Abstract
Arabinogalactan proteins (AGPs), a superfamily of plant hydroxyproline-rich glycoproteins, are present at cell surfaces. Although precise functions of AGPs remain elusive, they are widely implicated in plant growth and development. A well-characterized classical tomato (Lycopersicon esculentum) AGP containing a glycosylphosphatidylinositol plasma membrane anchor sequence was used here to elucidate functional roles of AGPs. Transgenic tobacco (Nicotiana tabacum) Bright Yellow-2 (BY-2) cells stably expressing green fluorescent protein (GFP)-LeAGP-1 were plasmolysed and used to localize LeAGP-1 on the plasma membrane and in Hechtian strands. Cytoskeleton disruptors and β-Yariv reagent (which binds and perturbs AGPs) were used to examine the role of LeAGP-1 as a candidate linker protein between the plasma membrane and cytoskeleton. This study used a two-pronged approach. First, BY-2 cells, either wild type or expressing GFP-microtubule (MT)-binding domain, were treated with β-Yariv reagent, and effects on MTs and F-actin were observed. Second, BY-2 cells expressing GFP-LeAGP-1 were treated with amiprophosmethyl and cytochalasin-D to disrupt MTs and F-actin, and effects on LeAGP-1 localization were observed. β-Yariv treatment resulted in terminal cell bulging, puncta formation, and depolymerization/disorganization of MTs, indicating a likely role for AGPs in cortical MT organization. β-Yariv treatment also resulted in the formation of thicker actin filaments, indicating a role for AGPs in actin polymerization. Similarly, amiprophosmethyl and cytochalasin-D treatments resulted in relocalization of LeAGP-1 on Hechtian strands and indicate roles for MTs and F-actin in AGP organization at the cell surface and in Hechtian strands. Collectively, these studies indicate that glycosylphosphatidylinositol-anchored AGPs function to link the plasma membrane to the cytoskeleton.
Arabinogalactan proteins (AGPs) are a class of cell surface plant proteoglycans that may mediate signal transduction at the cell wall-plasma membrane interface (Kjellbom et al., 1997; Gao and Showalter, 1999; Showalter, 2001; Kohorn, 2001). AGPs belong to a superfamily of Hyp-rich glycoproteins that have a wide taxonomic distribution in the plant kingdom (Fincher et al., 1983; Nothnagel, 1997; Showalter, 2001). AGPs are typically composed of 90% carbohydrate and 10% protein (Showalter and Varner, 1989; Showalter, 1993; Nothnagel, 1997). They consist of a peptide backbone on which Hyp residues are glycosylated with type II arabinogalactans and arabinosides (Pope, 1977; Qi et al., 1991). Moreover, several AGPs are characterized by a C-terminal glycosylphosphatidylinositol (GPI) anchor that allows for their attachment to the outer leaflet of the plasma membrane (Youl et al., 1998; Sherrier et al., 1999; Svetek et al., 1999; Sun et al., 2004). Despite the vast information on plant cell wall biochemistry and structure, little is known regarding the molecular components responsible for the dynamic connections between the cell wall, plasma membrane, and cytoskeleton (Knox, 1992; Carpita and Gibeaut, 1993; Wyatt and Carpita, 1993; Roberts, 1994; Darley et al., 2001; Martin et al., 2001; Gouget et al., 2006). Unlike animals, where extracellular matrix (ECM) proteins such as integrins are involved in the ECM-plasma membrane-cytoskeleton continuum and various signaling processes (Stupack and Cheresh, 2002; Katsumi et al., 2004), a number of proteins are suggested to play a role in mediating these connections in plants (Kohorn, 2000). In this context, AGPs may function as potential candidates at the cell surface to mediate signal transduction via the cell wall-plasma membrane-cytoskeleton continuum.
Several approaches are being used to elucidate AGP functions. One approach uses Yariv phenylglycosides (Yariv et al., 1962, 1967), which selectively bind and perturb AGPs to probe their functions (Serpe and Nothnagel, 1994; Willats and Knox, 1996; Showalter, 2001; Guan and Nothnagel, 2004). Another approach uses antibodies to track and/or perturb AGPs to provide functional insights. More recently, forward and reverse genetics approaches are being used to analyze specific AGP gene functions (van Hengel and Roberts, 2003; Gaspar et al., 2004). Although the specific functions of AGPs remain elusive, these studies have implicated AGPs in plant growth and developmental processes such as female gametogenesis (Acosta-Garcia and Vielle-Calzada, 2004), cell proliferation (Serpe and Nothnagel, 1994; Langan and Nothnagel, 1997), cell differentiation (Schindler et al., 1995), somatic embryogenesis (Thompson and Knox, 1998; van Hengel et al., 2001), cell expansion (Ding and Zhu, 1997), pollen germination and growth (Cheung et al., 1995), root regeneration and seed germination (van Hengel and Roberts, 2003), hormone responses (Park et al., 2003), and programmed cell death (PCD; Chaves et al., 2002).
Little is known regarding the role of AGPs in aspects related to the plant cytoskeleton. A recent study on the Arabidopsis (Arabidopsis thaliana) reb-1 mutant has suggested the connection between AGPs and microtubules (MTs; Andème-Onzighi et al., 2002). These mutants have a root epidermal bulger phenotype and show decreased amount of AGPs and disorganized cortical MT arrays (Andème-Onzighi et al., 2002). Ding and Zhu (1997) demonstrated that β-Yariv reagent treatment of the Arabidopsis seedling leads to epidermal cell bulging and phenocopies the reb-1 mutant. Studies are required to demonstrate that cell bulging is related to apparent changes in AGP-cortical MT connections. Another major protein component of the plant cytoskeleton, actin filaments help to maintain cell architecture and control tip growth polarity in most plants (Pierson and Cresti, 1992; Geitmann and Emons, 2000; Staiger, 2000). With studies showing the presence of AGPs on the growing tips of root hairs and pollen tubes (Samaj et al., 1999; Mollet et al., 2002) and a role for actin in signaling pathways initiated at the plasma membrane interface (Volkmann and Baluska, 1999; Staiger, 2000; Samaj et al., 2002), it remains to be demonstrated whether there are any connections between AGPs and actin.
A modular tomato (Lycopersicon esculentum) AGP, LeAGP-1 is a well-characterized Lys-rich AGP and is at our disposal for elucidating molecular interactions of AGPs (Gao et al., 1999; Gao and Showalter, 2000; Sun et al., 2004). A transgenic tobacco (Nicotiana tabacum) Bright Yellow-2 (BY-2) cell line expressing fusion protein green fluorescent protein (GFP)-LeAGP-1 was used to demonstrate that LeAGP-1 is a GPI-anchored plasma membrane AGP with potential roles in cell signaling pathways and matrix remodeling (Sun et al., 2004). Here, we employ this cell line as well as a cell line expressing GFP-MT-binding domain (MBD; of the MT-associated protein 4; Marc et al., 1998; Granger and Cyr, 2000) to study the selective perturbation of the cytoskeleton (MT and actin) and AGPs to demonstrate a cell surface network involving interactions among AGPs, MTs, and F-actin.
