Abstract
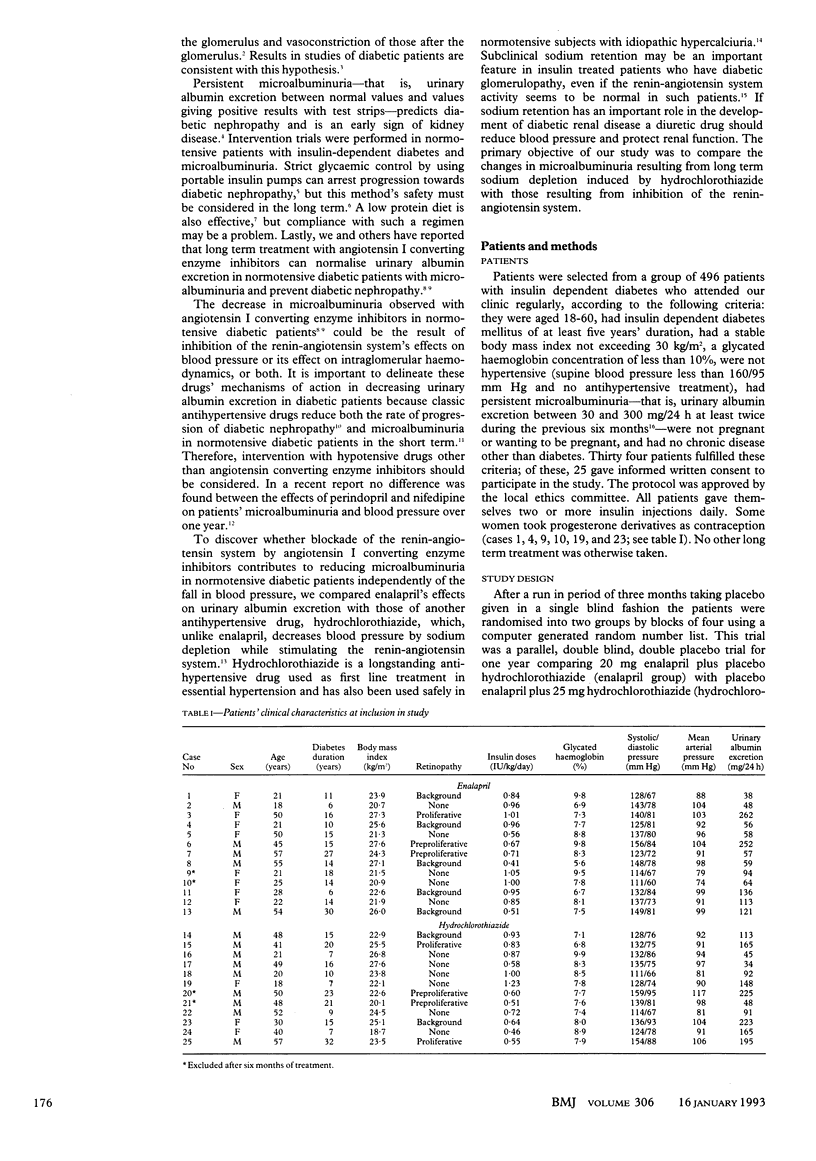
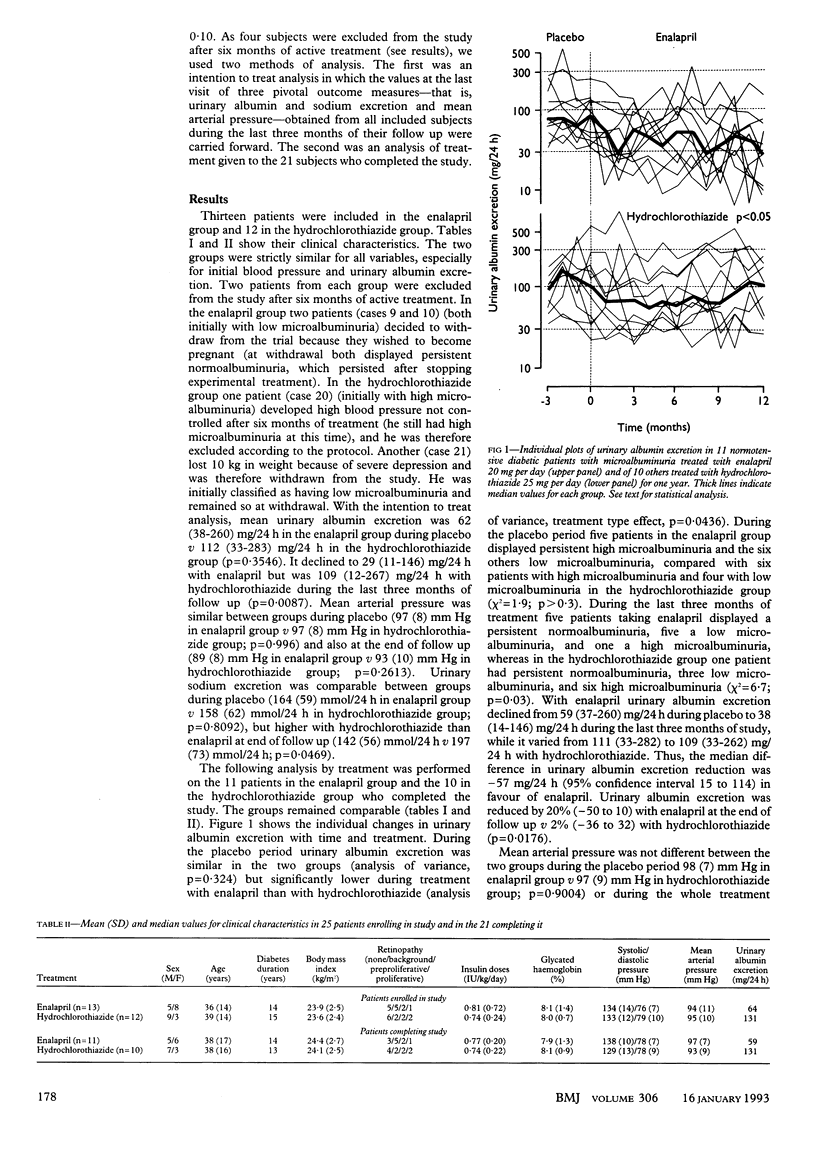
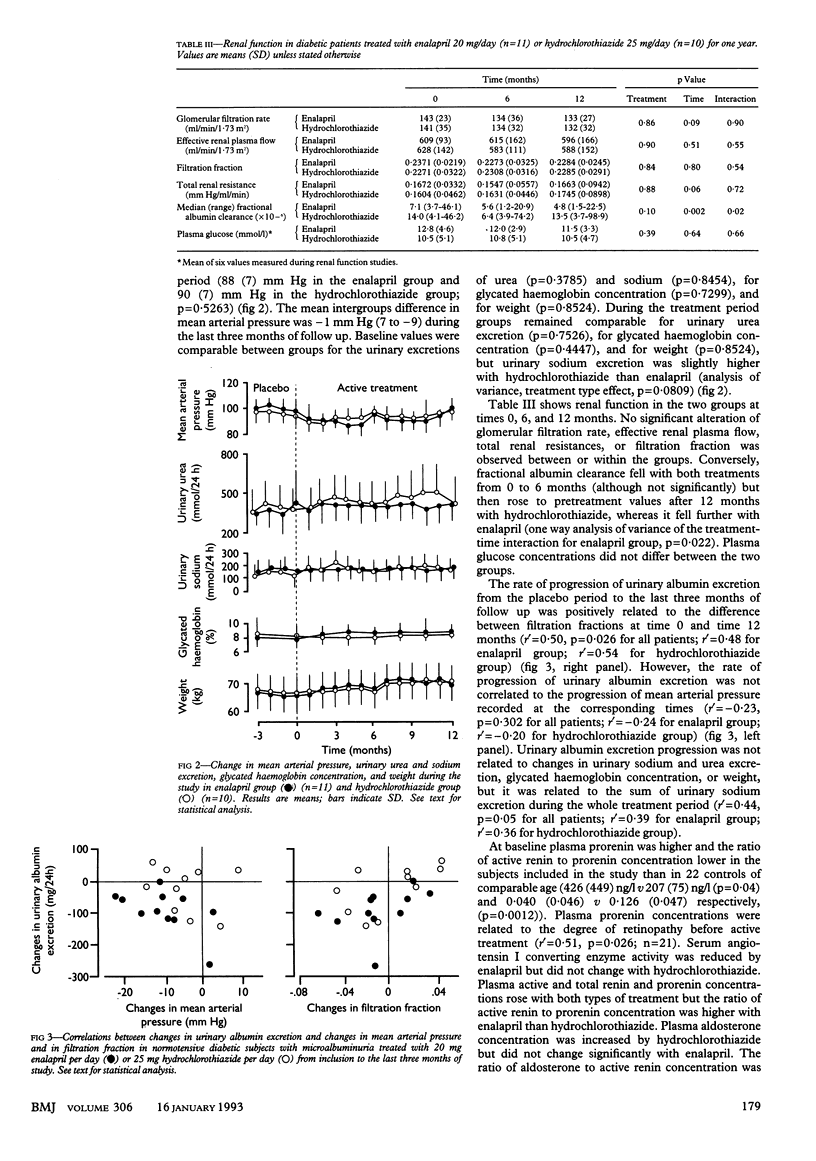
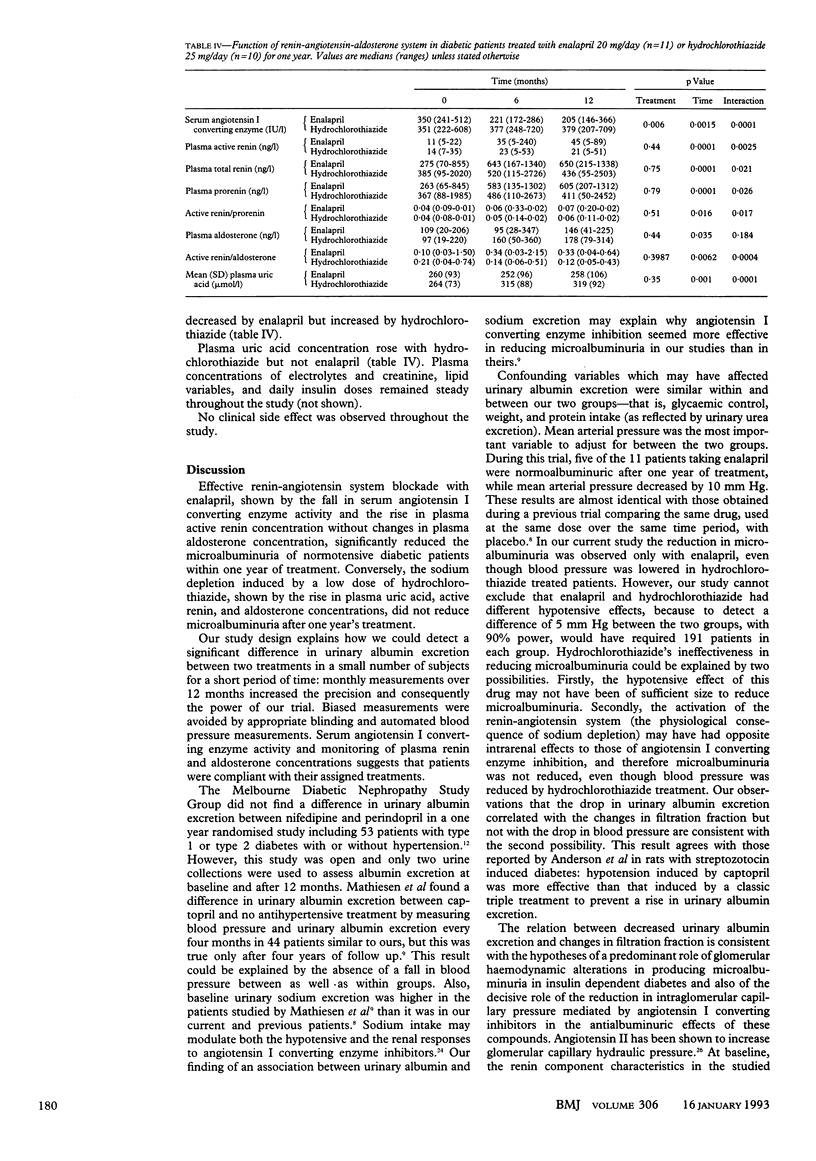
OBJECTIVE--To compare the effects of sodium depletion and of angiotensin I converting enzyme inhibition on microalbuminuria in insulin dependent diabetes. DESIGN--Randomised, double blind, double dummy parallel study of normotensive diabetic patients with persistent microalbuminuria (30-300 mg/24 h) treated with enalapril or hydrochlorothiazide for one year after a three month, single blind placebo period. SETTING--Diabetic clinic in a tertiary referral centre. PATIENTS--10 diabetic patients with low microalbuminuria (30-99 mg/24 h) and 11 with high microalbuminuria (100-300 mg/24 h). INTERVENTIONS--11 subjects (six with low microalbuminuria, five with high microalbuminuria) were given enalapril 20 mg plus placebo hydrochlorothiazide once daily and 10 (four with low microalbuminuria, six with high microalbuminuria) hydrochlorothiazide 25 mg plus placebo enalapril once daily. MAIN OUTCOME MEASURES--Monthly assessment of urinary albumin excretion and mean arterial pressure; plasma active renin and aldosterone concentrations and renal function studies at 0, 6, and 12 months. RESULTS--Median urinary albumin excretion decreased from 59 (range 37-260) to 38 (14-146) mg/24 h with enalapril and from 111 (33-282) to 109 (33-262) mg/24 h with hydrochlorothiazide (analysis of variance, p = 0.0436). During the last three months of treatment with enalapril five patients had persistent normoalbuminuria (2-3 times below 30 mg/24 h), five low microalbuminuria, and one high microalbuminuria; in the hydrochlorothiazide group one had normoalbuminuria, three low