Abstract
Like many enveloped viruses, human respiratory syncytial virus (RSV) assembles at and buds from lipid rafts. Translocation of the envelope proteins to these membrane subdomains is essential for production of infectious virus, but the targeting mechanism is poorly understood and it is not known if other virus proteins are required. Here we demonstrate that F protein of RSV intrinsically targets to lipid rafts without a requirement for any other virus protein, including the SH and G envelope proteins. Recombinant virus deficient in SH and G but retaining F protein expression was used to demonstrate that F protein still localized in rafts in both A549 and HEp-2 cells. Expression of a recombinant F gene by use of plasmid vectors demonstrated that F contains its own targeting domain and localized to rafts in the absence of other virus proteins. The domain responsible for translocation was then mapped. Unlike most other virus envelope proteins, F is unusual since the target signal is not contained within the cytoplasmic domain nor did it involve fatty acid modified residues. Furthermore, exchange of the transmembrane domain with that of the vesicular stomatitis virus G protein, a nonraft protein, did not alter F protein raft localization. Taken together, these data suggest that domains present in the extracellular portion of the protein are responsible for lipid raft targeting of the RSV F protein.
An essential step in replication for many viruses is assembly at and budding from the cell membrane, frequently using specialized domains termed lipid rafts. Lipid rafts are liquid-ordered, plasma membrane microdomains that are enriched in sphingolipids and cholesterol (44). At low temperatures, lipid rafts resist extraction with nonionic detergents and can be isolated on density gradients as a low-density fraction. Based on these criteria, lipid rafts are also termed detergent-resistant membranes (DRMs) (5, 6). The saturated hydrocarbons of the lipid raft sphingolipids and phospholipids create a tightly packed subdomain within the plasma membrane. Rafts may allow compartmentalization of the membrane, and raft-localized proteins that demonstrate an energetically favorable association with rafts cluster within these microdomains to create specialized regions for protein-protein interactions (44). This sequestration of membrane proteins is thought to enhance protein-protein interactions needed for diverse cell functions, including receptor ligand-mediated signaling and endocytosis (5, 15, 20).
Many enveloped viruses are thought to exploit membrane microdomains for assembly by providing a means to bring together virus structural proteins on the cell surface at the site of budding. Human immunodeficiency virus (HIV), measles virus, and influenza virus all appear to use rafts or raft-like membrane domains (28, 35, 42). For each virus, at least one of the envelope proteins demonstrates an intrinsic ability to associate with lipid rafts, potentially providing a nucleation event for assembly of new virus (41, 45, 47). Human respiratory syncytial virus (RSV) has also been shown to assemble at plasma membrane domains that are enriched in raft proteins, such as caveolin-1 (Cav-1) and GM1, indicating that RSV structural proteins are also able to target rafts (7, 8).
RSV has three envelope proteins referred to as the small hydrophobic protein (SH), G protein, and F protein. The G protein has been shown to facilitate binding of RSV to the target cell, but the function of SH remains unclear. F protein mediates virus-to-cell and cell-to-cell fusion, creating the characteristic syncytia for which the virus is named (14, 17, 22, 25, 46).
Unlike with other paramyxoviruses, the F protein is sufficient for cell-to-cell transmission since virus deficient in G and SH remains infectious (22, 37, 46). During infection, all three proteins have been shown to localize at rafts (18, 30, 40). F protein specifically has been found to localize in raft-like domains of infected cells and was found to be required for localization of M protein to DRMs (18). However, the mechanism whereby F targets to rafts has not been determined.
Since F protein is an integral factor in the propagation of infectious virus, it is important to determine if F protein can associate with lipid rafts or DRMs without the need for other viral proteins. The purpose of these studies was to evaluate whether F can directly target rafts and, if so, to identify the targeting domain within the protein. By use of both a recombinant virus expressing F in the absence of G and SH and an F protein expressed from a synthetic construct, the results demonstrate that F localizes to lipid rafts independently of other RSV proteins. Furthermore, the results show that the targeting domain is unusual in that it resides in the extracellular domain of the F protein.
MATERIALS AND METHODS
Cells and virus.
A549 cells (CCL-185; American Type Culture Collection, Manassas, VA) were grown in Ham's F-12 supplemented with 2% sodium bicarbonate, 1% penicillin-streptomycin, and 10% fetal calf serum. HEp-2 cells (CCL-23; ATCC) were maintained in Eagle's minimal essential medium supplemented with 1% penicillin-streptomycin and 10% fetal calf serum. RSV Long strain was maintained as described previously (10). rgRSV-SGF and rgRSV-F viruses were provided by Mark Peeples, Ohio State University, and were described previously (46). Both viruses encode the green fluorescent protein (GFP) at the 3′ end of the genome, and GFP expression was used to monitor growth and evaluate virus titers by fluorescence microscopy. rgRSV-F was derived from the cDNA of rgRSV-SGF by deletion of the G and SH genes. Virus was grown, virus titers were determined, and analysis by reverse transcription-PCR was done as described elsewhere (46). A549 and HEp-2 cells were infected at a multiplicity of infection of 1 and analyzed at 18 h postinfection.
Synthesis of RSV F gene, expression vector construction, and mutagenesis.
The native coding sequence of RSV was codon optimized for ectopic expression in human cells as described elsewhere (9). At the same time, potential splice sites were identified using the SpliceView algorithm (SpliceView, http://l25.itba.mi.cnr.it/∼webgene/wwwspliceview.html) and eliminated to produce an optimized coding sequence. Overlapping oligonucleotides (60 nucleotides each), corresponding to the optimized sequence of both sense and cDNA strands and terminal restriction enzyme sites, were purchased from Midland Company, Midland, TX (sequences are available upon request). The synthetic F gene was assembled as two fragments flanked by BamHI and EcoRI and EcoRI and NotI, respectively (see Fig. 2A). PCR-mediated assembly of both segments was performed under the following conditions: 94°C for 1 min, 50°C for 1 min, and 72°C for 2 min for 30 cycles, using all oligonucleotides except for those at the termini. Final products were isolated by a second round of PCR using primers complementary to the 5′ and 3′ ends that included the restriction enzyme sites used for insertion into the expression vector. Conditions for the second PCR were as follows: 90°C for 30 s, 55°C for 30 s, and 72°C for 1 min for 30 cycles. The two fragments were enzymatically digested with their respective restriction enzymes and sequentially cloned into pCDNA3 (Invitrogen, Carlsbad, CA). The resultant inserts were sequenced (UTMB Protein Chemistry Core), and errors were corrected by oligonucleotide-directed mutagenesis using a Quikchange kit (Stratagene, La Jolla, CA). The final pCDNA3 construct encoding full-length F was designated pCDNA3-SynF. All DNA constructs were purified using an Endofree plasmid maxi kit (QIAGEN, Valencia, CA).