RESULTS
Alterations in Localization of AGPs Induced by Cytoskeletal Disruptors and β-Yariv Reagent in BY-2 Cells Expressing GFP-LeAGP-1 Before and After Plasmolysis
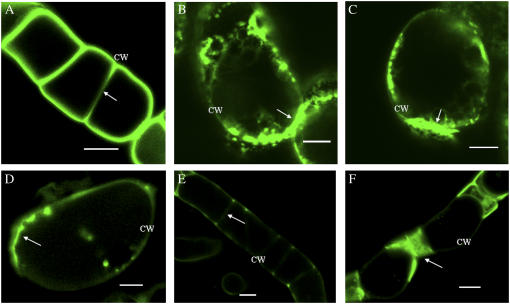
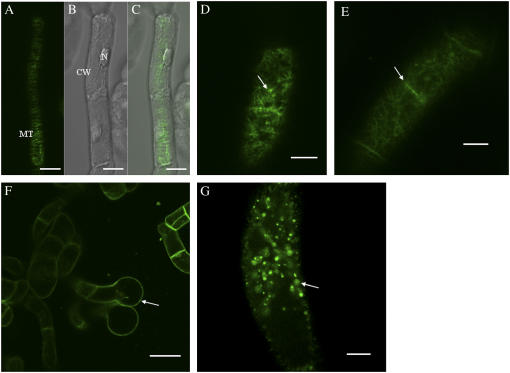
GFP-LeAGP-1 was expressed uniformly at the cell surface in transgenic BY-2 cells stably expressing GFP-LeAGP-1 (Fig. 1A). These transgenic cells were treated with cytoskeletal disruptors and β-Yariv reagent, and the resulting distribution of GFP fluorescence was analyzed using confocal laser scanning microscopy (CLSM). In the presence of 20 μm cytochalasin-D, an F-actin inhibitor, BY-2 cells showed a nonuniform distribution of GFP-LeAGP-1 at the cell surface and a pronounced accumulation of fluorescence at cell-cell adhesion zones (Fig. 1, B and C). BY-2 cells treated with 80 μm β-Yariv reagent for 7 h bound to GFP-LeAGP-1 on the cell surface and resulted in its nonuniform distribution (Fig. 1D). Prolonged treatment of 6-d-old cells with β-Yariv reagent (24 h) decreased GFP fluorescence on the cell surface (Fig. 1E), but in older cells (13 d) this treatment resulted in GFP-LeAGP-1 localization at end wall regions of adjoining cells (Fig. 1F). This pattern can be attributed to inaccessibility of β-Yariv reagent to the cell surfaces of adjoining cells.
Figure 1.
CLSM images showing the distribution of GFP fluorescence in tobacco BY-2 cells expressing GFP-LeAGP-1 following treatment with cytochalasin-D and β-Yariv reagent. BY-2 cells (6 d) were washed with fresh SH media and treated with cytochalasin-D or β-Yariv reagent. A, Control cells showed expression of the GFP-LeAGP-1 fusion protein on the cell surface (adjacent cell walls are marked with an arrow). B and C, Cytochalasin-D (20 μm for 2 h) treatment resulted in nonuniform distribution of GFP-LeAGP-1 fluorescence at the cell surface and enhanced accumulation at the cell-cell adhesion zone (arrows). D, Treatment with 80 μm β-Yariv reagent for 7 h showed an uneven distribution of LeAGP-1 on the cell surface. E and F, Prolonged β-Yariv treatment (80 μm for 24 h) resulted in a loss of GFP fluorescence at the cell surfaces and localization of GFP-LeAGP-1 at end wall regions between adjacent cells (arrows). Images are either a single optical section (F) or combined projections of multiple optical sections from a confocal Z-series (A–E). CW, Cell wall. Bars = 20 μm (A, E, and F) and 10 μm (B–D).
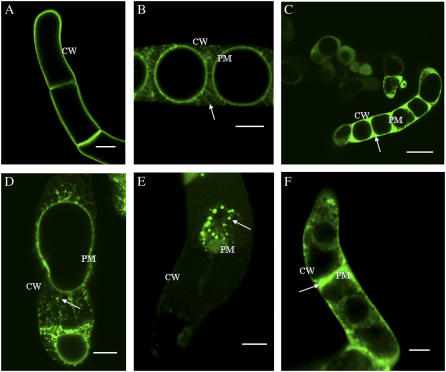
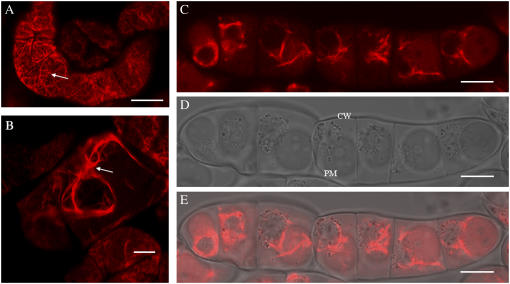
Previous studies employing immunolocalization and western analysis demonstrated that LeAGP-1 was localized to the plasma membrane and Hechtian strands (Sun et al., 2004). These previous results were confirmed using plasmolysis with 4% NaCl to reveal plasma membrane and Hechtian strand localization of GFP-LeAGP-1 (Fig. 2, A and B). This plasmolysis treatment was used as a control treatment in experimental analysis following treatments with cytoskeletal disruptors and β-Yariv reagent to reveal the locations of plasma membranes and Hechtian strands.
Figure 2.
Treatment of tobacco BY-2 cells (6 d) expressing GFP-LeAGP-1 with cytochalasin-D and β-Yariv reagent followed by plasmolysis. A, GFP-LeAGP-1 was localized on the surface of nonplasmolysed BY-2 cells. B, Plasmolysed (4% NaCl for 15 min) BY-2 cells showed GFP-LeAGP-1 was distributed on Hechtian strands (arrow) and plasma membranes. In the following sections, BY-2 cells were treated with cytochalasin-D or β-Yariv reagent followed by plasmolysis with 4% NaCl during the last 15 min. C and F, β-Yariv reagent treatment (100 μm for 1.5 h) caused relocalization of GFP-LeAGP-1 from Hechtian strands to the periplasmic space (arrow indicates plasmolysed cell). D, BY-2 cells (6 d) treated with a low concentration of cytochalasin-D (20 μm for 1 h) relocalized GFP-LeAGP-1 (arrow) from Hechtian strands to the periplasm. E, BY-2 cells (13 d) treated with a high concentration of cytochalasin-D (50 μm for 1 h) triggered disruption of a significant number of Hechtian strands and relocalized GFP-LeAGP-1 (arrow). Images were single optical sections (B and D) or combined projections of multiple optical sections from a confocal Z-series (A, C, E, and F). CW, Cell wall; PM, plasma membrane. Bars = 20 μm (A, B, D, and E), 50 μm (C), and 10 μm (F).
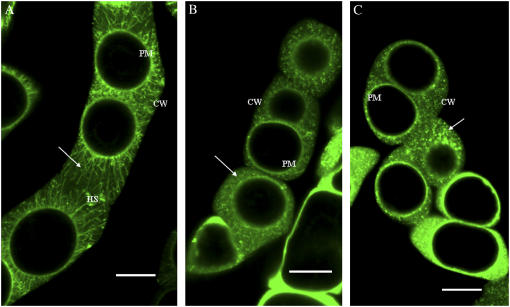
When transgenic BY-2 cells stably expressing GFP-LeAGP-1 were treated with β-Yariv reagent and subjected to plasmolysis, GFP-LeAGP-1 was no longer localized to Hechtian strands and was instead dispersed in the periplasmic space (Fig. 2, C and F). BY-2 cells treated with a low concentration of cytochalasin-D (20 μm for 1 h) exhibited a relocalization of LeAGP-1 on Hechtian strands (Fig. 2D), whereas treatment of older BY-2 cells (13 d) with higher concentrations of cytochalasin-D (e.g. 50 μm for 1 h) resulted in a dramatic relocalization of GFP-LeAGP-1 from Hechtian strands to the cytoplasm (Fig. 2E). In contrast, when the transgenic BY-2 cells were treated with amiprophosmethyl (APM; 30 μm for 45 min) followed by plasmolysis, this treatment resulted in a relocalization of GFP-LeAGP-1 from Hechtian strands to a punctate distribution pattern in the periplasmic space (Fig. 3).
Figure 3.
