Abstract
The ribosomal DNA origin binding protein Tif1p regulates the timing of rDNA replication and is required globally for proper S-phase progression and division of the Tetrahymena thermophila macronucleus. Here, we show that Tif1p safeguards chromosomes from DNA damage in the mitotic micronucleus and amitotic macronucleus. TIF1p localization is dynamically regulated as it moves into the micro- and macronucleus during the respective S phases. TIF1 disruption mutants are hypersensitive to hydroxyurea and methylmethanesulfonate, inducers of DNA damage and intra-S-phase checkpoint arrest in all examined eukaryotes. TIF1 mutants incur double-strand breaks in the absence of exogenous genotoxic stress, destabilizing all five micronuclear chromosomes. Wild-type Tetrahymena elicits an intra-S-phase checkpoint response that is induced by hydroxyurea and suppressed by caffeine, an inhibitor of the apical checkpoint kinase ATR/MEC1. In contrast, hydroxyurea-challenged TIF1 mutants fail to arrest in S phase or exhibit caffeine-sensitive Rad51 overexpression, indicating the involvement of TIF1 in checkpoint activation. Although aberrant micro- and macronuclear division occurs in TIF1 mutants and caffeine-treated wild-type cells, TIF1p bears no similarity to ATR or its substrates. We propose that TIF1 and ATR function in the same epistatic pathway to regulate checkpoint responses in the diploid mitotic micronucleus and polyploid amitotic macronucleus.
INTRODUCTION
The coordinate regulation of nuclear and cytoplasmic cell cycles ensures that daughter cells receive a complete complement of chromosomes. Consequently, perturbations in DNA replication, chromosome segregation, or nuclear division arrest the cell cycle before cytokinesis. Recent studies have identified an intra-S phase DNA damage checkpoint that protects chromosomes from lesions associated with the elongating replication fork (for review, see Lambert and Carr, 2005). Sources of genotoxic stress, such as the chemical mutagen methylmethanesulfonate (MMS) or depletion of DNA precursors with hydroxyurea (HU), trigger the activation of the damage sensor/transducer protein Ataxia Telangectasia and RAD3-related (ATR) (MEC1 in Saccharomyces cerevisiae). This phosphatidylinositol 3 (PI3)-kinase–related protein kinase initiates a signaling cascade by phosphorylating downstream effector kinases Chk1p (in mammals), Rad53p (Chk2p) in budding yeast, and additional regulatory proteins (Foss, 2001; Gilbert et al., 2001; Tanaka and Russell, 2001). Uninitiated replication origins are repressed, and elongating replication forks are stabilized until the impediment to DNA replication is resolved by recombination or repair. Inactivation of the damage checkpoint is lethal in all examined eukaryotes.
The coordinate regulation of nuclear and cytoplasmic cell cycles is subjected to unusual challenges in ciliated protozoa, such as Tetrahymena thermophila, because members of this ancient lineage contain two nuclei within a single cytoplasm. These nuclei serve nonoverlapping roles and harbor chromosomes that are organized and segregated in fundamentally different ways (for review, see Karrer, 2000). The transcriptionally silent, diploid micronucleus functions as the germline nucleus and contains five chromosome pairs that are transmitted by conventional mitosis and meiosis. In contrast, the transcriptionally active, polyploid (∼45 C) macronucleus has >250 distinct chromosomes that lack centromeres and segregate by a poorly understood amitotic mechanism. The macronuclear genome is not inherited after conjugation. Instead, a new macronuclear “anlage” is generated by differentiation of a toti-potent postzygotic micronucleus in progeny cells. Macronuclear chromosomes are produced by site-specific fragmentation and rearrangement of their micronuclear precursors. With the exception of the 21-kilobase rDNA minichromosome, which is amplified to ∼9000 copies, macronuclear chromosomes attain a copy number of ∼45 C.
Once macronuclear development is complete, T. thermophila divides by binary fission. Because the timing of micro- and macronuclear DNA replication and division are offset in the cell cycle, these nuclei must respond to different cell cycle cues. Although macronuclear chromosomes segregate randomly, chromosome copy number is maintained in a manner somewhat analogous to bacterial plasmids (for review, see Dobbs et al., 1994). The imprecision of amitotic macronuclear division raises the possibility that an ATR-like intra-S-phase checkpoint is dispensable for macronuclear chromosome homeostasis. Furthermore, unconventional mechanisms have evolved to compensate for the associated genic imbalances. Partial endoreplication cycles and the elimination of “excess DNA” in the form of macronuclear extrusion bodies maintain macronuclear DNA content and gene copy number within a narrow range (Cleffmann, 1968; Doerder and Debault, 1975; Bodenbender et al., 1992).
Because these compensating pathways do not operate in the diploid, mitotic micronucleus, one would predict that the micronucleus uses conventional DNA damage checkpoint pathways to maintain genome stability. One argument against this idea is that fact that micronuclear aneuploidy does not arrest vegetative cell cycle progression. “Functionally amicronucleate,” hypo-diploid “star” strains divide normally and have an unlimited life span (Allen, 1967), and some related tetrahymenid species lack a micronucleus. The integrity of the micronucleus only comes into play during conjugation, because it contains the sole source of transmitted nuclear genes.
We obtained preliminary evidence for a macronuclear S-phase checkpoint during our analysis of tif1-1::neo mutants, which among other things are partially defective in macronuclear S-phase progression (Morrison et al., 2005). TIF1-deficient cells exhibit a prolonged macronuclear S phase, that once complete, is followed by a further delay in macronuclear division and cytokinesis (Morrison et al., 2005). Whereas macronuclear divisions are frequently aberrant in TIF1 mutants, the observed defect in S phase progression and subsequent delay in macronuclear division and cytokinesis argue that macronuclear and cytoplasmic cell cycles are coordinately regulated. Tif1p binds essential cis-acting replication determinants in the rDNA origin, in vitro and in vivo (Saha and Kapler, 2000; Saha et al., 2001) and was recently shown to regulate rDNA origin activation, functioning in a repressive capacity to prevent precocious initiation during macronuclear S phase (Morrison et al., 2005). Because macronuclear S phase is prolonged in Tif1p-deficient strains, Tif1p might regulate initiation and/or the elongation of replication forks in other (non-rDNA) macronuclear chromosomes.
Here, we show that Tif1p is required to maintain genome integrity in the mitotic germline micronucleus. We demonstrate that wild-type T. thermophila elicits an intra-S-phase DNA damage checkpoint response that has the hallmarks of the highly conserved ATR-dependent pathway. Furthermore, we show that the intra-S-phase checkpoint pathway promotes chromosome homeostasis in both the diploid mitotic micronucleus and the polyploid amitotic macronucleus. Most significantly, we demonstrate that Tif1p is required to activate the intra-S-phase checkpoint response in both nuclear compartments. These observations, in conjunction with the previously described role for Tif1p at the rDNA origin (Morrison et al., 2005), suggest that this protein contributes to chromosome homeostasis through its action at origins and at elongating replication forks.
MATERIALS AND METHODS
Manipulation of T. thermophila Strains
The relevant features of the micro- and macronucleus in wild-type and TIF1-deficient strains are listed in Table 1. Standard methods were used for strain propagation, mating, and selection or screening for drug-resistant markers [100 μg/ml paromomycin (pm), 12.5% (wt/vol) 2-deoxygalactose (2-dgal), or 15 μg/ml cycloheximide (cycl); Sigma-Aldrich, St. Louis, MO] (Orias and Bruns, 1976). The homozygous tif1-1::neo null strain (TXk202), macronuclear TIF1/tif1-1::neo knockdown mutant (TXh48), and heterozygous TIF1/tif1-1::neo germline disruption strains (TXh102 and TXh106) were generated previously by biolistic transformation with the tif1-1::btu1-neo (neomycin phosphotransferase) disruption cassette (Morrison et al., 2005). The latter three strains were serially propagated in increasing concentrations of pm to obtain phenotypic assortants with elevated levels of the tif1-1::neo disruption and diminished amounts of the wild-type gene in the transcribed polyploid macronucleus (wild-type macronuclear TIF1 DNA copy number relative to untransformed control: TXh48, ∼25%; TXh102, ∼20%; and TXh106, ∼20%). The homozygous null strain TXk202 eventually senesced, and repeated attempts to generate another homozygous null strain were unsuccessful. The macronuclear anlage cotransformant TAM101 is heterozygous for the tif1–2 allele that contains 6xhis/5xmyc epitope tag on its carboxy terminus (Brown et al., 1999). Cotransformation of the developing macronucleus and screening for partial replacement of wild-type macronuclear TIF1 were performed as described previously (Morrison et al., 2005).