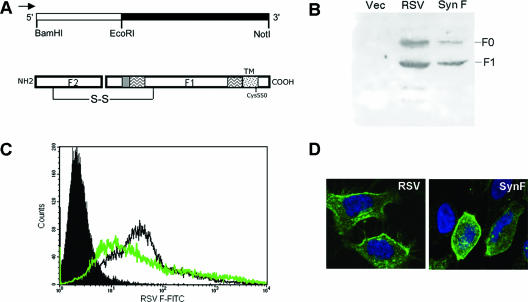
FIG. 2.
Synthetic F construct design and expression. A synthetic F gene was constructed for optimal expression in mammalian cells while preserving the amino acid sequence. (A) The gene was constructed in two sections and is flanked with restriction enzyme sites and contains a cytomegalovirus promoter indicated by the arrow. S-S indicates disulfide bond. (B) The synthetic F gene product was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting of HEp-2 cell lysates either infected with wild-type RSV (RSV) or transfected with the vector control (Vec), pCDNA3, or SynF gene. The F0 (uncleaved precursor) and F1 (cleaved) forms of F were detected using an anti-F1 antibody. Cell surface expression was demonstrated in HEp-2 cells by labeling cells on ice without permeabilization with an anti-RSV F antibody and analyzing by both (C) flow cytometry and (D) confocal microscopy. SynF-transfected cells (green line) were compared to RSV-infected cells (black line), and pCDNA3-transfected control cells (solid peak) were labeled with a primary antibody specific for RSV F and analyzed by flow cytometry. For each sample, 20,000 events were acquired. Surface expression of F was also seen in SynF-transfected cells that were labeled on ice with an anti-RSV F antibody (green) and analyzed by confocal microscopy. Cells were also labeled with DAPI (blue) for nuclear labeling. Images shown are optical slices from one field of cells.
F protein mutagenesis.
C-terminal truncations of F were generated by PCR using oligonucleotides that incorporated stop codons at the 3′ end of the synthetic F gene. The primers used were 5′ GAGGCGGCCGCTCATTAGCAATACAGCAGCAGGCCGAC 3′ for Δ551-574 (which had amino acids 551 to 574 deleted), 5′ GAGGCGGCCGCTCATTAGCTGCGGGCCTTGCAATACAG 3′ for Δ555-574, and 5′ GAGGCGGCCGCTCATTAATACAGCAGCAGGCCGAC 3′ for Δ550-574. The forward primer was the same for all F mutations and corresponded to the 5′ end of the SynF gene. AccuTaq polymerase (Sigma-Aldrich, St. Louis, MO) was used, and conditions were as follows: 95°C for 30 s, 55°C for 30 s, and 72°C for 2 min for 35 cycles. The resulting fragment was digested with EcoRI and NotI endonucleases and inserted into pCDNA3. All constructs were sequenced and changes confirmed. For single amino acid changes, necessary mutations were incorporated into oligonucleotides and introduced into the expression construct using a Quikchange kit. PCR conditions were as follows: 95°C for 30 s, 55°C for 1 min, and 68°C for 2 min for 12 cycles. The mutations were confirmed by sequencing each construct. For the substitution of the vesicular stomatitis virus G protein (VSV G) transmembrane (TM) domain and cytoplasmic tail, an XbaI restriction endonuclease site was introduced at homologous positions in VSV G and F coding sequences (amino acids 561 and 527, respectively). DNA fragments encoding the RSV F ectodomain and the VSV G transmembrane and cytoplasmic tails were then joined by sequential insertion into the expression vector.
Transient transfection of expression constructs.
Transient transfection of plasmid DNA into epithelial cell lines A549 and HEp-2 was accomplished by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions, with adjustments. Cells were grown to 90% confluence. Transfections were carried out in OptiMEM (Invitrogen) with a DNA-to-Lipofectamine 2000 reagent ratio of 1:2. Resultant DNA complexes were incubated with cells for 4 h at 37°C prior to replacing media with antibiotic-free medium.
Fluorescence confocal microscopy.
Adherent cells were grown on four-well culture slides (BD Falcon, California). All cell culture samples were labeled directly on slides. For indirect labeling of RSV F, the primary antibody was a mouse anti-RSV F antibody (MCA490; Serotec, Raleigh, NC) and the secondary antibody was an anti-mouse immunoglobulin G conjugated to fluorescein isothiocyanate (FITC) (STAR9B; Serotec) or Alexa Fluor 350 (Molecular Probes). For cholera toxin labeling, cells were labeled at 4°C with an Alexa Fluor 594-labeled cholera toxin B subunit (CTxB) (Molecular Probes). Colabeling for RSV F protein and transferrin receptor (TfR) was performed similarly to the described staining method involving CTxB, with replacement of CTxB by Alexa Fluor 594-labeled transferrin (Tfn) (Molecular Probes). Samples were fixed with 2% paraformaldehyde. DAPI (4′,6′-diamidino-2-phenylindole) mounting medium (Molecular Probes) was used to counterstain nuclear DNA where indicated. For confocal microscope analysis, a Zeiss LSM 510 UV Meta laser scanning confocal microscope was used at ×63 magnification. Images were analyzed using Zeiss imaging software.
Colocalization analysis.
Confocal microscope images were analyzed for colocalization of RSV proteins, cholera toxin subunit B, or transferrin by use of ImageJ (W. S. Rasband, National Institutes of Health, Bethesda, Md., 1997-2004, http://rsb.info.nih.gov/ij/). Acquired images were analyzed as individual green and red channels corresponding to anti-RSV F protein staining and CTxB or Tfn staining, respectively. Background was corrected using the background subtraction function on ImageJ. Regions of interest were selected based on patching patterns in both red and green channels. Areas that contained staining artifacts, as indicated by intense regions of fluorescence in both channels, were excluded from analysis. Manders overlap coefficient, a measure of overlap of the red and green signals, was generated using the Manders coefficient plug-in (27) which is included in the WCIF version of ImageJ (http://www.uhnresearch.ca/facilities/wcif/imagej/), with regions of interest for analysis set for the green channel. Coefficients for three separate images were averaged, and a paired Student t test value was calculated using Excel (Microsoft, Redmond, WA) to test overlap of F protein staining patterns with that of CTxB or Tfn staining.
Flow cytometry.
HEp-2 cells were infected with RSV Long strain at a multiplicity of infection of 1 and harvested after 24 h of infection. Cells transfected with the synthetic RSV F expression construct or vector control were fixed and labeled, as described above for F expression, by use of anti-RSV F primary antibody and FITC-conjugated secondary antibody. Control samples were labeled with the secondary antibody alone. Twenty thousand events were analyzed for each sample on a BD FACSort instrument using CellQuest software.
Separation of DRMs.