microalbuminuria, and six high microalbuminuria (chi 2 test = 6.7; p = 0.03). Mean arterial pressure did not differ before (98 (SD 7) with enalapril v 97 (9) mm Hg with hydrochlorothiazide) or during treatment (88 (7) with enalapril v 90 (7) mm Hg with hydrochlorothiazide (analysis of variance, p = 0.5263)). Glomerular filtration rate did not vary. The aldosterone to active renin ratio was decreased by angiotensin I converting enzyme inhibition and increased by sodium depletion, showing treatment efficacy. CONCLUSION--Angiotensin I converting enzyme inhibition by enalapril effectively reduces microalbuminuria in normotensive diabetic patients whereas hydrochlorothiazide is not effective. Changes in blood pressure and activity of the renin-angiotensin-aldosterone system may contribute to these different effects.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen A. R., Christiansen J. S., Andersen J. K., Kreiner S., Deckert T. Diabetic nephropathy in Type 1 (insulin-dependent) diabetes: an epidemiological study. Diabetologia. 1983 Dec;25(6):496–501. doi: 10.1007/BF00284458. [DOI] [PubMed] [Google Scholar]
- Anderson S., Rennke H. G., Garcia D. L., Brenner B. M. Short and long term effects of antihypertensive therapy in the diabetic rat. Kidney Int. 1989 Oct;36(4):526–536. doi: 10.1038/ki.1989.227. [DOI] [PubMed] [Google Scholar]
- Björck S., Mulec H., Johnsen S. A., Nordén G., Aurell M. Renal protective effect of enalapril in diabetic nephropathy. BMJ. 1992 Feb 8;304(6823):339–343. doi: 10.1136/bmj.304.6823.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björck S., Mulec H., Johnsen S. A., Nyberg G., Aurell M. Contrasting effects of enalapril and metoprolol on proteinuria in diabetic nephropathy. BMJ. 1990 Apr 7;300(6729):904–907. doi: 10.1136/bmj.300.6729.