Table 1.
T. thermophila strains used in this study
| Strain | Micronuclear genotype | Macronuclear genotype/phenotype |
|---|---|---|
| TXh102a | TIF1/tif1-1::neo | TIF1 disruption (partial replacement) pm resistant |
| TXh106a | TIF1/tif1-1::neo | TIF1 disruption (partial replacement) pm resistant |
| TXk202a | tif1-1::neo/tif1-1::neo | TIF1 disruption (complete null) pm resistant |
| TXh48a | TIF1/TIF1 | TIF1 disruption (partial replacement) pm resistant |
| TAM101b | tif1-2/tif1-2 | C terminal his-myc TIF1 Epitope tag (partial replacement) pm resistant |
| CU427c | TIF1/TIF1 CHX1/chx1-1 | pm sensitive Cycloheximide-sensitive |
| CU428c | TIF1/TIF1 | pm sensitive |
| SB210c | TIF1/TIF1 gal1-1/gal1-1 | pm sensitive 2-deoxygalactose-sensitive |
| SB1969c | TIF1/TIF1 ts29/ts29 | pm sensitive Temperature resistant |
chx1-1, cycloheximide-resistance; gal1-1, 2-deoxygalactose-resistance; tif1-1::neo, paromomycin resistance conferred by cadmium-inducible neomycin phosphotransferase transgene targeted to the TIF1 locus; and ts29, recessive temperature-sensitive allele at locus of unknown function.
a TIF1gene disruption: the MTT1neoBTU1transgene was introduced into the macronuclear anlagen or premeiotic germline micronucleus (note the micronuclear genotype indicates whether the strain is a [germline] micronuclear and macronuclear anlagen transformant). Partial replacements contain wild-type TIF1 macronuclear gene dosages that are ∼20–25% relative to wild type (macronucleus = 45 C).
b Macronuclear anlagen cotransformants carrying his-myc epitope-tagged TIF1 and MTT1neoMTT1 at the respective TIF1 and MTT1 loci.
c Heterokaryon strains.
Micronuclear Cytology in Mating Cells
For mating experiments, wild-type strains (CU427 and CU428) were distinguished from TIF1 knockout (TXk202), knockdown (TXh48), or A* strains by incorporation of MitoTracker dyes (Invitrogen, Carlsbad, CA). Starved vegetative cell cultures were incubated overnight with 0.1 μg of MitoTracker Green FM (wild type) or MitoTracker Red-CMXRos (mutants). Cells were subsequently mated at a concentration of 2 × 105 cells/ml. One-milliliter aliquots were harvested at defined intervals, stained with 0.1 μg/ml 4′,6′-diamidino-2-phenylindole (DAPI; Sigma-Aldrich), and examined by fluorescence microscopy (Morrison et al., 2005). The functionally amicronucleate A* strain served as a reference strain for aberrant development. This strain contains a DAPI-staining micronucleus that fails to transmit genetic information to progeny. Vegetative nuclei were also visualized in living cells with ApoFluor (0.001% acridine orange and 5 μg/ml Hoechst 33342; Sigma-Aldrich).
Immunocytological studies were performed using antibodies directed against human Rad51p, 5′-bromo-2′-deoxyuridine (BrdU), or the myc-epitope tag in the Tif1-2p fusion protein. Fixed cells were incubated with antibodies before mounting onto slides (Morrison et al., 2005) or mounted before antibody incubation (Loidl and Scherthan, 2004). For cell cycle studies, vegetative cultures were starved, refed, and harvested at 30-min intervals as described previously (Mohammad et al., 2003).
Molecular Biology Techniques
Standard molecular biology techniques, including genomic DNA isolation, polymerase chain reaction (PCR), and Northern blotting were performed as described previously (Mohammad et al., 2000; Saha et al., 2001). Micronuclear genome instability was examined with PCR primers that span chromosome breakage sequence elements that demarcate the sites of chromosome fragmentation in the developing macronucleus (Hamilton et al., 2005; Yakisich and Kapler, 2006). Total cellular RNA was prepared using an RNAeasy mini-kit (QIAGEN, Valencia, CA) according to the manufacturer's recommendations, resolved on formaldehyde agarose gels, and hybridized to random primer radiolabeled RAD51 or TIF1 coding region probes (Morrison et al., 2005).
For Western blot analysis, protein samples were prepared by direct lysis in 1× Laemmli buffer (5 × 104 cells), resolved on a 12% SDS-PAGE gel, and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA) according to the manufacturer's recommendations. Blocked membranes were incubated overnight at 4°C with mouse anti-human Rad51p polyclonal antiserum (1:2500 dilution; NeoMarkers, Fremont, CA) or rabbit polyclonal antiserum directed against the myc epitope tag (1:2500 dilution; Delta Biolabs, Gilroy, CA) to monitor Tif1p. Membranes were washed and then incubated with secondary anti-mouse (1:3000) or anti-rabbit (1:5000) antibody coupled to horseradish peroxidase (Jackson ImmunoResearch Laboratories, West Grove, PA) for 3 h at 4°C. After washing twice with H2O and once with phosphate-buffered saline/0.05%Tween 20 (15 min each) and five times with H2O (5 min each), the membranes were developed using enhanced chemiluminiscence (Millipore). Human Rad51p antibody detects a single DNA damage-inducible polypeptide of the size predicted for T. thermophila Rad51p (34 kDa; EMBL accession no. AF064516) (Campbell and Romero, 1998; Loidl and Scherthan, 2004).
DNA Damage and S-Phase Checkpoint Analysis
For studies involving the DNA-damaging agent MMS, wild type (CU428) or TIF1 knockdown (TXh48) cultures were grown to a concentration of 1 × 105 cells/ml. Cultures (30 ml) were treated with varying concentrations of MMS (Sigma-Aldrich). Cells were grown at 30°C for 1 h before a 30-min pulse labeling with 100 μg/ml BrdU. Samples (15 ml) were harvested and prepared for BrdU immunofluorescence analysis with mouse monoclonal anti-BrdU antibody (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom), and rhodamine-conjugated goat anti-mouse antibody (Jackson ImmunoResearch Laboratories) (Morrison et al., 2005). Secondary antibody treatment and DAPI staining were performed as noted above. MMS time courses were also used to examine Tif1p and Rad51p localization and abundance.
The intra-S-phase checkpoint was examined by treating cells with HU and/or caffeine (Sigma-Aldrich). HU inhibits ribonucleotide reductase and depletes dNTP stores in S phase, whereas caffeine inhibits the G1 checkpoint kinase Ataxia Telangectasia Mutated (ATM) and intra-S-phase checkpoint kinase ATR in all examined eukaryotes (for review, see Lambert and Carr, 2005). The effect of wortmannin (WM), which preferentially inhibits conventional (non-ATM/ATR) PI3-kinases, was also tested (Yakisich and Kapler, 2004). Stock solutions of HU and caffeine were prepared in water. Wortmannin stocks were prepared in dimethyl sulfoxide. Mock treatments involved the addition of drug vehicle alone to culture media. HU dose–response curves were generated by measuring tritiated thymidine incorporation into trichloroacetic acid-insoluble material and by assessing cell cycle progression with flow cytometry to determine the ID50 (Morrison et al., 2005).
Log phase cultures were subsequently grown for 12 h in HU-containing media to induce cell cycle S-phase arrest (or HU + caffeine), washed twice with 10 mM Tris, pH 7.5, and then resuspended in drug-free media to measure culture outgrowth, viability of treated cells, and cell division during the first cell cycle after drug removal. Cell viability was determined by serially diluting cells into 100 μl of growth media in 96-well microtiter dishes immediately after drug treatment. The number of confluent wells (clonally derived lines) was recorded 4 d later in plates that contained <33% positive wells and related to the number of input cells in the relevant dilution. For cell division analysis, DAPI-stained cells were examined microscopically at defined intervals after drug treatment and removal. The input cells for the latter analysis were synchronized by starvation and refeeding before the addition of HU and/or caffeine (Mohammad et al., 2003). The cell division index corresponds to the percentage of cells exhibiting a cytokinetic furrow, as determined by light microscopy.
