use of the Kessner index to assess the adequacy of prenatal care. This index was designed primarily for a US population and has been criticized because it is heavily weighted toward the timing of the initiation of prenatal care, does not distinguish timing of initiation from poor subsequent frequency of visits and may inaccurately measure overall adequacy of care for term and post-term pregnancies.20 However, there is currently no suitable alternative, and this index has recently been used by other investigators.21
In conclusion, this study of neonatal GBS disease in a Canadian population identified an association of elevated disease risk with intrauterine monitoring. No risk factor profile predicted all infant disease before labour, which suggests that universal screening may be more appropriate for prevention.
Acknowledgments
This study was supported in part by grants from the National Health Research Development Program, the Alberta Health Services Research Innovation Fund and the Alberta Heritage Foundation for Medical Research (AHFMR). Dr. Davies is a Medical Scholar of the AHFMR.
Footnotes
Presented in part at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, 26–29 Sept. 1999 (abstract 1734, session 166L, p. 684).
This article has been peer reviewed.
Contributors: Dr. Adair contributed to the conception and design of the study, oversaw much of the running of the study and of the analyses, and co-drafted the paper and revised it for critical intellectual content. Ms. Kowalsky, Ms. Robertson and Ms. Mucenski contributed to the acquisition of data and the interviews and helped draft the paper. Mr. Quon contributed to the acquisition of data and helped draft the paper. Ms. Ma was responsible for most of the initial statistical analyses and helped with revision of the paper. Dr. Stoffman contributed to the design of the study and revision of the paper. Dr. McGeer contributed to the conception and design of the study and revision of the paper. Dr. Davies was the principal investigator, contributed to the conception and design of the study, was responsible for overall coordination of the study and analyses, co-drafted and revised the paper critically for important intellectual content, and was responsible for the final editing and submission. All authors approved the final version of the manuscript.
Competing interests: None declared.
Correspondence to: Dr. H. Dele Davies, Professor and Chair, Pediatrics and Human Development, Michigan State University, College of Human Medicine, B240 Life Sciences Bldg, East Lansing MI 48824; fax 517 353-8464; daviesde@msu.edu
References
- 1.Allardice JG, Baskett TF, Seshia MM, Bowman N, Malazdrewicz R. Perinatal group B streptococcal colonization and infection. Am J Obstet Gynecol 1982; 142 (6 pt 1):617-20. [DOI] [PubMed]
- 2.Schuchat A, Deaver-Robinson K, Plikaytis BD, Zangwill KM, Mohle-Boetani J, Wenger JD. Multistate case–control study of maternal risk factors for neonatal group B streptococcal disease. The Active Surveillance Study Group. Pediatr Infect Dis J 1994;13:623-9. [DOI] [PubMed]
- 3.Eickhoff T, Klein J, Daly A, Ingall D, Finland M. Neonatal sepsis and other infections due to group B beta hemolytic streptococci. N Engl J Med 1964;271: 1221-8. [DOI] [PubMed]
- 4.Schrag S, Zywicki S, Farley M, Reingold A, Harrison L, Lefkowitz L, et al. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med 2000;342:15-20. [DOI] [PubMed]
- 5.Davies HD, LeBlanc J, Bortolussi R, McGeer A, PICNIC. The Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) study of neonatal group B streptococcal infections in Canada. Pediatr Child Health 1999; 4:257-63. [PMC free article] [PubMed]
- 6.Davies HD, Adair CE, Schuchat A, Low DE, Sauve RS, McGeer A. Physicians' prevention practices and incidence of neonatal group B streptococcal disease in 2 Canadian regions. CMAJ 2001;164:479-85. [PMC free article] [PubMed]
- 7.Davies HD, Raj S, Adair C, Robinson J, McGeer A. Population-based active surveillance for neonatal group B streptococcal infections in Alberta, Canada: implications for vaccine formulation. Pediatr Infect Dis J 2001;20:879-84. [DOI] [PubMed]
- 8.Bramer S, van Wijk FH, Mol BW, Adriaanse AH. Risk indicators for neonatal early-onset GBS-related disease. A case–control study. J Perinat Med 1997; 25: 469-75. [DOI] [PubMed]
- 9.Adams WG, Kinney JS, Schuchat A, Collier CL, Papasian CJ, Kilbride HW, et al. Outbreak of early onset group B streptococcal sepsis. Pediatr Infect Dis J 1993; 12:565-70. [DOI] [PubMed]
- 10.Yagupsky P, Menegus MA, Powell KR. The changing spectrum of group B streptococcal disease in infants: an eleven-year experience in a tertiary care hospital. Pediatr Infect Dis J 1991;10:801-8. [DOI] [PubMed]
- 11.Spaans WA, Knox AJ, Koya HB, Mantell CD. Risk factors for neonatal infection. Aust N Z J Obstet Gynaecol 1990;30:327-30. [DOI] [PubMed]
- 12.Rothman KJ. Modern epidemiology. Toronto: Little, Brown; 1996.