Transfected or infected cells were harvested by scraping and pelleting. Samples were disrupted by Dounce homogenization in TNE buffer (25 mM Tris, pH 7.5, 150 mM NaCl, 5 mM EDTA) containing serine and cysteine protease inhibitor cocktail (Roche, Indianapolis, IN) at 4°C. Nuclei were pelleted by spinning for 10 min at 1,000 × g, and Triton X-100 was added to postnuclear supernatants to give a final concentration of 0.25% (vol/vol). After 30 min on ice, homogenates were mixed with 60% Optiprep (Sigma-Aldrich) to give a 40% (vol/vol) solution (1.2 ml total). This was then overlaid with 2.4 ml of a 30% Optiprep solution (diluted with TNE). TNE was overlaid on the top of the gradient, and the tube was spun in an SW60 rotor at 45,000 rpm for 4 h at 4°C. After centrifugation, eight equal fractions were manually collected from the top of the gradient. Protein was precipitated using a methanol-chloroform extraction of 150 μl of each fraction (50), and each fraction was analyzed by Western blotting.
Western blot analysis.
Cell samples were lysed in sample loading buffer (50 mM Tris-HCl, pH 6.8, 1% sodium dodecyl sulfate, 10% glycerol, and 0.05% bromophenol blue) supplemented with 2.5% β-mercaptoethanol and protease inhibitor cocktail (Roche) and boiled for 5 min. Proteins were separated on a 4 to 20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel (Novex; Invitrogen) and transferred onto a nitrocellulose membrane. After blocking in 5% skim milk in Tris-buffered saline with Tween 20 (0.02 M Tris, 0.5 M NaCl, pH 7.5, 0.05% Tween 20), membranes were probed with a rabbit anti-RSV F1 antibody, raised against residues 255 to 275 and used at 1:500 (provided by Jose Melero, Instituto de Salud Carlos II, Madrid, Spain). Membranes were then probed with a secondary anti-rabbit antibody conjugated to horseradish peroxidase (Bio-Rad, Hercules, CA). Precipitated DRM fractions were also analyzed for TfR (non-DRM resident) (16) and Cav-1 (DRM resident) (24) with antibodies from Santa Cruz Biotechnology. For analysis of the RSV F/VSV G hybrid protein, blots were probed with rabbit antiserum reactive to the cytoplasmic tail domain (CTD) of VSV G (provided by Michael Whitt, University of Alabama—Birmingham).
RESULTS
RSV G and SH are not required for F to localize to lipid rafts.
Previous studies have demonstrated that RSV envelope proteins SH, G, and F are able to associate with lipid raft microdomains of the plasma membrane of infected cells (8, 30, 40). Using an RSV recombinant virus (46) deficient in G and SH (rgRSV-F), we were able to demonstrate that the F protein associates directly with lipid rafts and does not require G or SH for this localization. This was established using two independent methods. First, rgRSV-F-infected A549 cells were labeled for GM1 and F protein colocalization. GM1 is a glycosphingolipid that localizes to plasma membrane lipid rafts and is used as a raft marker (38). GM1 can be specifically labeled by binding of fluorescently conjugated CTxB (33). A549 cells grown on culture slides were infected with rgRSV-SGF or rgRSV-F for 18 h to allow F expression and virus budding. Cells were labeled simultaneously at 4°C with a fluorescently labeled CTxB and an antibody specific for RSV F protein. Raft domains in cells are too small to visualize by standard light microscopy and normally give a diffuse membrane staining pattern. To promote visualization of raft-associated F proteins, cells were labeled with a fluorescently conjugated secondary antibody specific for mouse immunoglobulin G, thus cross-linking the membrane-associated F proteins and rafts in which they were embedded. Similar cross-linking methods have been used in other studies to promote visualization of raft domains (13, 16). We confirmed previous reports (8, 30) that the F protein from wild-type virus colocalized with GM1 (Fig. 1A) and was associated with viral filaments. In the current experiments, the F protein from rgRSV-F-infected cells also demonstrated such colocalization and association with the filaments (Fig. 1B).
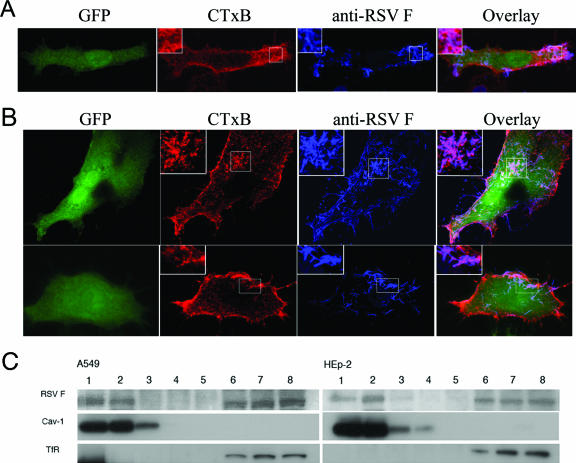
FIG. 1.
Colocalization of F protein with a lipid raft marker in the absence of G and SH. Cells were infected with rgRSV-SGF (A) or rgRSV-F (B), viruses engineered to express GFP as a marker of infection. rgRSV-F has the G and SH coding sequences removed and does not express the G and SH proteins. Infected A549 cells grown on culture slides were stained for surface RSV F protein and GM1 at 18 h postinfection. For staining, cells were incubated with a primary antibody specific for RSV F protein and CTxB (binds to GM1) on ice, followed by incubation with secondary antibody conjugated to FITC that binds the primary F antibody for indirect staining of F protein. Fixed cells were then analyzed by confocal microscopy. For panel B, two representative fields are shown (top and bottom rows). Colocalization is shown in the overlay panels and appears pink, and the portions indicated by the boxes are magnified approximately ×5 in the insets. The F protein was not detected in samples of uninfected A549 cells (not shown). (C) RSV F protein expression from both A549 and HEp-2 cells infected with rgRSV-F was analyzed for detergent insolubility as a measure of lipid raft association. Cells were treated with 0.25% Triton X-100 at 4°C and subsequently separated on a density gradient. The lipid raft domains are shown in fractions 1 and 2 as determined by Cav-1 localization. Lanes 6, 7, and 8, are the nonraft or detergent-soluble fractions as determined by TfR localization.
In a second approach to detect F protein raft association, detergent solubility tests were performed with extracts from RSV-infected A549 and HEp-2 cells. Lipid rafts resist extraction with Triton X-100 at low temperatures, and raft-associated proteins, such as Cav-1, remain associated with the low-density complex (6, 24). We tested whether F protein could be detected in DRM-containing fractions from both A549 and HEp-2 cells. After detergent extraction and density gradient fractionation, each fraction was analyzed by Western blotting and probed with both an antibody specific for amino acids 255 to 275 of the F1 subunit and antibodies for Cav-1 and a nonraft marker, TfR. F was consistently found to colocalize with Cav-1 in the DRM fraction (Fig. 1C). Some F protein was also detected in soluble fractions, which has been observed for F expressed during infection by wild-type virus (30) as well as for other well-characterized raft-associated proteins, including virus envelope proteins (12, 52).
Design of a synthetically engineered F gene and characterization of its protein expression.