APM treatment of BY-2 cells (6 d) expressing GFP-LeAGP-1. A, BY-2 cells plasmolysed with 4% NaCl for 10 to 15 min showed strands (arrow) connecting the cell wall and the plasma membrane. B and C, Relocalization of GFP-LeAGP-1 from Hechtian strands to a punctate distribution pattern in the periplasmic space was seen in BY-2 cells after 30 μm APM treatment for 1 h followed by plasmolysis with 4% NaCl during the last 15 min of treatment (arrows mark the punctate distribution pattern in the periplasmic space). All images are single optical sections. CW, Cell wall; PM, plasma membrane; HS, Hechtian strands. Bars = 20 μm (A–C).
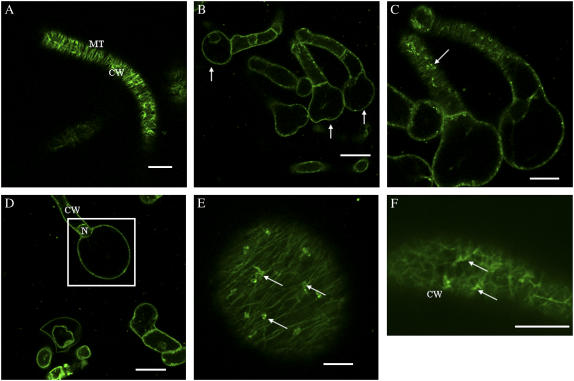
Alterations in Cortical MT Arrays and Cell Morphology Induced by β-Yariv Reagent in BY-2 Cells Expressing GFP-MBD
To visualize cortical MTs, BY-2 cells expressing GFP-MBD were used either directly (unwashed) or washed with fresh N. tabacum (NT-1) media prior to treatments with Yariv reagent and APM. Compared to unwashed control cells (Fig. 4A), unwashed cells treated with 100 μm Yariv for 24 h exhibited characteristic terminal cell bulger phenotypes (Fig. 4, B–D). Optical sectioning of these bulged cells revealed the formation of depolymerization products, or puncta (Fig. 4E). Treatment of unwashed control cells with 100 μm β-Yariv for 5 h resulted in disorganization and depolymerization of cortical MTs (Fig. 4F). Depolymerization of cortical MTs was evident by their decreased number and observation of oligomers of tubulin subunits, as reported by Sivaguru et al. (2003).
Figure 4.
Confocal images showing depolymerized cortical MT arrays and the “terminal bulger” phenotype in unwashed tobacco BY-2 cells (4 d) expressing GFP-MBD in response to β-Yariv treatment. A, Control BY-2 cells expressing GFP-MBD displayed transverse arrays of cortical MTs. B, C, D, and E, Treatment of BY-2 cells expressing GFP-MBD with 100 μm β-Yariv reagent for 24 h resulted in cell bulging (arrows in B indicate the terminal cell bulger) and characteristic depolymerization products, puncta, in C and E (arrows). D and E, MTs within the bulged cell (inset box in D) are shown as a single optical section in image (E). F, Fluorescent image showing disorganized and depolymerized MTs (arrows) after β-Yariv treatment (100 μm for 5 h). Images were single optical sections (A, E, and F) or combined projections of multiple optical sections from a confocal Z-series (B–D). CW, Cell wall; N, nucleus; PM, plasma membrane. Bars = 20 μm (A, B, D, and F), 10 μm (C), and 5 μm (E).
Control BY-2 cells expressing GFP-MBD were washed with fresh NT-1 media and revealed transverse arrays of cortical MTs (Fig. 5, A–C) similar to those seen in unwashed control cells. However, in contrast to unwashed cells, washed BY-2 cells treated with 5 μm Yariv reagent for 5 h displayed disorganization of cortical MTs and also displayed fluorescence decoration at the walls of adjoining cells (Fig. 5D). The washing treatment effectively lowers the concentration of Yariv reagent required to elicit cortical MT disorganization by washing away AGPs secreted into the media. Washed cells treated with higher concentrations of β-Yariv reagent (100 μm for 5 h) caused disorganization of cortical MTs resulting in terminal cell bulging (Fig. 5F) as well as enhanced fluorescent labeling of cortical MTs underlying the end walls of adjacent cells (Fig. 5E). A positive control treatment with APM demonstrated extreme depolymerization of cortical MTs with characteristic depolymerization products appearing within 45 min (Fig. 5G).
Figure 5.
β-Yariv treatment of washed BY-2 cells (4 d) expressing GFP-MBD. Control BY-2 cells showing transverse arrays of cortical MTs using a fluorescent filter (A) and the phase contrast image (B). Superimposed image of A and B is shown in image C. D, BY-2 cells treated with 5 μm β-Yariv reagent for 5 h showed disorganization (arrow) of the cortical MTs in most (70%) of the cells. E, Treatment with 80 μm β-Yariv reagent for 5 h resulted in disorganization of cortical MT arrays (arrow points to the deposition of fluorescence in the end wall region). F, Prolonged exposure (80 μm; 5 h) to β-Yariv reagent produced a terminal cell bulger phenotype (arrow). G, Positive control treatment with 30 μm APM for 1 h resulted in extreme depolymerization (arrow) of cortical MTs. Images are single optical sections (A–E and G) or combined projections of multiple optical sections from a confocal Z-series (F). CW, Cell wall; N, nucleus. Bars = 5 μm (A, E, and F), 10 μm (B–D and H), and 20 μm (G).
Alterations in F-Actin Arrays Induced by β-Yariv Reagent in BY-2 Cells
Wild-type BY-2 cells were fluorescently labeled with rhodamine-phalloidin to localize F-actin (Fig. 6A). BY-2 cells were washed with NT-1 media prior to treatment. BY-2 cells were treated with β-Yariv reagent or cytochalasin-D, fixed, and immunolabeled to observe the distribution of F-actin. Treatment with β-Yariv reagent (80 μm for 1 h) showed formation of thicker cortical actin filaments (Fig. 6B). A positive control treatment with cytochalasin-D, an F-actin inhibitor, depolymerized F-actin arrays (Fig. 6, C–E).
Figure 6.
F-actin (labeled with rhodamine-phalloidin) distribution in wild-type BY-2 cells (3 d) in response to treatment with Yariv reagent and cytochalasin-D. A, Control CSLM image showing F-actin arrays (arrow) in BY-2 cells. B, Yariv reagent treatment (80 μm for 1 h) resulted in formation of thicker actin cables (arrow). C to E, Positive control treatment with cytochalasin-D (20 μm for 1 h) depolymerizes actin strands. Fluorescent image (C) and phase contrast image (D) show depolymerized actin strands and their corresponding superimposed image is shown as image E. CW, Cell wall; PM, plasma membrane. Bars = 20 μm (A, C–E) and 10 μm (B).
DISCUSSION
Although AGP mutants in Arabidopsis have been reported with specific phenotypes, a clear understanding of AGP function, mode of action, and molecular interactions remains elusive (Park et al., 2003; Shi et al., 2003; van Hengel and Roberts, 2003; Acosta-Garcia and Vielle-Calzada, 2004; Gaspar et al., 2004; Motose et al., 2004). Consistent with the approach of identifying molecular interactions of AGPs, we employed β-Yariv reagent and cytoskeleton inhibitors to conduct in vivo studies at the cellular level to examine molecular interactions between AGPs and the cytoskeleton (MTs and F-actin). Specifically, we demonstrate that β-Yariv treatment triggers responses in cortical MT and F-actin networks; conversely, cytoskeleton inhibitors relocalize LeAGP-1 in Hechtian strands and the periplasmic space.