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D., Dodds R., Viberti G. Effect of protein restriction in insulin dependent diabetics at risk of nephropathy. Br Med J (Clin Res Ed) 1987 Mar 28;294(6575):795–798. doi: 10.1136/bmj.294.6575.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldt-Rasmussen B., Mathiesen E. R., Deckert T. Effect of two years of strict metabolic control on progression of incipient nephropathy in insulin-dependent diabetes. Lancet. 1986 Dec 6;2(8519):1300–1304. doi: 10.1016/s0140-6736(86)91433-9. [DOI] [PubMed] [Google Scholar]
- Feldt-Rasmussen B., Mathiesen E. R., Deckert T., Giese J., Christensen N. J., Bent-Hansen L., Nielsen M. D. Central role for sodium in the pathogenesis of blood pressure changes independent of angiotensin, aldosterone and catecholamines in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1987 Aug;30(8):610–617. doi: 10.1007/BF00277316. [DOI] [PubMed] [Google Scholar]
- Feldt-Rasmussen B., Mathiesen E. R., Jensen T., Lauritzen T., Deckert T. Effect of improved metabolic control on loss of kidney function in type 1 (insulin-dependent) diabetic patients: an update of the Steno studies. Diabetologia. 1991 Mar;34(3):164–170. doi: 10.1007/BF00418270. [DOI] [PubMed] [Google Scholar]
- Franken A. A., Derkx F. H., Man in't Veld A. J., Hop W. C., van Rens G. H., Peperkamp E., de Jong P. T., Schalekamp M. A. High plasma prorenin in diabetes mellitus and its correlation with some complications. J Clin Endocrinol Metab. 1990 Oct;71(4):1008–1015. doi: 10.1210/jcem-71-4-1008. [DOI] [PubMed] [Google Scholar]