RESULTS
Tif1p Is Required for the Maintenance of a Functional Micronucleus
The previously described array of macronuclear aberrations associated with Tif1p-deficient Tetrahymena (Morrison et al., 2005) indicates that this protein plays a global role in macronuclear chromosome biology. Preliminary studies suggested that Tif1p functions in the diploid micronucleus as well, because the intensity of micronuclear DAPI staining was reduced in tif1-1::neo mutant strains (Morrison et al., 2005). To investigate the contribution of Tif1p to micronuclear chromosome stability, we monitored the transmission of genetic markers from TIF1-deficient parents to progeny. First, we mated two heterozygous tif1-1::neo/TIF1 mutants to one another. Before mating, strains TXh102 and TXh106 were propagated in pm for >100 fissions to allow cells to reach sexual maturity and to select for phenotypic assortants with high levels of the tif1-1::neo disruption allele in the amitotic polyploid macronucleus. Mature cells were mated, and 48 clonal lines were established by plating cells at limiting dilutions 24 h after initiating the mating.
If the micronucleus of tif1-1::neo/TIF1 mutants is intact and low levels of Tif1p expression do not effect meiotic chromosome transmission, then one of four progeny should be homozygous wild type (12/48 clones). However, because the pairing efficiency of this mating was ∼80%, the predicted frequency of pm-sensitive progeny is 20% (9.6 clones). The 48 lines were expanded and tested for pm resistance and sexual immaturity. None of the clonal lines were pm sensitive (Table 2, cross 1), suggesting that they failed to transmit the wild-type allele or had aborted development altogether and consequently retained their parental macronucleus. The second prediction was confirmed in a subsequent cross, in which a tester strain was immediately mated to the presumed F1 progeny (at ∼20–30 fissions) to determine whether they were juveniles or sexually mature. True progeny require at least 70 fissions before they can form mating pairs (for review, see Karrer, 2000). All 48 clones formed mating pairs in this cross, indicating that they did not contain a new macronucleus. These results are consistent with the presence of a severely compromised micronucleus in heterozygous TIF1/tif1-1::neo mutants. An alternative possibility is that the observed macronuclear retention reflects a maternal requirement for Tif1p during new macronuclear anlage development.
Table 2.
Chromosome transmission in TIF1 heterozygote and heterokaryon strains
| Cross | Strain | Phenotype | Expected % | Observed % |
|---|---|---|---|---|
| 1 | TXh102 × TXh106 | pms clonal progeny | 20a | 0, n = 48 |
| Sexual immaturity | 100 | 0, n = 48 | ||
| 2a | TXh102 × SB210 | 2dgalr/pmr progeny | 50 | 0, n = 40 |
| 2b | TXh106 × SB210 | 2dgalr/pmr progeny | 50 | 0, n = 40 |
Resistance to 2-deoxygalactose, cycloheximide, or paromomycin is encoded in the micronucleus of SB210 (gal1-1), SB1969 (chk1-1), and TIF1:tif1-1::neo heterozygotes, respectively. For cross 1, n is the number of clonal progeny tested for pm resistance. For cross 2, n is the number of small-scale matings in which the nonclonal progeny pool was tested for pm resistance.
a The predicted frequency for pms progeny is 25%. However, because the pairing efficiency in this cross was ∼80%, the expected frequency of pms clones is ∼20% (9.6 clones of 48 rather than 12 of 48).
To distinguish between these possibilities, we directly mated TIF1/tif1-1::neo mutants to a wild-type tester strain. Because genetic exchange is reciprocal, half of the progeny that inherit the tif1-1::neo allele will undergo macronuclear development in a wild-type cytoplasm. Eighty clonal lines were established from two different heterozygous tif1-1::neo/TIF1 transformants (TXh102 and TXh106), and subsequently mated to the wild-type heterokaryon strain SB210, which contains a 2-deoxygalactose (2-dgal)–sensitive macronucleus and is homozygous for 2-dgal resistance in the transcriptionally silent micronucleus (1000 cells/mating). True progeny were selected en mass for 2-dgal resistance and then screened for pm resistance, conferred by the introduced tif1-1::neo disruption allele. Because many progeny were generated in each cross, all of the cultures should include pm-resistant progeny if micronuclear chromosomes from the tif1-1:neo/TIF1 parent were successfully transmitted. This was not the case, because all of the 2-dgal–resistant progeny died in pm (Table 2, crosses 2a and 2b). This result, in conjunction with the aborted development observed in cross 1, argues that the TIF1/tif1-1-1::neo micronucleus underwent extensive genome instability during vegetative propagation.
Aberrant Meiosis in Tif1p-deficient Cells
As cells enter meiosis, the spherical micronucleus migrates away from the macronucleus and elongates into a crescent that encircles a significant portion of the macronucleus. TIF1 mutants generate a very small crescent micronucleus that stains weakly with DAPI relative to wild-type controls (Morrison et al., 2005). Although these observations and genetic studies (Table 2) suggest that micronuclear DNA is lost, examination of the meiotic program provides more definitive information on the fate of germline chromosomes. Consequently, we mated homozygous tif1-1::neo and heterozygous TIF1/ tif1-1::neo mutants with a wild-type strain and compared meiotic progression, mating pair synchrony and chromosome composition to a control mating involving two wild-type strains. Cells were examined by DAPI staining, before prophase I through anaphase II.
In contrast to a mating involving two wild-type strains (our unpublished data), the progression of tif1-1::neo/TIF1 mutants through meiosis frequently lagged behind the wild-type partner (Figure 1A, micrographs i–iii). In mating pairs in which the wild-type micronucleus revealed multiple, extended metaphase or anaphase chromosomes, the tif1-1::neo/TIF1 partner displayed one or two condensed micronuclear DNA masses (Figure 1A, micrographs i and ii, arrows). The scarcity of anaphase structures in Tif1p-deficient mating partners suggested that the residual chromosomes failed to segregate.
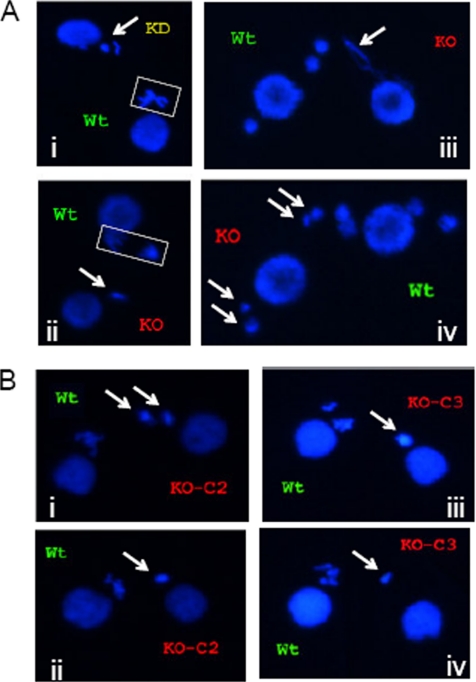
Figure 1.
Cytogenetic evidence for micronuclear genome instability, developmental delay, and/or meiotic arrest in TIF1-deficient strains. The wild-type strain (CU428) was mated with TIF1 mutants (TXh48 [KD, knockdown]; TXk202 [KO, knockout/null]), and mating pairs were examined at various times during development by DAPI fluorescent staining. Wild-type and mutant strains were prelabeled with MitoTracker Red and Green dyes, respectively, to identify each partner. (A) Meiotic aberrations in TIF1-deficient strains. Meiotic stages: i, wild type: metaphase, mutant: prophase (?); ii, wild type: anaphase, mutant: prophase (?); iii, wild type: postmeiotic pronuclei, mutant: meiotic crescent or aberrant anaphase (?); and iv, wild type and mutant: postmeiotic pronuclei. Arrows point to mutant nuclei. (B) Comparative cytogenetic analysis of siblings cells in subcloned parental lines KO-C2 and KO-C3 derived from the tif1-1::neo knockout strain TXh202. The newly generated clonal lines were briefly expanded and then mated with the wild-type strain CU428 to examine meiosis. Arrows point to condensed, micronuclear-derived DAPI-staining chromosomes in siblings within the same mating culture. Micrographs i and ii, representative tif1-1::neo/TIF1 knockout clone C2 siblings; micrographs iii and iv, representative tif1-1::neo knockout clone C3 siblings.