To further investigate the ability of F protein to localize to lipid rafts, we tested the ability of F protein to target rafts in the absence of all other viral proteins. F expression is usually studied using a vaccinia system (3, 17), which usually results in abnormally high expression levels. Plasmid-based F expression vectors were designed to overcome issues of excessive expression altering F localization as well as to eliminate any potential influence of vaccinia proteins on localization. Unfortunately, cDNA constructs of F corresponding to the virus coding sequence were very poorly expressed in mammalian cells. This may have been due to poor codon utilization or RNA secondary structure, as seen for HIV envelope protein genes (11). Gene expression may also be suboptimal since wild-type RSV replicates in the cytoplasm and the genome of the virus could contain cryptic RNA splice sites. To achieve optimal expression of F protein, the RSV F DNA sequence was optimized for high-level expression by eliminating potential splice sites and optimizing codon usage while retaining the native amino acid sequence (9). Oligonucleotide-mediated gene synthesis was used, and a synthetic RSV F gene (encoding SynF) was cloned into pCDNA3. RSV F is made as a precursor termed F0 and is cleaved at two sites to yield F2 and F1 subunits (Fig. 2A). Expression of F was first evaluated by Western blotting for detection of the F1 subunit using an anti-F1 antibody specific for amino acids 255 to 275 (Fig. 2B). F1 protein was detected both in cell lysates of the virus-infected cells and in those transfected with SynF. To determine if F was expressed on the cell surface, SynF-transfected HEp-2 cells were labeled using an antibody specific for RSV F without permeabilization of cells. Flow cytometry evaluation of expression levels on cells demonstrated F protein expression levels that were similar to those attained during RSV infection (Fig. 2C). Subcellular localization of F protein was determined by confocal microscopy for both RSV-infected and SynF-expressing cells, which demonstrated similar staining patterns and levels of expression (Fig. 2D). Optical slices (0.2 μm) of cells also demonstrated labeling on the cell surface (not shown). Similar expression patterns were observed for SynF-transfected A549 cells and 293 fibroblasts (Fig. 3 and data not shown). These data suggested that SynF was made and processed similarly to the F protein produced during virus infection of cells.
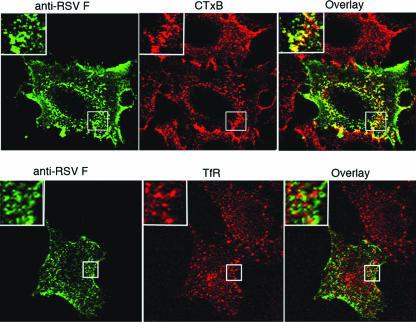
FIG. 3.
RSV F colocalizes with GM1 more than with transferrin receptor in the absence of other viral proteins. Cells were transfected with the SynF gene and labeled on ice with an anti-RSV F antibody (green) and either CTxB or Tfn (red). Samples were analyzed by confocal microscopy, with colocalization shown in yellow in the overlay panel. The portions indicated by the boxes are magnified approximately ×5 in the insets.
Localization of F protein in lipid rafts.
To determine if SynF protein colocalized with lipid rafts, transfected cells were stained for F protein and GM1 by use of F-specific antibody and fluorescently labeled CTxB, respectively, by the same methods described for Fig. 1. In these experiments, the F protein was detected at the cell surface and colocalized with GM1 (Fig. 3). In contrast, staining for TfR, a non-raft-associated protein, using fluorescently labeled Tfn showed that SynF was largely excluded from sites that stained for TfR (Fig. 3 and insets). SynF also localized to insoluble fractions after Triton X-100 extraction, as did Cav-1 (see Fig. 5), thus confirming the microscopy analysis.
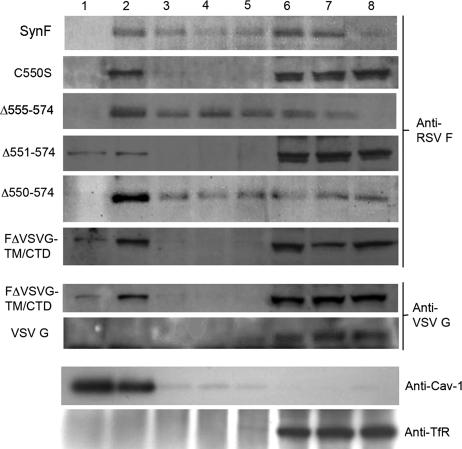
FIG. 5.
DRM association of F protein with mutations to the palmitoylation site, TM domain, and CTD. Detergent solubility of RSV F protein was examined in cells transfected with the various F mutation constructs or with VSV G. Postnuclear supernatants were treated with 0.25% Triton X-100 at 4°C and separated on a density gradient. Subsequent blots of the fractions were probed with either an anti-RSV F antibody or an antibody specific for the CTD of VSV G (anti-VSV G), as specified to the right of the respective blots. The SynF mutant is labeled to the left of the blot. Also shown are representative blots probed with anti-Cav-1 and anti-TfR.
RSV F palmitoylation, transmembrane domain, and cytoplasmic tail are dispensable for lipid raft association.
Lipid rafts are composed of sphingolipids and cholesterol, which allow a more ordered lipid phase than the surrounding membrane (43). The long acyl chains of sphingolipids account for this property (32). One mechanism to promote raft association is posttranslational modification with addition of palmitate or myristic acid groups to cysteine residues (32). HIV and murine leukemia virus are two examples for which palmitoylation of envelope protein TM cysteines are important for raft association (4, 26). RSV F contains one reported palmitoylation modification of Cys550 (Fig. 4A), which is predicted to be located in the TM domain adjacent to the cytoplasmic side of the plasma membrane (2). To determine if this modified residue was important for raft association, we mutated the Cys residue of SynF to serine (C550S) and determined raft association of the modified protein (41). Surprisingly, the F protein lacking this palmitoylation site maintained its ability to colocalize with GM1 (Fig. 4B) and DRMs (Fig. 5).
FIG. 4.
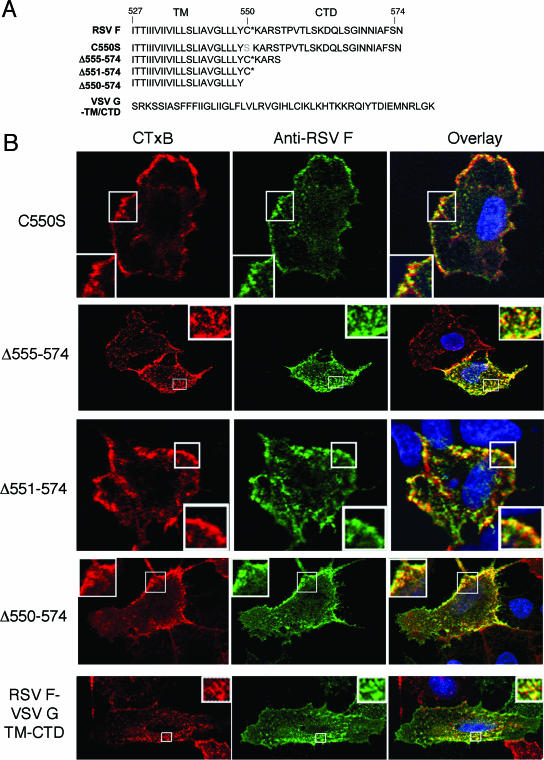
RSV F protein membrane-proximal palmitoylation, TM domain, and CTD are not required for lipid raft association. (A) SynF sequences for TM domain and CTD mutants are listed. The final construct is a chimeric protein between the VSV G TM domain and CTD and the ectodomain of F protein. *, palmitoylation site. (B) Cells were transfected with the RSV F protein mutants, and colocalization of GM1 and the altered F proteins was analyzed by confocal microscopy. Labeling was done as described in the legend for Fig. 3, and cells were analyzed by confocal microscopy. The portions indicated by the boxes are magnified approximately ×5 in the insets.