Previous studies have shown that β-Yariv disruption of AGPs at the cell wall plasma membrane interface results in inhibition of growth in cell cultures (Serpe and Nothnagel, 1994; Gao and Showalter, 1999; Guan and Nothnagel, 2004) and plants (Ding and Zhu, 1997; Roy et al., 1998). In nonplasmolyzed BY-2 cells, β-Yariv and cytochalasin-D treatment affects localization of GFP-LeAGP-1 and results in pronounced fluorescence at end wall regions (Fig. 1F) and cell-cell adhesion zones (Fig. 1, B and C), respectively. This pronounced fluorescence may result from β-Yariv's inaccessibility to end wall regions. Although the exact mechanism of AGP-Yariv binding is unknown, Yariv's structural configuration allows it to associate with AGP molecules to form aggregated AGP-Yariv complexes. Moreover, Yariv enters into the cell wall where it binds AGPs at the cell wall-plasma membrane interface, thereby disrupting interactions of AGPs with other molecular components in the cell wall and plasma membrane (Serpe and Nothnagel, 1994). A recent report demonstrated that sonic disruption of tobacco BY-2 cells results in a large pool of cell surface soluble AGPs that are localized at the zone of plasma membrane-cell wall interface known as periplasm (Lamport et al., 2006).
In plant cells, signal transduction of developmental and environmental cues is believed to be perceived through a route of cell wall, plasma membrane, and cytoskeleton (Wyatt and Carpita, 1993; Baluska et al., 2003). Unlike animal cells where a family of transmembrane receptors termed integrins participate in the ECM-plasma membrane-cytoskeleton continuum (Hynes, 1992), plant cells lack integrin homologs (Baluska et al., 2003). Integrins in animal cells interact with ECM proteins such as vitronectin, fibronectin, collagen, and laminin via an Arg-Gly-Asp motif (Hynes, 1999). In plant cells, plasmolysis reveals the existence of adhesion zones between the cell wall and protoplast (Lang-Pauluzzi, 2000). Thread-like structures known as Hechtian strands are seen in the adhesion zones that connect the cell wall to the plasma membrane (Hecht, 1912; Lang-Pauluzzi, 2000). Hechtian strands are suggested to play significant roles in signal transduction and cell-cell communication events (Zandomeni and Schopfer, 1994; Reuzeau et al., 1997; Canut et al., 1998; Glass et al., 2000). In plants, application of Arg-Gly-Asp peptides results in disruption of Hechtian strands accompanied by increased pathogen susceptibility (Mellersh and Heath, 2001) and loss in signaling between cell wall and plasma membrane (Kiba et al., 1998). Studies indicate that connections between the plasma membrane and cell wall can be mediated by a number of proteins, such as AGPs, wall-associated kinases, endo-1-4-β-d-glucanases, and cellulose synthases (Kjellbom et al., 1997; Kohorn, 2000, 2001). A Lys-rich tomato AGP, LeAGP-1, is plasma membrane bound via a C-terminal GPI anchor that tethers the protein to the plasma membrane (Sun et al., 2004). Consistent with this study, we show that plasmolysis of transgenic BY-2 cells localizes GFP-LeAGP-1 on the plasma membrane and in Hechtian strands (Figs. 2B and 3A). More importantly, we show that treatment of BY-2 cells with β-Yariv, a reagent that crosslinks AGPs, relocalizes GFP-LeAGP-1 within the periplasmic space from the Hechtian strands (Fig. 2, C and F).
There are contrasting reports on the presence of microfilaments and MTs within Hechtian strands. Certain studies indicate existence of microfilaments and MTs within the Hechtian strand (Lang-Pauluzzi, 2000; Lang-Pauluzzi and Gunning, 2000), whereas another study demonstrates their absence (Domozych et al., 2003). Consistent with our hypothesis that cytoskeleton inhibitors would disrupt the localization of LeAGP-1 on Hechtian strands, treatment of BY-2 cells with APM and cytochalasin-D relocalizes GFP-LeAGP-1 in the periplasmic space. APM is a specific depolymerization agent for cortical MTs (Granger and Cyr, 2000; Kundelchuk et al., 2002), while cytochalasin-D is an F-actin inhibitor used to probe microfilaments in eukaryotes (Cooper, 1987) and study the role of actin depolymerization in vesicle trafficking and cytoplasmic streaming. In this study, APM treatment relocalizes GFP-LeAGP-1 on Hechtian strands and results in a punctate distribution (Fig. 3, B and C). Interestingly, this APM treatment does not disrupt Hechtian strand formation (data not shown), consistent with previous studies that demonstrated no effects of cytoskeletal destabilizing drugs on the formation of Hechtian strands (Lang-Pauluzzi, 2000; Lang-Pauluzzi and Gunning, 2000). Treatment of BY-2 cells with both low and high concentrations of cytochalasin-D results in relocalization of GFP-LeAGP-1 on Hechtian strands (Fig. 2D) and in the cytoplasm (Fig. 2E). The appearance of GFP-LeAGP-1 in the cytoplasm most likely indicates disruption of vesicle trafficking resulting from actin depolymerization (Nebenführ et al., 1999; Gallagher and Benfey, 2005), although endocytosis of GFP-LeAGP-1 cannot be excluded as another alternative leading to this cytoplasmic localization.
Studies employing Yariv reagent in suspension cultured cells (Serpe and Nothnagel, 1994; Willats and Knox, 1996) and Arabidopsis seedlings (Ding and Zhu, 1997) indicate a connection between AGPs and cell expansion. Another study with the Arabidopsis reb-1 mutant reinforced this connection and reported phenotypic variations, including root epidermal cell bulging in the elongation zone (exclusively within the trichoblasts), immunolocalization of AGPs in the atrichoblast of the roots, and disrupted MTs within the swollen trichoblast cells (Andème-Onzighi et al., 2002). The REB-1 gene encodes a UDP-d-Glc-4-epimerase, which functions in d-Gal synthesis (Seifert et al., 2002). A recent immunocytochemical and biochemical study on reb-1 suggested a role for UDP-d-Glc-4-epimerase in galactosylation of AGPs and xyloglucans (Nguema-Ona et al., 2006).
In our studies, treatment of BY-2 cells expressing GFP-MBD with Yariv reagent demonstrates a terminal cell bulger phenotype that phenocopies the reb-1 epidermal cell bulger (Figs. 4, C–F). Yariv-induced terminal cell bulging of BY-2 cells indicates a role for AGPs in this event. Anisotropic growth in plant cells is maintained by the turgor pressure (internal and isotropic) exerted on the cell wall. Cellulose microfibrils play an important role in controlling the anisotropic growth (Williamson et al., 2001; Scheible et al., 2003). The direction of growth is controlled by deposition of cellulose microfibrils, which in turn are directed by the organization of cortical MTs (Baskin, 2001; Camilleri et al., 2002; Sugimoto et al., 2003; Lloyd, 2006). Previously, a study on a GPI-anchored protein, COBRA, revealed its role in anisotropic expansion and orientation of cellulose microfibrils (Roudier et al., 2005). In certain studies, it was also shown that loss in anisotropic growth is accompanied by abnormal cell swelling (Lane et al., 2001; Williamson et al., 2001). Our studies show that bulging takes place only in the terminal cells and not in the cells between them. In an elongating cell, growth occurs along the length of the cell, and the cellulose microfibrils in these cells provide structural support and shape by being deposited perpendicularly to the direction of growth and expansion. Compared to nonterminal cells, terminal cells are more exposed to Yariv reagent and thereby readily show a bulger phenotype. Although the exact mechanism underlying this cell bulging is not clear, the depolymerized MTs, cell bulging, and defects in directional growth (Fig. 4) indicate a connection between AGPs, MTs, terminal cell bulging, and anisotropic growth.