Asynchronous meiosis and the apparent loss or fusion of micronuclear chromosomes is a hallmark of micronuclei derived from the functionally amicronucleate star strains, which contain a cytologically visible, but genetically compromised micronucleus that fails to transmit genetic information to progeny (Allen, 1967). Both tif1-1::neo/TIF1 and A* strains exhibited this phenotype in crosses with wild-type tester strains (our unpublished data). In rare instances where a tif1-1::neo/TIF1 mutant micronucleus seemed to complete meiosis II, qualitative differences in the DAPI staining intensity of the four postmeiotic pronuclei were evident, suggesting that meiotic chromosome segregation was impaired (Figure 1A, micrograph iv, arrows). Whereas DAPI is not ideally suited for quantification, the often diminutive and highly variable size of micronuclei (crescents and pronuclei) and meiotic chromosomal masses supports the notion that micronuclear DNA content is diminished in the TIF1 mutant background. This prediction was subsequently borne out at the molecular level (see below).
Progressive micronuclear genome instability was evident in cytological studies of siblings derived from freshly established clone lines of the homozygous knockout strain TXk202. For example, when clonal line KO-C2 was mated with a wild-type partner (CU428), one mutant cell exhibited two large globular DNA masses during meiosis, whereas a sibling produced a single, more compact DNA mass (Figure 1B, micrographs i and ii). All siblings from the KO-C3 clone contained a single DAPI-staining micronuclear mass; however, the DAPI-staining intensity varied considerably (Figure 1B, micrographs iii and iv). Thus, the sterility of TIF1 mutants is reflected by an apparent diminution in DNA mass, chromosome number, and aberrant meiotic cell cycle progression. It should be noted that the homozygous null strain TXh202 eventually senesced, suggesting that the long-term absence of Tif1p affects macronuclear genic balance as well. Repeated attempts to generate new homozygous null strains failed with freshly generated heterozygous germline transformants, even when selective pressure for the disruption allele was removed immediately after identifying transformants (our unpublished data). We conclude that partial Tif1p depletion has a greater effect on the function of the diploid micronucleus relative to the polyploid macronucleus.
Molecular Analysis of Micronuclear Chromosomes Reveals Ongoing Genome Instability
The genetic and cytological studies described above indicate that Tif1p is required for micronuclear chromosome transmission. To gain insight into the fate of chromosomes at the molecular level, we subjected 10 freshly generated subclones of strain Txh48 to PCR analysis with primer combinations that span sites for programmed DNA fragmentation of the five germline chromosomes in the developing macronucleus (Hamilton et al., 2005). These primer sets will only amplify products derived from intact micronuclear chromosomes.
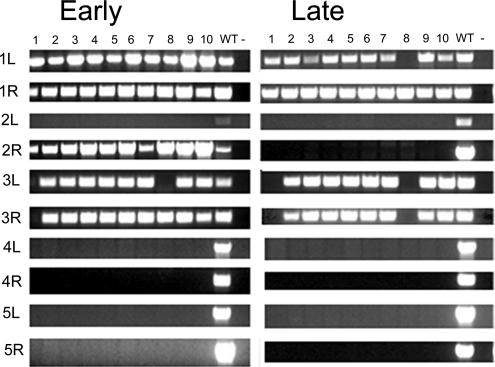
All 10 examined subclones failed to amplify markers derived from the left (L) and right (R) arms of chromosomes 4 and 5 (Figure 2, early). The chromosome 2L marker was absent from all 10 clones as well; however, the 2R marker was retained, consistent with the formation of a double-strand break (DSB). These results suggest that chromosome arms and possibly entire chromosomes are missing. Heterogeneity was detected among the subclones for other markers (1L, 2R, and 3R), indicating that the stochastic loss of these markers occurred after the parental TXh48 strain was generated. When these lines were reexamined ∼250 fissions later, additional markers were missing (Figure 2, late). These results indicate that the micronucleus of sterile Tif1p-deficient strains is hypodiploid and undergoes progressive and massive DNA loss during vegetative cell divisions.
Figure 2.
Micronuclear genome instability in TIF1-deficient T. thermophila. Ten clonal lines derived from the tif1-1::neo/TIF1 knockdown strain TXh48 were established and subjected to PCR analysis with primers sets that span sites for chromosome breakage sequence (CBS)-mediated chromosome fragmentation in the developing macronucleus. PCR primers derived from the right (R) and left (L) arms of all five micronuclear chromosomes were tested. 1–10, clonal TXh48 knockdown lines; WT, CU428. Early, ∼150 fissions after conferring resistance to high concentrations of pm (encoded by the tif1-1::neo disruption); late, ∼250 fissions later than “early.”
Elevated Levels of DNA Damage in MMS-treated tif1-1 Mutants
The micronuclear and macronuclear chromosome transmission defects associated with a Tif1p deficiency suggest that both nuclei accumulate DNA damage at an elevated rate, even in the absence of exogenous genotoxic stress (Morrison et al., 2005; this work). To test this prediction, we first asked whether tif1-1 mutants were hypersensitive to the alkylating agent MMS, which mutagenizes DNA and promotes activation of the intra-S-phase checkpoint response (Chang et al., 2002; Lupardus et al., 2002). BrdU incorporation was used to monitor the DNA damage response (Liu et al., 2003). Log phase wild-type and mutant cultures were exposed to various concentrations of MMS for 1 h, pulse labeled for 30 min with BrdU, and examined by indirect immunofluorescence. Due to the close physical association of micro- and macronuclei throughout most of the vegetative cell cycle, definitive information was not obtained for the micronucleus. Therefore, we focused our attention on the macronucleus.
Untreated wild-type and mutant strains exhibited a comparable percentage of BrdU-positive macronuclei in log phase cultures, indicating a similar cell cycle distribution in asynchronous populations (Table 3). MMS treatment inhibited BrdU incorporation in a dose-dependent manner in both genetic backgrounds; however, partial and complete inhibition occurred at lower MMS concentrations in the tif1-1::neo/TIF1 mutant background (Table 3). For example, Tif1p-deficient cells showed a marked decrease in BrdU-positive macronuclei in 0.06% MMS, whereas comparable inhibition occurred at a threefold higher concentration in the wild-type strain (0.18% MMS). This concentration abolished BrdU labeling in the mutant. These results raise several nonmutually exclusive possibilities. First, the elevated basal level of DNA damage in the TIF1 mutant lowers the threshold for exogenous damaging agents. Second, the TIF1 mutant fails to activate a checkpoint response, leading to the collapse of replication forks at DNA adducts. Third, the TIF1 mutant is defective in a repair or recombination pathway that removes or bypasses the lesion.
Table 3.
MMS DNA damage response
| % MMS (vol/vol) | % BrdU-positive macronuclei |
|
|---|---|---|
| Wild type | TIF1 mutant | |
| 0 | 40 | 39 |
| 0.03 | 38 | 36 |
| 0.06 | 36 | 26 |
| 0.12 | 32 | 23 |
| 0.18 | 23 | 0 |
| 0.24 | 0 | 0 |
More than 300 cells were examined at each MMS concentration.
Differential Regulation of TIF1 and RAD51 in MMS-treated Cells
Intrinsic and MMS-induced DNA damage can lead to the formation of DSBs during S phase. ATR activates the double-strand break repair response by phosphorylating Rad52p, which acts in concert with replication protein A (RP-A) and the RecA homologue Rad51p to promote homology-mediated repair at DSBs or stalled replication fork (for review, see Lambert and Carr, 2005). Romero and colleagues previously showed that RAD51 gene expression is induced by MMS and UV light (Campbell and Romero, 1998; Smith et al., 2004a), and heterologous antibodies have been used to monitor T. thermophila Rad51p abundance and localization (Loidl and Scherthan, 2004).