- 13.McDonald H, Vigneswaran R, O'Loughlin JA. Group B streptococcal colonization and preterm labour. Aust N Z Obstet Gynaecol 1989;29(3 pt 2):291-3. [DOI] [PubMed]
- 14.Persson K, Bjerre B, Elfstrom L, Polberger S, Forsgren A. Group B streptococci at delivery: high count in urine increases risk for neonatal colonization. Scand J Infect Dis 1986;18:525-31. [DOI] [PubMed]
- 15.Schuchat A, Oxtoby M, Cochi S, Sikes RK, Hightower A, Plikaytis B, et al. Population-based risk factors for neonatal group B streptococcal disease: results of a cohort study in metropolitan Atlanta. J Infect Dis 1990;162:672-7. [DOI] [PubMed]
- 16.Rosenstein NE, Schuchat A. Opportunities for prevention of perinatal group B streptococcal disease: a multistate surveillance analysis. The Neonatal Group B Streptococcal Disease Study Group. Obstet Gynecol 1997;90:901-6. [DOI] [PubMed]
- 17.Canadian Paediatric Society and the Society of Obstetricians and Gynaecologists of Canada. The prevention of early-onset group B streptococcal infections in the newborn [consensus statement]. Can J Infect Dis 1994;5:251-6. [DOI] [PMC free article] [PubMed]
- 18.American Academy of Pediatrics Committee on Infectious Diseases and Committee on Fetus and Newborn: guidelines for prevention of group B streptococcal (GBS) infection by chemoprophylaxis. Pediatrics 1992;90:775-8. [PubMed]
- 19.Alberta Vital Statistics. Annual review, 1995. Edmonton: Government of Alberta; 1995. p. 58.
- 20.Kotelchuck M. An evaluation of the Kessner Adequacy of Prenatal Care Index and a proposed Adequacy of Prenatal Care Utilization Index. Am J Public Health 1994;84:1414-20. [DOI] [PMC free article] [PubMed]
- 21.Schrag SJ, Zell ER, Lynfield R, Roome A, Arnold KE, Craig AS, et al. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N Engl J Med 2002;347:233-9. [DOI] [PubMed]
- 22.Premier's Council on Health Well-Being and Social Justice. Highlights of the Ontario Health Survey. Toronto: Ontario Ministry of Health; 1992.
- 23.Stein AD, Courval JM, Lederman RI, Shea S. Reproducibility of responses to telephone interviews: demographic predictors of discordance in risk factor status. Am J Epidemiol 1995;141:1097-105. [DOI] [PubMed]
- 24.Sayle AE, Savitz DA, Thorp JM Jr, Hertz-Picciotto I, Wilcox AJ. Sexual activity during late pregnancy and risk of preterm delivery. Obstet Gynecol 2001;97:283-9. [DOI] [PubMed]
- 25.Single E, Wortley S. A comparison of alternative measures of alcohol consumption in the Canadian National Survey of alcohol and drug use. Addiction 1994;89:395-9. [DOI] [PubMed]
- 26.Tarter R, Kirisci L. The Drug Use Screening Inventory for adults: psychometric structure and discriminative sensitivity. Am J Drug Alcohol Abuse 1997; 23: 207-19. [DOI] [PubMed]
- 27.Aday L. Designing and conducting health surveys. San Francisco: Jossey-Bass; 1989. p. 177-261.
- 28.Babor TF, Brown J, Del Boca FK. Validity of self-reports in applied research on addictive behaviors: Fact or fiction? Addict Behav 1990;12:5-13.