Yariv reagent depolymerizes and disorganizes cortical MTs in washed or unwashed GFP-MBD-expressing cells (Figs. 4 and 5). In comparison to the unwashed cells (Fig. 4), the washed cells (Fig. 5) demonstrate no reduction in the fluorescence of GFP-MBD, and any differences observed in fluorescence intensity are due either to BY-2 cells present in different focal planes or low fluorescent filter settings. Similarly, Yariv reagent also affects the organization of the F-actin. Although Yariv does not result in depolymerization of the F-actin, it results in thicker cortical F-actin filaments (Fig. 6). Previously, Gao and Showalter (1999b) reported that β-Yariv treatment of Arabidopsis cells for 72 h results in cytoplasmic shrinkage and absence of nucleus, structural changes that are characteristic of PCD. In another study, treatment of unwashed BY-2 cells with higher concentration (approximately 80 μm) of Yariv reagent results in PCD within 72 h (Chaves et al., 2002). Our studies show that the architectural changes in the cell shape and cytoskeleton take place within 5 h (for washed cells; Fig. 5) and 24 h (for unwashed cells; Fig. 4) of β-Yariv treatment. Thus, defects in the cytoskeleton precede PCD and may be responsible for PCD similar to the way disruption of the ECM-plasma membrane-cytoplasmic continuum can lead to PCD in animals (Ku et al., 1999; Bursch et al., 2000; Suetsugu and Takenawa, 2003).
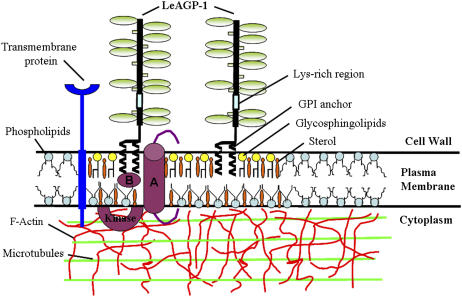
Based on our studies, we propose a cell surface network model involving interactions between AGPs and the cytoskeleton (i.e. MTs and F-actin) mediated by either a direct interaction with transmembrane protein(s) or by an indirect interaction involving lipid rafts (Fig. 7). Transmembrane proteins such as wall-associated kinases, cellulose synthases, endo-1-4-β-d-glucanases, Pro-rich extensin-like receptor kinases, formins, phospholipase-D, and lectin receptor kinases (Kohorn, 2000, 2001; Baluska et al., 2003; Nakhamchik et al., 2004; Gouget et al., 2006) may interact directly with AGPs to mediate such cell wall-plasma membrane-cytoskeleton connections. Most of these proteins have either an extracellular or a transmembrane domain that may interact with AGPs to mediate signaling at the plant cell surface. Also, we cannot rule out the possibility of Yariv-induced clustering of cell surface AGPs resulting in cytoskeletal changes. AGPs are abundant plant cell surface proteins, and any changes in the distribution of AGPs (crowding/steric effects) may result in changes in distribution of other membrane proteins/components, thereby producing a combined mass action that affects the cytoskeleton without any direct interactions.
Figure 7.
A hypothetical cell surface network model involving interactions between LeAGP-1 and the cytoskeleton (i.e. MTs and F-actin). GPI-anchored LeAGP-1 is localized to lipid rafts, which contain lipids such as glycosphingolipids and sterols (such as stigmasterol, campesterol, and β-sitosterol), and interacts with MTs and F-actin in the cytoplasm either by a transmembrane protein (in the phospholipid bilayer) or by molecules (A and B) associated with the lipid rafts. GPI-anchored LeAGP-1 in lipid rafts may mediate interactions in two possible ways: A, binding of LeAGP-1 to a transmembrane receptor in the lipid microdomain mediates the interactions; and B, ligand-LeAGP-1 receptor complex-induced translocation of a signal molecule outside the lipid raft to activate a cytoplasmic kinase. Also shown are the different constituents of LeAGP-1 that include a Pro/Hyp-rich protein backbone decorated with arabinogalactan polysaccharides, short arabinosides, and an unglycosylated Lys-rich peptide region.
Another possible mode of interaction involves lipid rafts. Lipid rafts were first discovered in animal cells and are specialized lipid microdomains enriched in cholesterol, glycosphingolipids, GPI-anchored proteins, and various molecules involved in cell signaling (van Meer, 2002; Zajchowski and Robbins, 2002). Lipid rafts are categorized as specialized centers for signaling cascades because of the identification of a number of signaling molecules within these lipid microdomains (Zajchowski and Robbins, 2002). In animals, lipid rafts are implicated in protein sorting, signal transduction, pathogen entry, and endocytosis (Simons and Toomre, 2000; Ikonen, 2001; Pike, 2004). A recent study in Arabidopsis showed that detergent-resistant membranes/lipid rafts are enriched in GPI-anchored proteins, including GPI-anchored AGPs like AtAGP4 (Borner et al., 2005). LeAGP-1 is also a GPI-anchored protein likely to be associated with lipid rafts. This GPI anchor in the lipid microdomain can potentially interact with receptors for the signaling ligands. Based on studies in animal cells (Zajchowski and Robbins, 2002), GPI-anchored AGPs in lipid rafts may mediate signaling in plants. Two possible scenarios come to mind, as depicted in Figure 7: (A) binding of the GPI-anchored protein to a transmembrane receptor present within the lipid microdomain may initiate signaling; and (B) binding of an extracellular ligand to LeAGP-1 receptor present in rafts may translocate another signal molecule out of the lipid microdomain leading to activation of intracellular/cytoplasmic kinase. While testing of this model remains to be done, our studies to date clearly indicate that GPI anchored-AGPs play a role in the plasma membrane-cytoskeleton connections.
MATERIALS AND METHODS
Cell Cultures and Growth Conditions
Two transgenic tobacco (Nicotiana tabacum) BY-2 cell lines were used to conduct the studies, a cell line expressing GFP-LeAGP-1 and another cell line expressing GFP-MBD. The BY-2 suspension cell cultures expressing GFP-MBD were maintained in liquid Murashige and Skoog media (4.3 g/L Murashige and Skoog salts [Sigma], 30 g/L Suc, 1 mg/mL thiamine HCl, 100 mg/L myoinositol, 0.44 mg/L 2,4-dichlorophenoxyacetic acid, pH 5.8) and BY-2 cell line expressing GFP-LeAGP-1 were maintained in liquid Schenk and Hildebrandt (SH) media (3.2 g/L SH basal salt; Sigma), 1 g/L SH vitamin powder, 1 mg/mL kinetin, 1 mg/mL p-chlorophenoxy acetic acid, 1 mg/mL 2,4-dichlorophenoxyacetic acid, 34 g Suc, pH 5.8) on a rotary shaker (120 rpm) at 24°C and subcultured weekly (1:10) into fresh culture media.
Plasmolysis Treatment
BY-2 cells expressing GFP-LeAGP-1 were washed with fresh SH media or NT-1 media and treated with 4% NaCl solution for 10 to 15 min at the end of respective pharmacological treatments.
Pharmacological Treatments
β-Yariv Reagent Treatment
Washed and unwashed BY-2 cells were subjected to Yariv treatments. BY-2 cells (3 d) expressing GFP-MBD were washed with fresh NT-1 media (three times) and treated with different concentrations of β-Yariv reagent for different time periods. Also, unwashed BY-2 cells expressing GFP-MBD were treated with 100 μm β-Yariv for 24 h. In another treatment, BY-2 cells (3 d) expressing GFP-LeAGP-1 were washed with fresh SH media (three times) and treated with 80 μm β-Yariv reagent for 1 h. After the inhibitor treatment, cells were fixed for cortical F-actin using rhodamine-phalloidin (Molecular Probes).