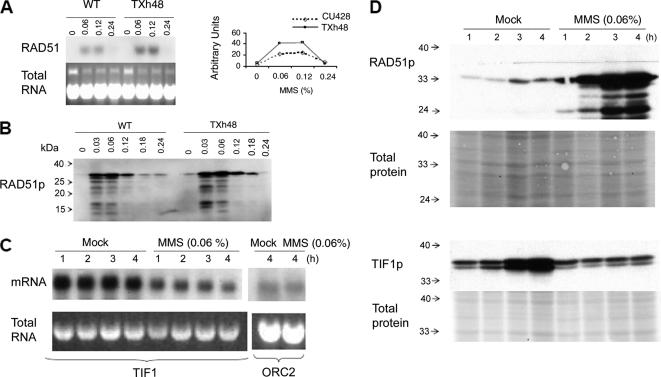
To assess DNA damage in tif1-1 mutants and examine the integrity of the repair response, we first assayed the accumulation of RAD51 mRNA and protein in MMS-treated cells. The basal levels of RAD51 mRNA were barely detectable by Northern blot analysis in untreated wild-type and mutant strains (Figure 3A). The steady-state RNA level increased in response to MMS in both backgrounds and was approximately twofold higher in the tif1-1 mutant (range 0.03–0.18% MMS). RAD51 mRNA levels dropped precipitously at high MMS concentrations (0.24% MMS and above) (Figure 3A; our unpublished data); however, microscopic examination revealed that most cells had lost motility and were dying. Similar responses were observed at the protein level: Rad51p was induced by MMS and consistently higher in the mutant (Figure 3B, 1-h induction).
Figure 3.
Regulation of RAD51 and TIF1 by MMS. (A) RAD51 Northern blot analysis after a 1-h exposure to MMS [0–0.24% (vol/vol)]. Top left, hybridization with a RAD51 coding region probe. Bottom left, ethidium bromide staining of total DNA before transfer to GeneScreen Plus membranes (New England Biolabs, Beverly, MA). Right, graphic representation of RNA hybridization signals quantified on a PhosphorImager (Bio-Rad, Hercules, CA). Dotted line, wild type (CU428); solid line, TIF1 knockdown mutant (TXh48). (B) Western blot analysis of Rad51p in wild-type and mutant whole cell lysates (1-h MMS treatment). (C) Northern blot analysis of TIF1 mRNA in MMS-treated wild-type cells. Strain CU427, same MMS treatment regimen as in C. Probes, TIF1 and origin recognition complex, subunit 2 (ORC2) protein-coding regions. Bottom, ethidium bromide staining of RNA preparations for normalization. (D) Rad51p and Tif1p levels in mock and MMS-treated cells (0.06% MMS; 1–4 h). The Tif1p Western blot probe was directed against the myc epitope in the tif1-2–tagged strain TAM101. Membranes were stained with Ponceau S to compare the amount of total protein loaded in each lane.
Because 0.06% MMS induced the most robust RAD51 response, we examine TIF1 mRNA and protein levels across a 4-h interval at this MMS concentration. Northern blot analysis showed a rapid and substantial decline in TIF1 mRNA following the addition of MMS, whereas ORC2 mRNA levels were unaffected (Figure 3C). This decline may reflect the arrest of cell cycle progression, because TIF1 mRNA levels peak before the onset of macronuclear S phase, well before the peak for maximal TIF1 in vitro DNA binding activity (Morrison et al., 2005).
To monitor Tif1p, we transformed Tetrahymena with a TIF1 derivative encoding a reiterated his-myc epitope tag on the carboxy terminus (tif1-2). The tagged allele was targeted to the endogenous TIF1 locus during macronuclear development (Cassidy-Hanley et al., 1997), generating a partial gene replacement in which the transgene was under the control of its native promoter. Transformants expressing an unlinked marker were screened for epitope-tagged tif1-2 by PCR and by Western blotting (our unpublished data). Cotransformant strain TAM101 was analyzed further.
To best compare the TIF1 and RAD51 S-phase responses, we synchronized wild-type and TIF1-tagged strains by growing cultures to stationary phase and briefly starving cells for 8 h before refeeding in the absence or presence of MMS (0.06%, 0- to 4-h time course). Western blot analysis showed modest and comparable increases in Rad51p and Tif1-2p protein levels over time in mock-treated cells, consistent with a single round of cell division and hence a doubling in cell number (Figure 3D, compare signal to mock controls). Whereas Rad51 protein levels increased significantly over this time interval in MMS-treated cells, Tif1-2 protein levels remained constant. A doublet was detected at the expected molecular weight for tagged Tif1-2p, suggesting that this protein is subjected to posttranslational modification.
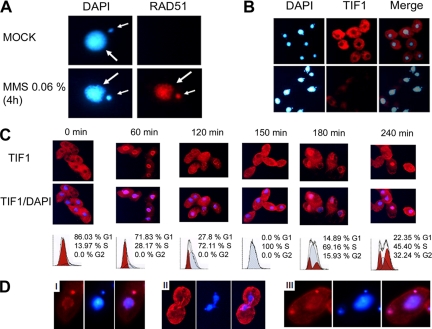
Indirect immunofluorescence was then used to examine the subcellular localization of Tif1p. Concurrent staining of the macronucleus and cytoplasm was observed in a subpopulation of wild-type cells; however, Tif1p was markedly diminished in the macronucleus in most cells (Figure 4, B and C). To our surprise, the Tif1p immunofluorescence signal was significantly reduced in MMS-treated cells (Figure 4B, 0.06% MMS for 4 h). In contrast, Rad51p, which was not detected in untreated cells, produced a robust micro- and macronuclear immunofluorscence signal in MMS-treated cells (Figure 4A). Because Tif1p levels do not decline (Figure 3D), this result raises the possibility that the myc epitope tag might be partially masked after DNA damage.
Figure 4.
Immunolocalization of Rad51p and Tif1p in control and MMS-treated cells. (A) Rad51p immunolocalization in wild-type cells (0.06% MMS; 4 h). Red, Rad51p immunofluorescence; blue, DAPI. Small arrow, micronucleus, large arrow, macronucleus. (B) Immunolocalization of Tif1p in asynchronous control and MMS-treated cultures (strain TAM101; 0.06% MMS, 4 h). Red, Tif1p immunofluorescence; blue, DAPI. (C) Cell cycle localization of Tif1p. Strain TAM101 was synchronized by starvation and refeeding and assayed at 30-min intervals for TIF1p localization (red, TIF1p immunofluorescence; blue, DAPI) and DNA content (flow cytometry). Alternating time points are shown. (D) Immunolocalization of Tif1p late in the cell cycle (240 min). Note the micronuclear localization of Tif1p in micrographs i and iii and exclusion from dividing macronuclei in micrograph ii.
Localization of Rad51p to the micronucleus was previously reported during meiosis (Loidl and Scherthan, 2004), whereas a separate study of vegetative cells reported Rad51p staining of just the macronucleus in MMS-treated cultures (Campbell and Romero, 1998). The discrepancy in vegetative micronuclear staining between our work and the previous study may reflect the more robust reactivity of heterologous antibodies that were used in our analysis. The cumulative RAD51 data suggest that MMS promotes the formation of DSBs in both nuclear compartments and indicate that the micro- and macronucleus elicit comparable responses.
Cell Cycle-regulated Localization of Tif1p
The heterogeneous pattern of Tif1p staining in asynchronous cultures raised the possibility that nuclear localization of this protein might be cell cycle regulated. To address this possibility, TAM101 cells were synchronized by starvation and refeeding, and then they were harvested at 30-min intervals for flow cytometry and immunofluorescence analysis (Figure 4C). Dynamic changes in micro- and macronuclear localization were observed across the cell cycle. Tif1p localized exclusively to the cytoplasm in starved cells. Before the onset of macronuclear S phase (Figure 4C, 60 min), a fraction of Tif1p associated with the macronucleus, forming intensely staining perinuclear foci. Diffuse, homogeneous macronuclear staining was observed during the peak interval for macronuclear DNA replication (Figure 4C, 120–180 min), and a return to punctate perinuclear staining was seen in a subpopulation at late S-phase cells (Figure 4C, 180 min), suggesting that Tif1p relocalizes to the periphery in G2. The mixed localization and heterogeneous flow cytometry profiles at 240 min are indicative of increased asynchrony.