In addition to palmitoylation, subdomains of the envelope proteins can facilitate targeting to rafts. For Newcastle disease virus fusion protein and influenza virus spike glycoproteins, DRM association mapped to the cytoplasmic tail domain (12, 52). We made a series of C-terminal truncations to test if the RSV F CTD contained a raft-targeting motif (Fig. 4A). Each C-terminally truncated F protein retained its ability both to colocalize with GM1 (Fig. 4B) and to associate with DRM fractions (Fig. 5). Furthermore, a mutated F protein lacking both the CTD and the palmitoylated C550 showed such localization.
The retention of lipid raft association in the truncated F proteins indicated that the raft-targeting region must be present in the TM domain or the ectodomain of F protein. For influenza virus, the TM domain of the hemagglutinin protein has previously been demonstrated to be responsible for DRM association (45). VSV G is considered non-raft associated since it is absent from DRM fractions and does not colocalize with GM1 by microscopy (6, 28, 52). To determine if the TM region of F protein was responsible for raft association, the RSV F TM domain and CTD were replaced with the corresponding region of VSV G (Fig. 4A). The chimeric protein was expressed at the cell surface similarly to the SynF protein; furthermore, it retained association with GM1 (Fig. 4B) and was DRM associated (Fig. 5). In contrast, VSV G remained in the soluble portion of the density gradient (Fig. 5).
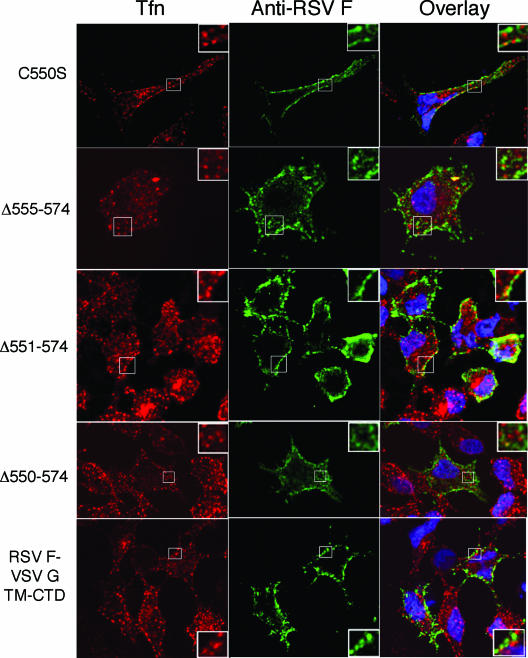
Transferrin receptor does not enrich in rafts and so should not colocalize strongly with RSV F protein (16). To test this, live A549 cells expressing RSV F and modified F proteins were labeled for F protein expression and for TfR. By confocal microscopy, none of the modified F proteins demonstrated strong colocalization with TfR (Fig. 6). To obtain a measure of colocalization, the images of cells colabeled for RSV F and GM1 or TfR were evaluated by determining Manders overlap coefficients (Table 1) . This calculation is based on the overlap of staining patterns, with values ranging from 0 to 1 indicating no to complete overlap, respectively (27, 53). With CTxB colocalization, each of the modified F proteins gave high Manders coefficient values that were comparable to that of SynF (wild-type protein sequence). In contrast, the colocalization of wild-type or modified F proteins with Tfn was low (Manders coefficient average of 0.502) and was significantly lower (P < 0.01) than that for CTxB staining (Manders coefficient average of 0.830). While not showing a result of 0 (indicating a low-level overlap of signal), this analysis indicated that the wild-type and modified F proteins each were enriched in domains stained with CTxB and were weakly associated with domains stained with Tfn. Furthermore, the modified proteins gave similar Manders coefficients, indicating that raft association must be dictated by the ectodomain of F.
FIG. 6.
Transferrin receptor localization in cells expressing RSV F proteins with mutations to the TM domain and CTD. A549 cells transfected with RSV F proteins were labeled for F protein (green) and for TfR with Tfn (red) as described in the legend for Fig. 3. Samples were analyzed by confocal microscopy, and a representative image is shown for each transfection group. The portions indicated by the boxes are magnified approximately ×5 in the insets.
TABLE 1.
Colocalization of RSV F proteins with CTxB and Tfna
| Protein | Overlap coefficient (mean ± SD)
|
P value (CTxB vs Tfn)b | |
|---|---|---|---|
| CTxB | Tfn | ||
| SynF | 0.848 ± 0.01 | 0.552 ± 0.06 | 0.016 |
| C550S mutant | 0.792 ± 0.01 | 0.607 ± 0.06 | 0.037 |
| Δ555-574 | 0.881 ± 0.05 | 0.528 ± 0.02 | 0.004 |
| Δ551-574 | 0.837 ± 0.03 | 0.511 ± 0.04 | 0.005 |
| Δ550-574 | 0.850 ± 0.01 | 0.386 ± 0.07 | 0.006 |
| F/VSV G TM-CTD | 0.773 ± 0.04 | 0.432 ± 0.03 | 0.013 |
Images of transfected A549 cells labeled for RSV F protein and CTxB or Tfn were acquired by confocal microscopy. Overlap between fluorescently labeled RSV F proteins and CTxB or Tfn signals was determined for three samples in each transfection group. For Manders coefficients of colocalization, 1 is high colocalization and 0 is low. The overlap coefficients of RSV F protein with nuclei stained with DAPI for select images ranged between 0.1 and 0.2.
CTxB versus Tfn (column 4) refers to CTxB colocalization with RSV F protein (column 2) versus Tfn colocalization with RSV F protein (column 3).
DISCUSSION
The objectives of this study were to determine if RSV F protein was able to target to lipid rafts independently of other viral proteins, including the G and SH envelope proteins, and to determine if palmitoylation, the cytoplasmic tail domain, and the transmembrane domain were involved in F association with rafts.