APM Treatment
Three-day-old tobacco BY-2 cells expressing GFP-MBD and GFP-LeAGP-1 were washed with fresh media (three times) before the treatment. Washed BY-2 cells were treated with 30 μm APM (10 mm stock in dimethyl sulfoxide; Austratec Pty) for 1 h.
Cytochalasin-D Treatment
Three-day-old wild-type tobacco BY-2 cells and tobacco cells expressing GFP-LeAGP-1 were washed with fresh media (three times) and were treated with 20, 25, and 50 μm of cytochalasin-D (10 mm stock in dimethyl sulfoxide; Sigma) for different time periods. Following the inhibitor-drug treatment, BY-2 cells were fixed for cortical F-actin using rhodamine-phalloidin.
After the pharmacological treatments, CLSM was conducted to examine the distribution and localization of fluorescence in respective cell lines.
Rhodamine-Phalloidin Staining
Wild-type BY-2 cells were washed with fresh Murashige and Skoog media (2 × 1 min) and attached to poly-l-Lys (Sigma)-coated glass slides (1 mg/mL). After 10 min, the cells were fixed with 3.8% formaldehyde/phosphate-buffered saline (PBS) for 30 min at room temperature and washed with PBS, pH 7.4 (3 × 5 min). Cells were permeabilized with 0.1% Triton-X-100/PBS for 10 min and washed again with PBS (3 × 5 min). Cells were then incubated with 1% bovine serum albumin/PBS for 25 min and labeled with rhodamine-phalloidin/PBS (5 μL of methanolic stock [6.6 μm] in 200 μL of PBS) for 20 min. After washing (3 × 5 min), the cells were mounted on slides with 50% glycerol/PBS and observed under CLSM.
CLSM
Cultured cells were placed on a drop of water on glass slides that were layered with coverslips. The glass slides were positioned onto the inverted platform of a CLSM (510; Zeiss) and the cells were imaged using the 488-nm line of argon laser. Images were recorded with ×10 and ×40 objectives (NA 0.75; Zeiss) and a 488-/543-nm dual dichroic excitation mirror with a 510- to 540-nm emission filter. All images were obtained either with a fluorescein isothiocyanate or a Texas red filter set. All images were processed with Zeiss imaging software and Adobe Photoshop.
Acknowledgments
We thank Dr. Richard Cyr (Pennsylvania State University) for supplying us with the GFP-MBD tobacco BY-2 cell cultures. We are grateful to Jeff Thuma for his advice and technical assistance with microscopy at Ohio University's CLSM facility.
This work was supported by the National Science Foundation (grant no. IBN–0110413) and by the Ohio University Graduate Student Senate (original work grant).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Allan M. Showalter (showalte@ohio.edu).
References
- Acosta-Garcia G, Vielle-Calzada JP (2004) A classical arabinogalactan protein is essential for the initiation of female gametogenesis in Arabidopsis. Plant Cell 16: 2614–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andème-Onzighi C, Sivaguru M, Judy-March J, Baskin TI, Driouich A (2002) The reb-1 mutation of Arabidopsis alters the morphology of trichoblast. The expression of arabinogalactan-proteins and the organization of cortical microtubules. Planta 215: 949–958 [DOI] [PubMed] [Google Scholar]
- Baluska F, Samaj J, Wojtaszek P, Volkmann D, Menzel D (2003) Cytoskeleton-plasma membrane-cell wall continuum in plants. Emerging links revisited. Plant Physiol 133: 482–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin TI (2001) On the alignment of cellulose microfibrils by cortical microtubules: a review and a model. Protoplasma 215: 150–171 [DOI] [PubMed] [Google Scholar]
- Borner GHH, Sherrier DJ, Weimar T, Michaelson LV, Hawkins ND, MacAskill A, Napier JA, Beale MH, Lilley KS, Dupree P (2005) Analysis of detergent-resistant membranes in Arabidopsis. Evidence for plasma membrane lipid rafts. Plant Physiol 137: 104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursch W, Hochegger K, Torok L, Marian B, Ellinger A, Hermann RS (2000) Autophagic and apoptotic types of programmed cell death exhibit different fates of cytoskeletal filaments. J Cell Sci 113: 1189–1198 [DOI] [PubMed] [Google Scholar]
- Camilleri C, Azimzadeh J, Pastuglia M, Bellini C, Grandjean O, Bouchez D (2002) The Arabidopsis TONNEAU2 gene encodes a putative novel protein phosphatase 2A regulatory subunit essential for the control of the cortical cytoskeleton. Plant Cell 14: 833–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canut H, Carrasco A, Galaud JP, Cassan C, Bouyssou H, Vita N, Ferrara P, Pont LR (1998) High affinity RGD-binding sites at the plasma membrane of Arabidopsis thaliana links the cell wall. Plant J 16: 63–71 [DOI] [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3: 1–30 [DOI] [PubMed] [Google Scholar]
- Chaves I, Regalado A, Chen M, Ricardo C, Showalter AM (2002) Programmed cell death induced by (b-D-galactosyl)3 Yariv reagent in Nicotiana tabacum BY-2 suspension-cultured cells. Physiol Plant 116: 548–553 [Google Scholar]
- Cheung AY, Wang H, Wu HM (1995) A floral transmitting tissue-specific glycoprotein attracts pollen tubes and stimulates their growth. Cell 82: 383–393 [DOI] [PubMed] [Google Scholar]
- Cooper JA (1987) Effects of cytochalasin and phalloidin on actin. J Cell Biol 105: 1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darley CP, Forrester AM, McQueen-Mason SJ (2001) The molecular basis of plant cell wall extension. Plant Mol Biol 47: 179–195 [PubMed] [Google Scholar]
- Ding L, Zhu JK (1997) A role of arabinogalactan-proteins in root epidermal cell expansion. Planta 203: 289–294 [DOI] [PubMed] [Google Scholar]
- Domozych DS, Roberts R, Danyow C, Flitter R, Smith B, Providence K (2003) Plasmolysis, Hechtian strand formation, and localized membrane-wall adhesion in the desmid, Closterium acerosum (Chlorophyta). J Phycol 39: 1194–1206 [Google Scholar]
- Fincher GB, Stone BA, Clarke AE (1983) Arabinogalactan-proteins: structure, biosynthesis, and function. Annu Rev Plant Physiol 34: 47–70 [Google Scholar]
- Gallagher KL, Benfey PN (2005) Not just another hole in the wall: understanding intercellular protein trafficking. Genes Dev 19: 189–195 [DOI] [PubMed] [Google Scholar]
- Gao M, Kieliszewski MJ, Lamport DTA, Showalter AM (1999) Isolation, characterization and immunolocalization of a novel, modular tomato arabinogalactan-protein corresponding to the LeAGP-1 gene. Plant J 18: 43–55 [DOI] [PubMed] [Google Scholar]
- Gao M, Showalter AM (1999) Yariv reagent treatment induces programmed cell death in Arabidopsis cell cultures and implicates arabinogalactan proteins involvement. Plant J 19: 321–331 [DOI] [PubMed] [Google Scholar]
- Gao M, Showalter AM (2000) Immunolocalization of LeAGP-1, a modular arabinogalactan protein, reveals its developmentally regulated expression in tomato. Planta 210: 865–874 [DOI] [PubMed] [Google Scholar]
- Gaspar YM, Nam J, Schultz CJ, Lee L-Y, Gilson PR, Gelvin SB, Bacic A (2004) Characterization of the Arabidopsis lysine-rich arabinogalactan-protein AtAGP17 mutant (rat1) that results in a decreased efficiency of Agrobacterium transformation. Plant Physiol 135: 2162–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geitmann A, Emons AM (2000) The cytoskeleton in plant and fungal cell tip growth. J Microsc 198: 218–245 [DOI] [PubMed] [Google Scholar]