- Hall J. E., Guyton A. C., Jackson T. E., Coleman T. G., Lohmeier T. E., Trippodo N. C. Control of glomerular filtration rate by renin-angiotensin system. Am J Physiol. 1977 Nov;233(5):F366–F372. doi: 10.1152/ajprenal.1977.233.5.F366. [DOI] [PubMed] [Google Scholar]
- Hannedouche T. P., Delgado A. G., Gnionsahe D. A., Boitard C., Lacour B., Grünfeld J. P. Renal hemodynamics and segmental tubular reabsorption in early type 1 diabetes. Kidney Int. 1990 Apr;37(4):1126–1133. doi: 10.1038/ki.1990.95. [DOI] [PubMed] [Google Scholar]
- Hostetter T. H., Troy J. L., Brenner B. M. Glomerular hemodynamics in experimental diabetes mellitus. Kidney Int. 1981 Mar;19(3):410–415. doi: 10.1038/ki.1981.33. [DOI] [PubMed] [Google Scholar]
- Keeton T. K., Campbell W. B. The pharmacologic alteration of renin release. Pharmacol Rev. 1980 Jun;32(2):81–227. [PubMed] [Google Scholar]
- Marre M., Chatellier G., Leblanc H., Guyene T. T., Menard J., Passa P. Prevention of diabetic nephropathy with enalapril in normotensive diabetics with microalbuminuria. BMJ. 1988 Oct 29;297(6656):1092–1095. doi: 10.1136/bmj.297.6656.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marre M., Claudel J. P., Ciret P., Luis N., Suarez L., Passa P. Laser immunonephelometry for routine quantification of urinary albumin excretion. Clin Chem. 1987 Feb;33(2 Pt 1):209–213. [PubMed] [Google Scholar]
- Marre M., Leblanc H., Suarez L., Guyenne T. T., Ménard J., Passa P. Converting enzyme inhibition and kidney function in normotensive diabetic patients with persistent microalbuminuria. Br Med J (Clin Res Ed) 1987 Jun 6;294(6585):1448–1452. doi: 10.1136/bmj.294.6585.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen E. R., Hommel E., Giese J., Parving H. H. Efficacy of captopril in postponing nephropathy in normotensive insulin dependent diabetic patients with microalbuminuria. BMJ. 1991 Jul 13;303(6794):81–87. doi: 10.1136/bmj.303.6794.