- 29.Friedenreich C, Slimani N, Riboli E. Measurement of past diet: review of previous and proposed methods. Epidemiol Rev 1992;14:177-96. [DOI] [PubMed]
- 30.Streiner D, Norman G. Health measurement scales: a practical guide to their development and use. Oxford: Oxford Medical Publications; 1989. p. 15-82.
- 31.Sudman S, Bradburn NM. Asking questions. San Francisco: Jossey-Bass; 1983. p. 54-88.
- 32.Bercini DH. Pretesting questionnaires in the laboratory: an alternative approach. J Expo Anal Environ Epidemiol 1992;2:241-8. [PubMed]
- 33.Jabine TB, Straf ML, Tanur JM, Tourangeau R. Cognitive aspects of survey methodology: building a bridge between disciplines. Washington: National Academy Press; 1984. p. 13-20.
- 34.Jobe JB, Mingay DJ. Cognitive research improves questionnaires. Am J Public Health 1989;79:1053-5. [DOI] [PMC free article] [PubMed]
- 35.Schlesselman: Case–control studies: design, conduct, analysis. New York: Oxford University Press; 1982. p. 177-8.
- 36.Hosmer DW, Lemeshow S. Applied logistic regression. New York: John Wiley & Sons; 1989. p. 138-9.
- 37.Alberta Health and Wellness. Alberta reproductive health: pregnancy outcomes report. Edmonton: Government of Alberta; 2001. p. 32.
- 38.Amann ST, Fagnant RJ, Chartrand SA, Monif GR. Herpes simplex infection associated with short-term use of a fetal scalp electrode: a case report. J Reprod Med 1992;37:372-4. [PubMed]
- 39.Cordero L, Anderson CW, Zuspan FP. Scalp abscess: a benign and infrequent complication of fetal monitoring. Am J Obstet Gynecol 1983;146:126-30. [DOI] [PubMed]
- 40.Driscoll DJ, Raab K. Herpes scalp infection associated with fetal electrode placement. Wis Med J 1976;75(2):9-10. [PubMed]
- 41.Goldkrand JW. Intrapartum inoculation of herpes simplex virus by fetal scalp electrode. Obstet Gynecol 1982;59:263-5. [PubMed]
- 42.Guill MA, Aton JK, Rogers RB. Neonatal herpes simplex associated with fetal scalp monitor. J Am Acad Dermatol 1982;7:408-9. [DOI] [PubMed]
- 43.Kaye EM, Dooling EC. Neonatal herpes simplex meningoencephalitis associated with fetal monitor scalp electrodes. Neurology 1981;31:1045-7. [DOI] [PubMed]
- 44.Parvey LS, Ch'ien LT. Neonatal herpes simplex virus infection introduced by fetal-monitor scalp electrodes. Pediatrics 1980;65:1150-3. [PubMed]
- 45.Brown ZA, Benedetti J, Ashley R, Burchett S, Selke S, Berry S, et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med 1991;324:1247-52. [DOI] [PubMed]
- 46.Yancey MK, Duff P, Clark P, Kurtzer T, Frentzen BH, Kubilis P. Peripartum infection associated with vaginal group B streptococcal colonization. Obstet Gynecol 1994;84:816-9. [PubMed]
- 47.Yancey MK, Duff P, Kubilis P, Clark P, Frentzen BH. Risk factors for neonatal sepsis. Obstet Gynecol 1996;87:188-94. [DOI] [PubMed]
- 48.Schuchat A, Zywicki SS, Dinsmoor MJ, Mercer B, Romaguera J, O'Sullivan MJ, et al. Risk factors and opportunities for prevention of early-onset neonatal sepsis: a multicenter case-control study. Pediatrics 2000;105(1 pt 1):21-6. [DOI] [PubMed]
- 49.Rouse DJ, Goldenberg RL, Cliver SP, Cutter GR, Mennemeyer ST, Fargason CA Jr. Strategies for the prevention of early-onset neonatal group B streptococcal sepsis: a decision analysis [review]. Obstet Gynecol 1994;83:483-94. [DOI] [PubMed]
- 50.Mohle-Boetani JC, Schuchat A, Plikaytis BD, Smith JD, Broome CV. Comparison of prevention strategies for neonatal group B streptococcal infection: a population-based economic analysis. JAMA 1993;270:1442-8. [PubMed]
- 51.Alberta Health and Wellness. Alberta reproductive health: pregnancy outcomes. Edmonton: Government of Alberta; 1999. p. 16-36.