- Glass NL, Jacobson DJ, Shiu PKT (2000) The genetics of hyphal fusion and vegetative incompatibility in filamentous ascomycete fungi. Annu Rev Genet 34: 165–186 [DOI] [PubMed] [Google Scholar]
- Gouget A, Senchou V, Govers F, Sanson A, Barre A, Rougé P, Pont-Lezica R, Canut H (2006) Lectin receptor kinases participate in protein-protein interactions to mediate plasma membrane-cell wall adhesions in Arabidopsis. Plant Physiol 140: 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger CL, Cyr RJ (2000) Microtubule reorganization in tobacco BY-2 cells stably expressing GFP-MBD. Planta 210: 502–509 [DOI] [PubMed] [Google Scholar]
- Guan Y, Nothnagel EA (2004) Binding of arabinogalactan proteins by Yariv phenylglycoside triggers wound-like responses in Arabidopsis cell cultures. Plant Physiol 135: 1346–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht K (1912) Studien ober den vorgang der plasmolyse. Beitr Biol Pflanz 11: 133–189 [Google Scholar]
- Hynes RO (1992) Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69: 11–25 [DOI] [PubMed] [Google Scholar]
- Hynes RO (1999) Cell adhesion: old and new questions. Trends Cell Biol 9: 33–37 [PubMed] [Google Scholar]
- Ikonen E (2001) Roles of lipid rafts in membrane transport. Curr Opin Cell Biol 13: 470–477 [DOI] [PubMed] [Google Scholar]
- Katsumi A, Orr AW, Tzima E, Schwartz MA (2004) Integrins in mechanotransduction. J Biol Chem 279: 12001–12004 [DOI] [PubMed] [Google Scholar]
- Kiba A, Sugimoto M, Toyoda K, Ichinose Y, Yamada T, Shiraishi T (1998) Interaction between cell wall and plasma membrane via RGD motif is implicated in plant defense responses. Plant Cell Physiol 39: 1245–1249 [Google Scholar]
- Kjellbom P, Snogerup L, Stohr C, Reuzeau C, McCabe PF, Pennell RI (1997) Oxidative cross-linking of plasma membrane arabinogalactan proteins. Plant J 12: 1189–1196 [DOI] [PubMed] [Google Scholar]
- Knox JP (1992) Cell adhesion, cell separation and plant morphogenesis. Plant J 2: 137–141 [Google Scholar]
- Kohorn BD (2000) Plasma membrane-cell wall contacts. Plant Physiol 124: 31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn BD (2001) WAKs: cell wall associated kinases. Curr Opin Cell Biol 13: 529–533 [DOI] [PubMed] [Google Scholar]
- Ku N-O, Zhou X, Toivola DM, Omary MB (1999) The cytoskeleton of digestive epithelia in health and disease. Am J Physiol Gastrointest Liver Physiol 277: 1108–1137 [DOI] [PubMed] [Google Scholar]
- Kundelchuk OP, Tarasenko LV, Blume YB (2002) Influence of amiprophosmethyl on the root cell structure in the herbicide-sensitive and -resistant lines of Nicotiana plumbaginifolia. Russ J Plant Physiol 49: 381–386 [Google Scholar]
- Lamport DTA, Kieliszewski MJ, Showalter AM (2006) Salt-stress upregulates arabinogalactan-proteins: using salt-stress to analyse AGP function. New Phytol 169: 479–492 [DOI] [PubMed] [Google Scholar]
- Lane DR, Wiedemeier A, Peng L, Höfte H, Vernhettes S, Desprez T, Hocart CH, Birch RJ, Baskin TI, Burn JE, et al (2001) Temperature-sensitive alleles of RSW2 link the KORRIGAN endo-1,4-β-glucanase to cellulose synthesis and cytokinesis in Arabidopsis. Plant Physiol 126: 278–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan KJ, Nothnagel EA (1997) Cell surface arabinogalactan-proteins and their relation to cell proliferation and viability. Protoplasma 196: 87–98 [Google Scholar]
- Lang-Pauluzzi I (2000) The behaviour of the plasma membrane during plasmolysis: a study by UV microscopy. J Microsc 198: 188–198 [DOI] [PubMed] [Google Scholar]
- Lang-Pauluzzi I, Gunning BES (2000) A plasmolytic cycle: the fate of cytoskeletal elements. Protoplasma 212: 174–183 [Google Scholar]
- Lloyd C (2006) Microtubules make tracks for cellulose. Science 312: 1482–1483 [DOI] [PubMed] [Google Scholar]
- Marc J, Granger C, Brincat J, Fisher D, Kao T, McCubbin A, Cyr R (1998) A GFP-MAP4 reporter gene for visualizing cortical microtubule rearrangement in living epidermal cells. Plant Cell 10: 1927–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Bhatt K, Baumann K (2001) Shaping in plant cells. Curr Opin Plant Biol 4: 540–549 [DOI] [PubMed] [Google Scholar]
- Mellersh DG, Heath MC (2001) Plasma membrane-cell wall adhesion is required for expression of plant defense responses during fungal penetration. Plant Cell 13: 413–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollet JC, Kim S, Jauh GY, Lord EM (2002) Arabinogalactan proteins, pollen tube growth, and the reversible effects of Yariv phenylglycoside. Protoplasma 219: 89–98 [DOI] [PubMed] [Google Scholar]
- Motose H, Sugiyama M, Fukuda H (2004) A proteoglycan mediates inductive interaction during plant vascular development. Nature 429: 873–878 [DOI] [PubMed] [Google Scholar]
- Nakhamchik A, Zhao Z, Provart NJ, Shiu S-H, Keatley SK, Cameron RK, Goring DR (2004) A comprehensive expression analysis of the Arabidopsis proline-rich extensin-like receptor kinase gene family using bioinformatic and experimental approaches. Plant Cell Physiol 45: 1875–1881 [DOI] [PubMed] [Google Scholar]
- Nebenführ A, Gallagher LA, Dunahay TG, Frohlick JA, Mazurkiewicz AM, Meehl JB, Staehelin LA (1999) Stop-and-go movements of plant golgi stacks are mediated by the acto-myosin system. Plant Physiol 121: 1127–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguema-Ona E, Andème-Onzighi C, Aboughe-Angone S, Bardor M, Ishii T, Lerouge P, Driouich A (2006) The reb1-1 mutation of Arabidopsis. Effect on the structure and localization of galactose-containing cell wall polysaccharides. Plant Physiol 140: 1406–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothnagel EA (1997) Proteoglycans and related components in plant cells. Int Rev Cytol 174: 195–291 [DOI] [PubMed] [Google Scholar]
- Park MH, Suzuki Y, Chono M, Knox JP, Yamaguchi I (2003) CsAGP1, a gibberellin-responsive gene from cucumber hypocotyls, encodes a classical arabinogalactan protein and is involved in stem elongation. Plant Physiol 131: 1450–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson ES, Cresti M (1992) Cytoskeleton and cytoplasmic organization of pollen and pollen tubes. Int Rev Cytol 140: 73–125 [Google Scholar]
- Pike LJ (2004) Lipid rafts: heterogeneity on the high seas. Biochem J 378: 281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope DG (1977) Relationships between hydroxyproline-containing proteins secreted into the cell wall and medium by suspension-cultured Acer pseudoplatanus cells. Plant Physiol 59: 894–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W, Fong C, Lamport DTA (1991) Gum arabic glycoprotein is a twisted hairy rope. Plant Physiol 96: 848–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuzeau C, Mcnally JG, Pickard BG (1997) The endomembrane sheath: a key structure for understanding the plant cell? Protoplasma 200: 1–9 [DOI] [PubMed] [Google Scholar]
- Roberts K (1994) The plant extracellular matrix: in a new expansive mood. Curr Opin Cell Biol 6: 688–694 [DOI] [PubMed] [Google Scholar]