Incorporation of envelope proteins into the plasma membrane is thought to be a key nucleation step in viral assembly and budding. Several enveloped viruses, including measles virus and HIV, have been shown to incorporate their envelope proteins into specialized microdomains of the plasma membrane termed lipid rafts (4, 28, 35). Rafts may provide a means to localize membrane proteins within the cell membrane to enhance protein-protein interactions. For an enveloped virus, such interactions are important, as envelope proteins must associate and interact with capsid in the cytosol and also cellular components for proper assembly to take place. Human respiratory syncytial virus has three envelope proteins, SH, G, and F, together with matrix and capsid proteins. The matrix protein has previously been demonstrated to be associated with DRMs (18). Similarly, in cells infected with RSV, the envelope proteins were shown to associate with GM1 (8), a marker of rafts (38), and F and G were found in detergent-insoluble fractions (30). However, it was unclear if raft association of the envelope proteins was driven by contact with matrix proteins or by signals present in the envelope proteins themselves.
For measles virus, the F envelope protein associates with rafts independently of not only its H envelope protein but all other viral proteins (47). To address whether RSV F protein also exhibited an intrinsic ability to associate with rafts, we began by using a virus deficient in G and SH. We were able to demonstrate that the F protein was able to colocalize with GM1, perhaps even more distinctly than in rgRSV-SGF-infected cells. F protein fractionated with DRMs of both A549 and HEp-2 cells. This demonstrated that F contains targeting sequences sufficient to bring F to lipid rafts in the absence of other virus envelope proteins.
In addition to the RSV envelope proteins being localized to lipid rafts, the matrix protein, nucleoprotein, and phosophoprotein also migrate with DRM fractions on gradients (30, 31). In order to determine whether F protein required any of these other RSV proteins for raft localization, a synthetic F gene product (SynF) was constructed based on the wild-type gene sequence that eliminated cryptic splice sites and was codon optimized for expression in human cells while retaining the wild-type amino acid sequence. This approach avoided potential problems from expressing F from a vaccinia-driven system, including introduction of vaccinia proteins that may alter cell functions, such as membrane trafficking and localization to the plasma membrane (21, 49).
Expression of SynF in A549 cells did not produce filaments detectable by our methods, as seen with RSV and rgRSV-F infection, suggesting that factors other than the F protein are required for filament formation. To further support this, Oomens et al. observed filament formation in cells infected with a virus with all three of the envelope proteins replaced with a VSV G/RSV F CTD hybrid protein (36). Regardless, the F protein still associated with GM1 and the SynF protein was distributed to DRM fractions. Additionally, F protein was largely excluded from locations that labeled for transferrin receptor, a marker for nonraft regions (16). Together, these observations indicated that signals present in the F protein are sufficient to target this protein to lipid rafts in the absence of all other virus proteins.
Palmitoylation of Cys residues in the TM domain of transmembrane proteins has been shown to be one of the requirements for lipid raft targeting (32). Murine leukemia virus and HIV contain glycoproteins with these acylation modifications on Cys residues that are crucial to DRM association (26, 41). RSV F protein contains one Cys residue located in the TM proximal to the cytoplasmic leaflet of the membrane. In infected cells, this Cys residue is modified by palmitoylation (2). Replacement of Cys550 with a Ser residue or deletion of Cys550 did not prevent association of F protein with rafts, indicating that fatty acid modification on this residue was not crucial for lipid raft association. Similar observations were found with Epstein-Barr virus LMP1, which has one palmitoylated Cys in its TM but does not require this modification for raft association (19). The role of the palmitoylation remains unclear, but it may help to anchor the envelope protein to the membrane or possibly to enhance lipid raft association determined by other signals in the F protein.
It has been demonstrated that the CTD region of both Newcastle disease virus F protein and influenza hemagglutinin contained important signals for determining DRM association (12, 52). To determine if this region was important for RSV F in raft targeting, truncations of F that removed portions or all of the 24-amino-acid CTD of RSV F were made. Unlike with other viruses, CTD truncation did not alter RSV F protein association with lipid rafts nor did the combined absence of Cys550 and the CTD.
The TM of membrane-spanning proteins may contain other acylated amino acids or other properties which make association with the raft regions favorable. The RSV F TM domain and CTD were replaced with those of VSV G, which is known not to associate with DRMs (28). Inclusion of the VSV G TM domain produced membrane localization, and surprisingly, raft association was maintained. All F protein mutations that were generated demonstrated colocalization with GM1 by microscopy. DRM fractionation confirmed localization of the mutated F to the insoluble fractions. There was variation in the distributions of the mutated F proteins among the insoluble, soluble, and intermediate fractions. This variation could be explained by fractionation errors or possibly by differences in strength of association of each modified F protein with the lipid rafts. The latter may reflect weak versus strong association of the different mutated F proteins with the lipid rafts (13). This variation could be evaluated further by careful examination of the effects of various temperatures or detergent concentrations/types on DRM association (29).
The DRM analyses of cells infected with rgRSV-F and those expressing SynF or SynF mutant proteins did not demonstrate cosedimentation exclusively with insoluble fractions, but rather a portion of the F protein was detected in soluble fractions as well. Past studies with virus-infected cells also showed F present in detergent-soluble as well as -insoluble fractions (30), which was attributed to budded RSV remaining associated with the cell membrane at non-lipid raft regions. The mechanism by which the budded virus remained cell associated was not determined. In our studies, it appears that F association with nonraft regions is a function of the F protein itself, since wild-type-virus-infected and rgRSV-F-infected cells show similar distributions of F protein in insoluble fractions. Similarly, F protein expressed independently of the other RSV proteins was found in both soluble and insoluble fractions. Therefore, it is likely that G or SH or other virus proteins are not involved in controlling F raft/nonraft region localization. Independent analysis by microscopy of the raft distribution of F protein supported the DRM results. Again, while the bulk of F was associated with GM1-rich regions of the cell (detected by cholera toxin labeling), some F protein was associated with transferrin receptor (detected with labeled transferrin). This distribution of F protein in both raft and nonraft fractions may indicate two different forms of F protein or may represent the affinity of F for different membrane microdomains. Partitioning to independent regions of the cell membrane may provide a means for the virus to affect the function of the cell in multiple ways. For example, raft-associated HIV Nef protein can downmodulate major histocompatibility complex class I expression to a greater extent than the non-raft-associated form of Nef (1). RSV F may demonstrate a similar phenomenon.
Overall, the data suggest that neither the TM domain nor the CTD contains domains that direct the association of the F protein with lipid rafts. This indicates that the raft-targeting region of F protein is located in the ectodomain of the protein. This is atypical, but other examples where ectodomains are important for raft association have been reported previously. The prion protein PrP associates with rafts through a targeting domain located at its N terminus (48). Epidermal growth factor receptor (EGFR) also contains raft-targeting information in its extracellular region (51). Interestingly, RSV is known to activate EGFR, with a resultant inflammatory response characterized by release of interleukin-8 (34). The mechanism for this activation has not been determined. It is possible that RSV interacts with EGFR in these raft regions to trigger the inflammatory response. RSV infection is also known to alter function of other raft-residing proteins, such as RhoA (30). Raft association through interaction with raft-resident proteins has also been described previously for the c-Cbl-associated protein, which binds to the raft-resident flotillin through a sorbin homology domain (23). RSV F protein may contain a similar domain involved in such an interaction. Future studies will examine the F ectodomain for its ability to target to lipid rafts.