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAreavey D., Brown W. B., Murray G. D., Robertson J. I. Exchangeable sodium in DOC-salt and post-DOC-salt hypertension in rats. J Hypertens. 1985 Jun;3(3):275–279. doi: 10.1097/00004872-198506000-00013. [DOI] [PubMed] [Google Scholar]
- Mimran A., Insua A., Ribstein J., Bringer J., Monnier L. Comparative effect of captopril and nifedipine in normotensive patients with incipient diabetic nephropathy. Diabetes Care. 1988 Nov-Dec;11(10):850–853. doi: 10.2337/diacare.11.10.850. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E., Chachati A., Christensen C. K., Close C. F., Deckert T., Hommel E., Kastrup J., Lefebvre P., Mathiesen E. R., Feldt-Rasmussen B. Microalbuminuria: an early marker of renal involvement in diabetes. Uremia Invest. 1985;9(2):85–95. doi: 10.3109/08860228509088195. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. Long-term antihypertensive treatment inhibiting progression of diabetic nephropathy. Br Med J (Clin Res Ed) 1982 Sep 11;285(6343):685–688. doi: 10.1136/bmj.285.6343.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen C. E. Prediction of clinical diabetic nephropathy in IDDM patients. Alternatives to microalbuminuria? Diabetes. 1990 Jul;39(7):761–767. doi: 10.2337/diab.39.7.761. [DOI] [PubMed] [Google Scholar]
- Morelli E., Loon N., Meyer T., Peters W., Myers B. D. Effects of converting-enzyme inhibition on barrier function in diabetic glomerulopathy. Diabetes. 1990 Jan;39(1):76–82. doi: 10.2337/diacare.39.1.76. [DOI] [PubMed] [Google Scholar]
- Neels H. M., van Sande M. E., Scharpé S. L. Sensitive colorimetric assay for angiotensin converting enzyme in serum. Clin Chem. 1983 Jul;29(7):1399–1403. [PubMed] [Google Scholar]