Previous studies showed that micronuclear and macronuclear S phases are offset, with micronuclear DNA replication occurring later in the cell cycle (for review, see Karrer, 2000). Tif1p localized to the micronucleus at late time points in the cell cycle (i.e., 240 min), consistent with a role during micronuclear S phase. Cells that displayed intense micronuclear staining exhibited punctate perinuclear staining of the replicated but an undivided macronucleus (Figure 4D, micrograph i). Tif1p was present in both postmitotic micronuclei, indicating that this association persists through mitosis. In contrast, macronuclear staining was generally lost before or during macronuclear division (Figure 4D, micrograph ii). The localization of Tif1p to S-phase micro- and macronuclei suggests that its primary role occurs when chromosomes are actively replicating.
Identification of a Macronuclear Intra-S-Phase Checkpoint
The elongated macronuclear S phase of TIF1 mutants could result from damage-induced activation of an intra-S phase checkpoint. However, the elevated rate of aberrant macronuclear division suggests that this checkpoint can be overridden in Tif1p-deficient Tetrahymena (Morrison et al., 2005). A bioinformatics search of the T. thermophila genome database (http://tgd.org) identified putative orthologues of the three intra-S-phase checkpoint kinases: ATR, CHK1, and CHK2, but it failed to yield a candidate orthologue for the G1 checkpoint kinase ATM. The e values for the predicted Tetrahymena proteins relative to humans were very high: ATR, 2.0−40; CHK1, 2.0−29; and CHK2, 2.0−61. Consequently, we asked whether wild-type and Tif1p-deficient Tetrahymena elicit a classical intra-S-phase checkpoint response. To do so, we used the ribonucleotide reductase inhibitor HU, which induces S phase arrest and activates the intra-S-phase checkpoint in all reported eukaryotes. We then tested the effect of caffeine, a potent inhibitor of the phosphatidylinositol kinase-related protein kinases ATM and ATR, on HU-arrested Tetrahymena (for review, see Abraham, 2001).
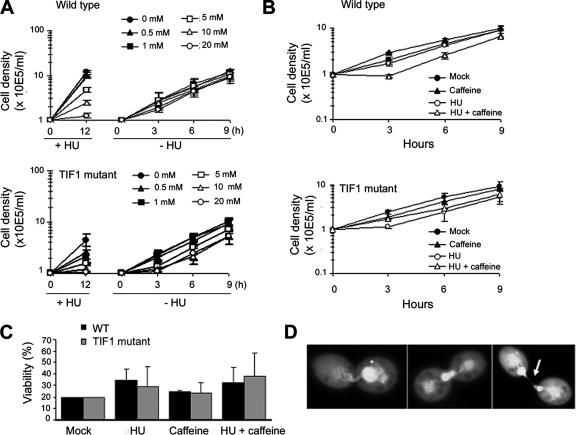
HU dose–response curves were generated by using tritiated thymidine and flow cytometry to monitor DNA synthesis and cell cycle progression. Pulse labeling with thymidine revealed no substantive difference in the rate of DNA synthesis in wild-type and Tif1p-deficient strains (Supplemental Figure S1). HU inhibited thymidine incorporation in a concentration-dependent manner, with the majority of cells arresting with a G1 DNA content (tested HU concentrations 0.1–20 mM, ID50 = 10 mM; flow cytometry, our unpublished data). The recovery from HU arrest was subsequently assessed in cultures treated with increasing concentrations of HU and then reseeded at a fixed cell density in drug-free media. No lag in the outgrowth of wild-type cells was observed at all tested HU concentrations, indicating that HU-induced cell cycle arrest is reversible (Figure 5A; our unpublished data).
Figure 5.
Response of wild-type and tif1-1::neo mutants to HU and caffeine. (A) Wild-type (CU428) and tif1-1::neo/TIF1 mutant (TXh48) strains were grown to a density of 3 × 104/ml and incubated for 12 h in growth media containing a range of HU concentrations (0–20 mM, +HU; stock solution in water) to induce S-phase–specific cell cycle arrest. Outgrowth of cultures after removal of the drug (−HU), refed cells were adjusted to a density of 1 × 105/ml and counted at 3-h intervals. The mean and SEs for three experiments are shown. (B) Outgrowth of cultures treated for 12 h with 20 mM HU and/or 0.3 mM caffeine (stock solution in water) and released into drug-free media. Cell densities were adjusted to 1 × 105/ml after washing out the drug(s), and the cultures were counted at 3-h intervals. The mean and SE for two experiments are shown. (C) Cell viability analysis in cultures treated with HU and/or caffeine. Wild-type and TIF1-deficient strains were incubated for 12 h in media containing no drug, 0.3 mM caffeine, 20 mM HU, or both. Cells were washed repeatedly to remove the drugs and plated out at decreasing concentrations into two 96-well dishes/dilution. For comparative analysis, the percentage of wells that was positive for growth in mock-treated cultures (plating density 0.3 cells/well) was normalized to 20 for each of the three experiments. The representative data for drug-treated strains were similarly normalized and correspond to the mean and SE of the three experiments. (D) Examples of macronuclear division defects in wild-type cells treated for 12 h with 20 mM HU and 0.3 mM caffeine and then propagated for 7 h in drug-free media.
Caffeine was added to the HU regimen to determine whether S-phase arrest can be reversed by inhibition of an ATR-like protein kinase. Caffeine alone (0.3 or 1 mM) was not toxic and did not induce cell cycle arrest or aberrant macronuclear division (Figure 5, A and B, wild type, 0.3 mM caffeine; 1 mM caffeine, our unpublished data). The addition of caffeine to wild-type cells partially reversed HU inhibition (Figure 5A) and caused a modest lag in outgrowth of the culture upon removal of both drugs (Figure 5B). To assess whether this lag corresponds to a recovery period for the resumption of replication or reflects a decrease in cell viability, HU + caffeine-treated cells were plated into 96-well dishes at limiting dilutions, and the outgrowth of clonal lines was quantified relative to mock and single drug regimens. The exposure to caffeine + HU did not decrease cell viability (Figure 5C, wild type). However, this regimen greatly perturbed DNA partitioning during macronuclear division (Figure 5D).
Grossly abnormal macronuclear divisions were observed in dividing wild-type cells examined 7 h after removal of HU and caffeine (Figure 5D, penetrance ∼40%). The most frequently observed phenotype consisted of dividing cells that had two properly partitioned daughter macronuclei and contained an additional large bolus of DNA at the cleavage furrow (Figure 5D, middle and right micrographs; note the wisp of DNA connecting the two daughter cells in the micrograph in the right micrograph [arrow]). The small fraction of wild-type cells that failed to arrest in HU exhibited similarly severe macronuclear division defects (our unpublished data). Amacronucleate cells were occasionally observed, along with dividing cells with large differences in macronuclear DAPI staining (Figure 5D, left micrograph). The severity of these phenotypes lessened at later times during the recovery, and normal macronuclear division was restored by 36 h (our unpublished data).
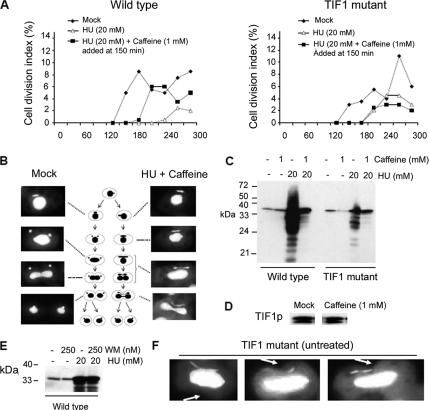
To further investigate the effect of caffeine on the intra-S-phase checkpoint response, we synchronized wild-type cells by starvation and refeeding and examined cytokinesis and nuclear division during the first cell cycle in the presence of HU or HU + caffeine. As expected, the majority of the synchronized wild-type cells arrested cell division in HU-supplemented media (Figure 6A, WT, open triangles). Rare escapees divided >1.5 h later than untreated controls. Furthermore, the addition of caffeine after the onset of S phase (150 min postrefeeding) suppressed cell cycle arrest in a significant fraction of HU-treated cells (Figure 6A, wild type, closed squares) and accelerated the timing of cytokinesis relative to HU treatment alone. Whereas the appearance of dividing cells during the 150- to 300-min interval after refeeding was reduced to 14% in cultures treated with HU alone, the addition of caffeine largely reversed the HU inhibition (HU + caffeine: 60%).
Figure 6.