While the CTD of F protein may not be needed for raft association, it likely serves other important roles in virus assembly. In particular, F protein is required for M protein to associate with DRM (18); however, the mechanism of association has not been investigated yet. As with influenza virus, RSV M protein polymerization may cause rafts to congregate through its interaction with F and create large viral assembly centers (39, 52). Along with viral proteins, host raft residents would also be sequestered, and this could have an impact on normal cellular functioning.
Future work will determine the requirements for these components in viral assembly and budding. The F and M proteins are envelope-associated proteins required in the production of infectious virus. Since M requires F to go to rafts but F can locate to rafts independently of M, it is possible that F homing to rafts is an important step in virus assembly and budding. The mutant proteins described in this work provide a means to test this concept and define F and M interaction sites.
Acknowledgments
We thank Eugene Knutson and Tom Albrecht of the Infectious Disease and Toxicology Optical Imaging Core of UTMB for their excellent assistance with the confocal microscope imaging and analysis. We thank Drew Deniger, Mohammad F. Saeed, and Tonya Colpitts for scientific discussion of the experiments. We thank Jose Melero of the Instituto de Salud Carlos II for the anti-F antibodies used for Western blotting. We thank Michael Whitt of the University of Alabama for both the VSV G cytoplasmic tail domain antibody and the construct expressing the VSV G transmembrane domain and cytoplasmic tail domain (pCAV 711V). We thank Mark Peeples of The Children's Research Institute at Ohio State University at Columbus, Ohio, for the use of his recombinant viruses and advice on their maintenance and for excellent scientific discussion.
E.H.F. was supported by the Robert D. Watkins Graduate Fellowship through the American Society for Microbiology.
Footnotes
Published ahead of print on 27 September 2006.
REFERENCES
- 1.Alexander, M., Y. C. Bor, K. S. Ravichandran, M. L. Hammarskjold, and D. Rekosh. 2004. Human immunodeficiency virus type 1 Nef associates with lipid rafts to downmodulate cell surface CD4 and class I major histocompatibility complex expression and to increase viral infectivity. J. Virol. 78:1685-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arumugham, R. G., R. C. J. Seid, S. Doyle, S. W. Hildreth, and P. R. Paradiso. 1989. Fatty acid acylation of the fusion glycoprotein of human respiratory syncytial virus. J. Biol. Chem. 264:10339-10342. [PubMed] [Google Scholar]
- 3.Ball, L. A., K. K. Young, K. Anderson, P. L. Collins, and G. W. Wertz. 1986. Expression of the major glycoprotein G of human respiratory syncytial virus from recombinant vaccinia virus vectors. Proc. Natl. Acad. Sci. USA 83:246-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharya, J., P. J. Peters, and P. R. Clapham. 2004. Human immunodeficiency virus type 1 envelope glycoproteins that lack cytoplasmic domain cysteines: impact on association with membrane lipid rafts and incorporation onto budding virus particles. J. Virol. 78:5500-5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, D. A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111-136. [DOI] [PubMed] [Google Scholar]
- 6.Brown, D. A., and J. K. Rose. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68:533-544. [DOI] [PubMed] [Google Scholar]
- 7.Brown, G., J. Aitken, H. W. Rixon, and R. J. Sugrue. 2002. Caveolin-1 is incorporated into mature respiratory syncytial virus particles during virus assembly on the surface of virus-infected cells. J. Gen. Virol. 83:611-621. [DOI] [PubMed] [Google Scholar]
- 8.Brown, G., H. W. Rixon, and R. J. Sugrue. 2002. Respiratory syncytial virus assembly occurs in GM1-rich regions of the host-cell membrane and alters the cellular distribution of tyrosine phosphorylated caveolin-1. J. Gen. Virol. 83:1841-1850. [DOI] [PubMed] [Google Scholar]
- 9.Chambers, R. S., and S. A. Johnston. 2003. High-level generation of polyclonal antibodies by genetic immunization. Nat. Biotechnol. 21:1088-1092. [DOI] [PubMed] [Google Scholar]
- 10.Chonmaitree, T., N. J. Roberts, Jr., R. G. Douglas, Jr., C. B. Hall, and R. L. Simons. 1981. Interferon production by human mononuclear leukocytes: differences between respiratory syncytial virus and influenza viruses. Infect. Immun. 32:300-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dayton, E. T., D. M. Powell, and A. I. Dayton. 1989. Functional analysis of CAR, the target sequence for the Rev protein of HIV-1. Science 246:1625-1629. [DOI] [PubMed] [Google Scholar]
- 12.Dolganiuc, V., L. McGinnes, E. J. Luna, and T. G. Morrison. 2003. Role of the cytoplasmic domain of the Newcastle disease virus fusion protein in association with lipid rafts. J. Virol. 77:12968-12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Favoreel, H. W., T. C. Mettenleiter, and H. J. Nauwynck. 2004. Copatching and lipid raft association of different viral glycoproteins expressed on the surfaces of pseudorabies virus-infected cells. J. Virol. 78:5279-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldman, S. A., S. Audet, and J. A. Beeler. 2000. The fusion glycoprotein of human respiratory syncytial virus facilitates virus attachment and infectivity via an interaction with cellular heparan sulfate. J. Virol. 74:6442-6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gustavsson, J., S. Parpal, M. Karlsson, et al. 1999. Localization of the insulin receptor in caveolae of adipocyte plasma membrane. FASEB J. 13:1961-1971. [PubMed] [Google Scholar]
- 16.Harder, T., P. Scheiffele, P. Verkade, and K. Simons. 1998. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 141:929-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heminway, B. R., Y. Yu, Y. Tanaka, et al. 1994. Analysis of respiratory syncytial virus F, G, and SH proteins in cell fusion. Virology 200:801-805. [DOI] [PubMed] [Google Scholar]
- 18.Henderson, G., J. Murray, and R. P. Yeo. 2002. Sorting of the respiratory syncytial virus matrix protein into detergent-resistant structures is dependent on cell-surface expression of the glycoproteins. Virology 300:244-254. [DOI] [PubMed] [Google Scholar]
- 19.Higuchi, M., K. M. Izumi, and E. Kieff. 2001. Epstein-Barr virus latent-infection membrane proteins are palmitoylated and raft-associated: protein 1 binds to the cytoskeleton through TNF receptor cytoplasmic factors. Proc. Natl. Acad. Sci. USA 98:4675-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horejsi, V., K. Drbal, M. Cebecauer, et al. 1999. GPI-microdomains: a role in signalling via immunoreceptors. Immunol. Today 20:356-361. [DOI] [PubMed] [Google Scholar]