- Pedersen M. M., Schmitz A., Pedersen E. B., Danielsen H., Christiansen J. S. Acute and long-term renal effects of angiotensin converting enzyme inhibition in normotensive, normoalbuminuric insulin-dependent diabetic patients. Diabet Med. 1988 Sep;5(6):562–569. doi: 10.1111/j.1464-5491.1988.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Pham-Huu-Trung M. T., Corvol P. A direct determination of plasma aldosterone. Steroids. 1974 Nov;24(5):587–508. doi: 10.1016/0039-128x(74)90013-0. [DOI] [PubMed] [Google Scholar]
- Romanelli G., Giustina A., Bossoni S., Caldonazzo A., Cimino A., Cravarezza P., Giustina G. Short-term administration of captopril and nifedipine and exercise-induced albuminuria in normotensive diabetic patients with early-stage nephropathy. Diabetes. 1990 Nov;39(11):1333–1338. doi: 10.2337/diab.39.11.1333. [DOI] [PubMed] [Google Scholar]
- Rudberg S., Aperia A., Freyschuss U., Persson B. Enalapril reduces microalbuminuria in young normotensive type 1 (insulin-dependent) diabetic patients irrespective of its hypotensive effect. Diabetologia. 1990 Aug;33(8):470–476. doi: 10.1007/BF00405108. [DOI] [PubMed] [Google Scholar]
- Sagnella G. A., Markandu N. D., Buckley M. G., Singer D. R., MacGregor G. A. Relationships between circulating ANP, cyclic GMP and sodium excretion in normal human subjects. J Hum Hypertens. 1993 Apr;7(2):181–182. [PubMed] [Google Scholar]
- Sutton R. A., Walker V. R. Responses to hydrochlorothiazide and acetazolamide in patients with calcium stones. Evidence suggesting a defect in renal tubular function. N Engl J Med. 1980 Mar 27;302(13):709–713. doi: 10.1056/NEJM198003273021302. [DOI] [PubMed] [Google Scholar]