Identification of an intra-S-phase checkpoint defect in Tif1p-deficient T. thermophila. (A) Wild-type (CU428) and tif1-1::neo/ TIF1 mutant (TXh48) strains were grown to saturation, starved, and then released into drug-free media (filled diamonds) or media containing 20 mM HU (open triangles and filled squares). Caffeine (1 mM) was added before the onset of macronuclear S phase (T = 150 min) (filled squares), and cell division was monitored by light microscopy. The cell division index corresponds to the percentage of cells with a cytokinetic furrow. (B) DAPI analysis of micro- and macronuclear division in mock and HU + caffeine-treated wild-type cells (same treatment as in A). (C) Rad51p Western blot analysis in synchronous wild-type and TIF1 mutant cultures, 5 h after refeeding with media containing HU, caffeine, or both. (D) Tif1p Western blot analysis of untreated and caffeine-treated wild-type strain (CU428; 1 mM caffeine for 4 h). (E) Rad51p Western blot analysis in synchronous wild-type cultures, 5 h after refeeding with media containing WM, HU, or both. (F) DAPI analysis documenting aberrant micro- and macronuclear division in tif1-1::neo mutant cells (TXh48) grown in normal culture media (no HU added). Arrow: cytokinetic furrow.
Caffeine-induced suppression of HU cell cycle arrest was similarly observed when both drugs were added at the time of refeeding, with HU + caffeine-treated cells divided later than mocked-treated and earlier that cells treated with HU alone (Supplemental Figure 2). The cumulative data indicate that macronuclear S-phase progression is mediated by a caffeine-sensitive factor, a hallmark of ATR. Abrogation of the intra-S-phase checkpoint allows macronuclear division and cytokinesis to occur in the absence of complete replication of macronuclear chromosomes.
The Caffeine-sensitive Intra-S-Phase Checkpoint Is Active in the Mitotic Micronucleus
Previous studies revealed that the mitotic micronucleus is dispensable to vegetative Tetrahymena (for review, see Karrer, 2000). However, the micronucleus contains all of the nuclear genes that are transmitted during conjugation. Because amicronucleate strains fail to undergo cell cycle arrest, it was unclear whether the intra-S-phase checkpoint pathway described above regulates micronuclear genome stability. To best address this question, we examined micro- and macronuclear division during the first cell cycle of synchronized cultures in the presence of HU + caffeine. Microscopic examination of dividing cells (Figure 6B) revealed defects in micronuclear division. The micronucleus normally divides and is partitioned to daughters before the onset of macronuclear division (Figure 6B, left panels and accompanying schematic). In contrast, micronuclear division was delayed in cells treated with HU + caffeine and frequently occurred concurrently with macronuclear division and cytokinesis (Figure 6B, right panel, bottom three micrographs). In these instances, segregating daughter micro- and macronuclei were typically connected by an extended segment of DAPI-staining material (Figure 6B, bottom micrograph). These results indicate that an ATR-like factor regulates the micronuclear cell cycle. Inhibition of this pathway eliminates the temporal regulation of micronuclear division and cytokinesis.
tif1-1::neo Mutants Fail to Activate the Intra-S-Phase Checkpoint Response
The previously documented defect in macronuclear S-phase progression, “cut” macronuclear division phenotype (Morrison et al., 2005), and hypersensitivity of tif1-1::neo/TIF1 mutants to MMS (Table 2) are consistent with a role for Tif1p in the S-phase DNA damage checkpoint response, similar to the caffeine-sensitive ATR-like target. To explore this possibility, we examined the response of tif1-1::neo mutants to HU and caffeine. tif1-1::neo mutants were slightly more sensitive to HU than wild type, and they displayed a modest, but reproducible dose-dependent lag in outgrowth after removal of the drug (Figure 5A). Although HU treatment alone slowed the outgrowth of tif1-1::neo mutants, this lag period was not extended when the presumed ATR response was blocked with caffeine (Figure 5B). By comparison, HU + caffeine generated a lag in outgrowth and slower doubling time in wild-type cells. Furthermore, macronuclear division defects were not exaggerated in HU + caffeine-treated tif1-1::neo mutants. Although the incidence of aberrant macronuclear division was ∼40% in HU + caffeine-treated wild-type cells, the severity of the cell division defect did not increase in frequency (∼20%) or magnitude in HU + caffeine-treated tif1-1::neo/TIF1 mutants relative to mock controls (our unpublished data).
Similar to HU + caffeine-treated wild-type cells, examination of synchronized tif1-1::neo/TIF1 cultures revealed simultaneous defects in micro- and macronuclear division, even in the absence of HU (Figure 6F). Because the ATR-dependent RAD51 response is muted in TIF1 mutants (Figure 6D) and Rad51p is targeted to both the micro- and macronucleus after genotoxic stress (Figure 4B), this result suggests that Tif1p is a component of the micronuclear S-phase checkpoint. Finally, similar to wild-type cells, tif1-1::neo mutant cell viability was not decreased after HU and/or caffeine exposure (Figure 5C), supporting the contention that the polyploid macronucleus is largely buffered from the effects of genotoxic stress.
The most compelling evidence for the involvement of Tif1p in the S-phase checkpoint response came from the analysis of cultures that were synchronized by starvation and then released into media containing HU, caffeine or HU + caffeine. As reported previously (Morrison et al., 2005), cytokinesis is delayed in tif1-1::neo mutants relative to wild-type controls (Figure 6A, filled diamonds). In contrast to wild-type cells, a significant fraction of TIF1p-deficient cells continued to divide in the presence of 20 mM HU (Figure 6A and Supplemental Figure S2, open triangles). These cells divided earlier than the rare wild-type cells that escape the HU block. Caffeine treatment alone had no effect on the incidence or timing of cell division in wild-type or mutant strains (Supplemental Figure S2). Furthermore, the addition of caffeine at the beginning of S phase (Figure 6A; T = 150 min, filled squares) or immediately upon refeeding of the mutant (Supplemental Figure S2; T = 0 min, filled squares) did not generate an increase in the percentage of dividing HU-treated cells. The failure to arrest in HU or exhibit and more pronounced defect in HU + caffeine argues that Tif1p is required to activate the intra-S-phase checkpoint response.
The final marker that we examined for S-phase checkpoint activation was Rad51p, which participates in recombination-mediated repair at stalled forks or replication-induced DSBs (for review, see Lambert and Carr, 2005). As noted, the basal levels of RAD51 mRNA and protein are elevated in tif1-1::neo mutants (Figure 3, A and B). To examine the contribution of Tif1p and the ATR-like checkpoint to the Rad51p response, synchronized cells were released into media containing HU and/or caffeine. HU-treated wild-type cells exhibited a dramatic increase in Rad51 protein levels (Figure 6C). The majority of this response was eliminated by the addition of caffeine, consistent with inactivation of the checkpoint target. In contrast, HU generated a very modest increase in the Rad51p level in the tif1-1::neo mutant. Caffeine had minimal effect on the Rad51p level, indicating a central role for Tif1p in the DNA damage checkpoint response. The absence of homology between TIF1p and ATR orthologues argues that TIF1p is not the caffeine-sensitive target. In support of this contention, caffeine did not alter Tif1 protein levels or the relative abundance of the two Tif1 protein isoforms (Figure 6D).
To verify that caffeine was not targeting a previously documented wortmannin-sensitive non-ATM/ATR PI3-kinases (Smith et al., 2004b; Yakisich and Kapler, 2004), we tested whether WM could suppress HU-induced activation of the intra-S-phase checkpoint response by monitoring the production of Rad51p in wild-type cells treated with HU, WM, or both, under the same conditions used for Figure 6C. WM at 250 nM failed to repress the induction of Rad51p (Figure 6E). This concentration exacerbates nuclear division defects in cells expressing paclitaxel-hypersensitive β-tubulin allele btu1-1 (Smith et al., 2004b), and it inhibits programmed nuclear death during Tetrahymena development (Yakisich and Kapler, 2004). Conversely, 1 mM caffeine did not block acidification of the old parental macronucleus in mating progeny, as is observed in WM-treated cells (our unpublished data). We conclude that the caffeine-sensitive intra-S-phase checkpoint is activated by an ATR-like protein kinase. Consistent with this model, we have identified a strong candidate ATR orthologue in the T. thermophila genome database as well as other conserved components of this checkpoint pathway.