- 21.Husain, M., and B. Moss. 2003. Intracellular trafficking of a palmitoylated membrane-associated protein component of enveloped vaccinia virus. J. Virol. 77:9008-9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karron, R. A., D. A. Buonagurio, A. F. Georgiu, et al. 1997. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc. Natl. Acad. Sci. USA 94:13961-13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura, A., C. A. Baumann, S. H. Chiang, and A. R. Saltiel. 2001. The sorbin homology domain: a motif for the targeting of proteins to lipid rafts. Proc. Natl. Acad. Sci. USA 98:9098-9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurzchalia, T. V., and R. G. Parton. 1999. Membrane microdomains and caveolae. Curr. Opin. Cell Biol. 11:424-431. [DOI] [PubMed] [Google Scholar]
- 25.Levine, S., R. Klaiber-Franco, and P. R. Paradiso. 1987. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J. Gen. Virol. 68:2521-2524. [DOI] [PubMed] [Google Scholar]
- 26.Li, M., C. Yang, S. Tong, A. Weidmann, and R. W. Compans. 2002. Palmitoylation of the murine leukemia virus envelope protein is critical for lipid raft association and surface expression. J. Virol. 76:11845-11852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manders, E. M. M., F. J. Verbeek, and J. A. Aten. 1993. Measurement of co-localisation of objects in dual-colour confocal images. J. Microsc. 169:375-382. [DOI] [PubMed] [Google Scholar]
- 28.Manié, S. N., S. Debreyne, S. Vincent, and D. Gerlier. 2000. Measles virus structural components are enriched into lipid raft microdomains: a potential cellular location for virus assembly. J. Virol. 74:305-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matto, M., C. M. Rice, B. Aroeti, and J. S. Glenn. 2004. Hepatitis C virus core protein associates with detergent-resistant membranes distinct from classical plasma membrane rafts. J. Virol. 78:12047-12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCurdy, L. H., and B. S. Graham. 2003. Role of plasma membrane lipid microdomains in respiratory syncytial virus filament formation. J. Virol. 77:1747-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald, T. P., A. R. Pitt, G. Brown, H. W. Rixon, and R. J. Sugrue. 2004. Evidence that the respiratory syncytial virus polymerase complex associates with lipid rafts in virus-infected cells: a proteomic analysis. Virology 330:147-157. [DOI] [PubMed] [Google Scholar]
- 32.Melkonian, K. A., A. G. Ostermeyer, J. Z. Chen, M. G. Roth, and D. A. Brown. 1999. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J. Biol. Chem. 274:3910-3917. [DOI] [PubMed] [Google Scholar]
- 33.Merritt, E. A., T. K. Sixma, K. H. Kalk, B. A. van Zanten, and W. G. Hol. 1994. Galactose-binding site in Escherichia coli heat-labile enterotoxin (LT) and cholera toxin (CT). Mol. Microbiol. 13:745-753. [DOI] [PubMed] [Google Scholar]
- 34.Monick, M. M., K. Cameron, J. Staber, et al. 2005. Activation of the epidermal growth factor receptor by respiratory syncytial virus results in increased inflammation and delayed apoptosis. J. Biol. Chem. 280:2147-2158. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen, D. H., and J. E. Hildreth. 2000. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 74:3264-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oomens, A. G., A. G. Megaw, and G. W. Wertz. 2003. Infectivity of a human respiratory syncytial virus lacking the SH, G, and F proteins is efficiently mediated by the vesicular stomatitis virus G protein. J. Virol. 77:3785-3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oomens, A. G., and G. W. Wertz. 2004. trans-complementation allows recovery of human respiratory syncytial viruses that are infectious but deficient in cell-to-cell transmission. J. Virol. 78:9064-9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parton, R. G. 1994. Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae. J. Histochem. Cytochem. 42:155-166. [DOI] [PubMed] [Google Scholar]
- 39.Rajendran, L., and K. Simons. 2005. Lipid rafts and membrane dynamics. J. Cell Sci. 118:1099-1102. [DOI] [PubMed] [Google Scholar]
- 40.Rixon, H. W., G. Brown, J. Aitken, et al. 2004. The small hydrophobic (SH) protein accumulates within lipid-raft structures of the Golgi complex during respiratory syncytial virus infection. J. Gen. Virol. 85:1153-1165. [DOI] [PubMed] [Google Scholar]
- 41.Rousso, I., M. B. Mixon, B. K. Chen, and P. S. Kim. 2000. Palmitoylation of the HIV-1 envelope glycoprotein is critical for viral infectivity. Proc. Natl. Acad. Sci. USA 97:13523-13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scheiffele, P., A. Rietveld, T. Wilk, and K. Simons. 1999. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J. Biol. Chem. 274:2038-2044. [DOI] [PubMed] [Google Scholar]
- 43.Silvius, J. R., D. del Giudice, and M. Lafleur. 1996. Cholesterol at different bilayer concentrations can promote or antagonize lateral segregation of phospholipids of differing acyl chain length. Biochemistry 35:15198-15208. [DOI] [PubMed] [Google Scholar]
- 44.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 45.Takeda, M., G. P. Leser, C. J. Russell, and R. A. Lamb. 2003. Influenza virus hemagglutinin concentrates in lipid raft microdomains for efficient viral fusion. Proc. Natl. Acad. Sci. USA 100:14610-14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Techaarpornkul, S., N. Barretto, and M. E. Peeples. 2001. Functional anal-ysis of recombinant respiratory syncytial virus deletion mutants lacking the small hydrophobic and/or attachment glycoprotein gene. J. Virol. 75:6825-6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vincent, S., D. Gerlier, and S. N. Manié. 2000. Measles virus assembly within membrane rafts. J. Virol. 74:9911-9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walmsley, A. R., F. Zeng, and N. M. Hooper. 2003. The N-terminal region of the prion protein ectodomain contains a lipid raft targeting determinant. J. Biol. Chem. 278:37241-37248. [DOI] [PubMed] [Google Scholar]
- 49.Ward, B. M., and B. Moss. 2000. Golgi network targeting and plasma membrane internalization signals in vaccinia virus B5R envelope protein. J. Virol. 74:3771-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wessel, D., and U. I. Flugge. 1984. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138:141-143. [DOI] [PubMed] [Google Scholar]
- 51.Yamabhai, M., and R. G. Anderson. 2002. Second cysteine-rich region of epidermal growth factor receptor contains targeting information for caveolae/rafts. J. Biol. Chem. 277:24843-24846. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, J., A. Pekosz, and R. A. Lamb. 2000. Influenza virus assembly and lipid raft microdomains: a role for the cytoplasmic tails of the spike glycoproteins. J. Virol. 74:4634-4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zinchuk, V., O. Zinchuk, and T. Okada. 2005. Experimental LPS-induced cholestasis alters subcellular distribution and affects colocalization of Mrp2 and Bsep proteins: a quantitative colocalization study. Microsc. Res. Tech. 67:65-70. [DOI] [PubMed] [Google Scholar]