- Roudier F, Fernandez AG, Fujita M, Himmelspach R, Borner GHH, Schindelman G, Song S, Baskin TI, Dupree P, Wasteneys GO, et al (2005) COBRA, an Arabidopsis extracellular glycosyl-phosphatidyl inositol-anchored protein, specifically controls highly anisotropic expansion through its involvement in cellulose microfibril orientation. Plant Cell 17: 1749–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Jauh GY, Hepler PK, Lord EM (1998) Effects of Yariv phenylglycoside on cell wall assembly in the lily pollen tube. Planta 204: 450–458 [DOI] [PubMed] [Google Scholar]
- Samaj J, Braun M, Baluska F, Ensikat H-J, Tsumaraya Y, Volkmann D (1999) Specific localization of arabinogalactan-protein epitopes at the surface of maize root hairs. Plant Cell Physiol 40: 874–883 [Google Scholar]
- Samaj J, Ovecka M, Hlavacka A, Lecourieux F, Meskiene I, Lichtscheidl I, Lenart P, Salaj J, Volkmann D, Bogre L, et al (2002) Involvement of the mitogen-activated protein kinase SIMK in regulation of root hair tip-growth. EMBO J 21: 3296–3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheible WR, Fry B, Kochevenko A, Schindelasch D, Zimmerli L, Somerville S, Loria R, Somerville CR (2003) An Arabidopsis mutant resistant to thaxtomin A, a cellulose synthesis inhibitor from Streptomyces species. Plant Cell 15: 1781–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler T, Bergfeld R, Schopfer P (1995) Arabinogalactan proteins in maize coleoptiles: developmental relationship to cell death during xylem differentiation but not to extension growth. Plant J 7: 25–36 [DOI] [PubMed] [Google Scholar]
- Seifert GJ, Barber C, Wells B, Dolan L, Roberts K (2002) Galactose biosynthesis in Arabidopsis: genetic evidence for substrate channeling from UDP-D-galactose into cell wall polymers. Curr Biol 12: 1840–1845 [DOI] [PubMed] [Google Scholar]
- Serpe MD, Nothnagel EA (1994) Effects of Yariv phenylglycosides on Rosa cell-suspensions: evidence for the involvement of arabinogalactan-proteins in cell proliferation. Planta 193: 542–550 [Google Scholar]
- Sherrier DJ, Prime TA, Dupree P (1999) Glycosylphosphatidylinositol-anchored cell-surface proteins from Arabidopsis. Electrophoresis 20: 2027–2035 [DOI] [PubMed] [Google Scholar]
- Shi H, Kim Y, Guo Y, Stevenson B, Zhu J-K (2003) The Arabidopsis SOS5 locus encodes a putative cell surface protein and is required for normal cell expansion. Plant Cell 15: 19–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter AM (1993) Structure and function of plant cell wall proteins. Plant Cell 5: 9–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter AM (2001) Arabinogalactan proteins: structure, expression and function. Cell Mol Life Sci 58: 1399–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter AM, Varner JE (1989) Plant hydroxyproline-rich glycoproteins. In A Marcus, ed, The Biochemistry of Plants. Academic Press, New York, pp 485–536
- Simons K, Toomre D (2000) Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1: 31–39 [DOI] [PubMed] [Google Scholar]
- Sivaguru M, Pike S, Gassmann W, Baskin TI (2003) Aluminum rapidly depolymerizes cortical microtubules and depolarizes the plasma membrane: evidence that these responses are mediated by a glutamate receptor. Plant Cell Physiol 44: 667–675 [DOI] [PubMed] [Google Scholar]
- Staiger CJ (2000) Signaling to the actin cytoskeleton in plants. Annu Rev Plant Physiol Plant Mol Biol 51: 257–288 [DOI] [PubMed] [Google Scholar]
- Stupack DG, Cheresh DA (2002) Get a ligand, get a life: integrins, signaling and cell survival. J Cell Sci 115: 3729–3738 [DOI] [PubMed] [Google Scholar]
- Suetsugu S, Takenawa T (2003) Regulation of cortical actin network in cell migration. Int Rev Cytol 229: 245–286 [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Himmelspach R, Williamson RE, Wasteneys GO (2003) Mutation or drug-dependent microtubule disruption causes radial swelling without altering parallel cellulose microfibril deposition in Arabidopsis root cells. Plant Cell 15: 1414–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Zhao ZD, Hare MC, Kieliszewski MJ, Showalter AM (2004) Tomato LeAGP-1 is a plasma membrane-bound, glycosylphophatidylinositol-anchored arabinogalactan-protein. Physiol Plant 120: 319–327 [DOI] [PubMed] [Google Scholar]
- Svetek J, Yadav MP, Nothnagel EA (1999) Presence of a glycosylphosphatidylinositol lipid anchor on rose arabinogalactan proteins. J Biol Chem 274: 14724–14733 [DOI] [PubMed] [Google Scholar]
- Thompson HJM, Knox JP (1998) Stage-specific responses of embryogenesis carrot cell suspension cultures to arabinogalactan protein-binding β-glucosyl Yariv reagent. Planta 205: 32–38 [Google Scholar]
- Willats WGT, Knox JP (1996) A role for arabinogalactan proteins in plant cell expansion: evidence from studies on the interaction of β-glucosyl Yariv reagent with seedlings of Arabidopsis thaliana. Plant J 9: 919–925 [DOI] [PubMed] [Google Scholar]
- Williamson RE, Burn JE, Birch R, Baskin TI, Arioli T, Betzner AS, Cork A (2001) Morphology of rsw1, a cellulose-deficient mutant of Arabidopsis thaliana. Protoplasma 215: 116–127 [DOI] [PubMed] [Google Scholar]
- Wyatt SE, Carpita NC (1993) The plant cytoskeleton-cell-wall continuum. Trends Cell Biol 3: 413–417 [DOI] [PubMed] [Google Scholar]
- Yariv J, Lis H, Katchalski E (1967) Precipitation of arabic acid and some seed polysaccharides by glycosylphenylazo dyes. Biochem J 105: 1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yariv J, Rapport MM, Graf L (1962) The interaction of glycosides and saccharides with antibody to the corresponding phenylazo glycoside. Biochem J 85: 383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youl JJ, Bacic A, Oxley D (1998) Arabinogalactan-proteins from Nicotiana alata and Pyrus communis contain glycosylphosphatidylinositol membrane anchors. Proc Natl Acad Sci USA 95: 7921–7926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hengel AJ, Roberts K (2003) AtAGP30, an arabinogalactan-protein in the cell walls of the primary root, plays a role in root regeneration and seed germination. Plant J 36: 256–270 [DOI] [PubMed] [Google Scholar]
- van Hengel AJ, Tadesse Z, Immerzeel P, Schols H, Van Kammen A, de Vries SC (2001) N-Acetlyglucosamine and glucosamine-containing arabinogalactan proteins control somatic embryogenesis. Plant Physiol 125: 1880–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G (2002) The different hues of lipid rafts. Science 296: 855–857 [DOI] [PubMed] [Google Scholar]
- Volkmann D, Baluska F (1999) The actin cytoskeleton in plants: from transport networks to signaling networks. Microsc Res Tech 47: 135–154 [DOI] [PubMed] [Google Scholar]
- Zajchowski LD, Robbins SM (2002) Lipid rafts and little caves: compartmentalized signalling in membrane microdomains. Eur J Biochem 269: 737–752 [DOI] [PubMed] [Google Scholar]
- Zandomeni K, Schopfer P (1994) Mechanosensory microtubule reorientation in the epidermis of maize coleoptiles subjected to bending stress. Protoplasma 182: 96–101 [DOI] [PubMed] [Google Scholar]