DISCUSSION
DNA damage checkpoints facilitate the repair of potentially catastrophic lesions before DNA replication. ATR is an S-phase–specific sensor/transducer kinase that activates downstream targets that stabilize stalled replication forks and inhibit initiation from late firing origins (for review, see Abraham, 2001). Although this pathway is conserved from yeast to human, it is unclear whether the demands on this pathway are diminished in polyploid organisms or tissues. Members of the ancient eukaryotic branch, the Ciliophora, are unusual in that they contain two nuclei within a single cytoplasm, the diploid micronucleus and polyploid macronucleus. In this study we provide evidence that a common S-phase checkpoint pathway functions in both types of nuclei. One component of the T. thermophila checkpoint seems to be functionally analogous to mammalian ATR, because its ability to induce S-phase arrest is inhibited by caffeine. The second component, Tif1p, has no obvious homologue in yeast and higher eukaryotes; however, we have identified a candidate orthologue in a distantly related ciliate, Paramecium tetraurelia (e value 6−13, 27% sequence identity, 56% sequence similarity; Kapler and Sperling, unpublished data). Sequence conservation outside of ciliates is limited principally to the carboxy terminus of Tif1p, which resembles the oligomerization domain of whirly transcription factors in plants, that like Tif1p, assemble into homotetramers and bind single-strand DNA in a sequence-specific manner (Desveaux et al., 2000, 2002). It is plausible that Tif1p functions in the intra-S-phase checkpoint pathway are broadly conserved.
Tif1p and the Cell Cycle
TIf1p undergoes dynamic relocalization during normal cell cycles (Figure 4, C and D). It is exclusively in the cytoplasm of G1 phase cells. Intense localization to the macronuclear periphery occurs before and during early macronuclear S phase. This distribution suggests that Tif1p may initially be targeted to rDNA-containing nucleoli. Tif1p is found throughout the macronucleus later in S phase, and it relocalizes to the periphery or is excluded from the macronucleus before cytokinesis. Tif1p is similarly targeted to the micronucleus during S phase, and it is transiently retained in postmitotic micronuclei. It is unclear how Tif1p is differentially localized to distinct nuclei that inhabit the same cytoplasm. By analogy, the subcellular localization of the TIF4 Orc2p cross-reactive subunit Tt-p69 is similarly regulated (Mohammad et al., 2003). We speculate that these proteins are imported into micro- and macronuclei by nucleus-specific “licensing factors.” Whatever the mechanism, we show that Tif1p is most prominently associated with replicating nuclei, suggesting that its primary role occurs during S phase.
One well-defined role for Tif1p is to regulate the timing of rDNA replication initiation (Morrison et al., 2005). Several lines of investigation illustrate a more global role for Tif1p in the macronucleus, because it is required for normal macronuclear S-phase progression, the coordinate regulation of macronuclear division and cytokinesis (Morrison et al., 2005), and functions in the checkpoint response to intrinsic or extrinsic inhibitors of DNA replication (this work). tif1-1::neo mutants contain elevated basal levels of Rad51p (Figure 3) and are hypersensitive to external sources of genotoxic stress (Table 3 and Figures 5 and 6), arguing that tif1-1::neo mutants accumulate DNA damage in unperturbed cell cycles. Moreover, because MMS and HU destabilize elongating replication forks and Tif1p localizes to the micro- and macronucleus during S phase, we speculate that the role of Tif1p in checkpoint activation occurs at the replication fork. By analogy, yeast and metazoan checkpoint sensor and effector proteins, such as Xenopus laevis ATM and ATR (Shechter et al., 2004), and S. cerevisiae Mrc1 (Szyjka et al., 2005), are targeted to elongating replication forks during unperturbed cycling cells.
Macronuclear genome stability is influenced by many factors, some of which efficiently compensate for the aberrant amitotic divisions associated with the loss of TIF1 or natural fluctuations in chromosome transmission. However, the conventional mitotic micronucleus lacks these compensatory mechanisms and is more dependent on Tif1p. Nine of 10 chromosomal markers were absent from the micronucleus in unselected TIF1/tif1-1::neo mutant cell populations (Figure 2). Although the micronuclear chromosome loss leads to germline sterility, TIF1/tif1-1::neo mutants fail to undergo cell cycle arrest. One possible reason is that Tif1p is required to activate the intra-S-phase checkpoint (Figure 6).
Induction of the Intra-S-Phase Checkpoint by Genotoxic Stress
Our studies indicate that wild-type Tetrahymena elicits a classic intra-S-phase checkpoint response. Cell cycle arrest is induced by HU and suppressed by further addition of caffeine, a known inhibitor of ATM and ATR kinases (Figure 6A and Supplemental Figure S2; see model in Figure 7). Cells that escape HU arrest undergo aberrant micro- and macronuclear division (Figures 5D and 6B), indicating that both nuclei are regulated by the caffeine-sensitive factor. Tif1p functions in S-phase checkpoint activation as well. tif1-1::neo mutants are hypersensitive to MMS and HU (Table 3 and Figure 5A) and fail to arrest in the presence of HU (Figure 6A). Aberrant macronuclear division occurs frequently, producing a cut phenotype, in which the macronucleus is bissected by the cleavage furrow (Morrison et al., 2005). Aberrant micronuclear division is also observed in the absence of exogenous genotoxic stress (Figure 6F). Micronuclear genome instability is rampant as no chromosome is spared (Figure 2).
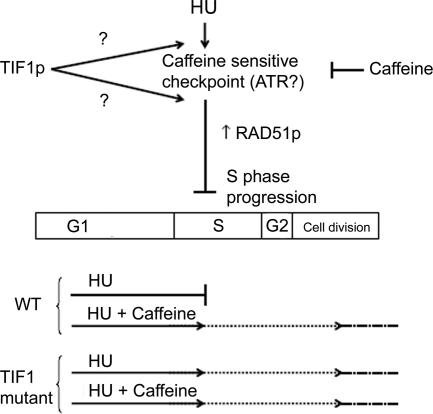
Figure 7.
Proposed models for the epistatic relationship between TIF1p and the presumed ATR (caffeine-sensitive) intra-S-phase checkpoint protein (see text for details).
Several lines of evidence argue that Tif1p and the presumed ATR kinase function in the same epistatic pathway (Figure 7). First, HU blocks cell cycle progression in wild-type cells, but it fails to induce cell cycle arrest in a significant fraction of tif1-1::neo mutant cells. Caffeine suppresses HU-induced cell cycle arrest in wild cells, but it fails to do so in the mutant, suggesting a role for TIF1 in the ATR response (Figures 6A and 7, bottom schematic). Second, HU-treated tif1-1::neo cells and HU + caffeine-treated wild-type cells divide with similar kinetics (Figure 6A). Cell division is associated with aberrant micro- and macronuclear division in both situations (Figure 6, B and F). Third, the induced expression of RAD51, a marker for checkpoint activation, is largely suppressed in tif1-1::neo mutants (Figure 6C). HU-treatment leads to a substantive increase in RAD51 mRNA and protein in wild-type cells, the majority of which is suppressed by caffeine (Figure 7, top schematic). In contrast, TIF1 mutants exhibit a very modest increase in Rad51 protein, and this induction is refractory to the addition of caffeine.
Rad51p interacts with the ATR substrate Rad52p in a recombination pathway that repairs DSBs and lesions ahead of stalled replication forks (for review, see Lambert and Carr, 2005). Although Rad51p has not been commonly used to assess checkpoint activation, we show that the up-regulation of RAD51 mRNA and protein is induced by HU and suppressed by further addition of caffeine. Consequently, robust expression of RAD51 is part of the Tetrahymena intra-S-phase checkpoint response. RAD51 induction served as a reliable marker that illustrated the contribution of TIF1 to checkpoint activation.
It remains to be determined whether Tif1p acts upstream or downstream of ATR in the intra-S-phase checkpoint pathway (Figure 7, TIF1 alternate arrows). Bioinformatic analyses suggest the presence of ATR, CHK1, and CHK2 orthologues in the T. thermophila genome. We predict that Tif1p is not a direct substrate of ATR, because it lacks the clustered SQ/TQ motifs present in most ATM/ATR substrates (Traven and Heierhorst, 2005). Consistent with this prediction, caffeine does not alter the abundance or stoichiometry of Tif1p isoforms (Figure 6E). Because Tif1p does not display homology to other downstream mediator or adapter proteins, we propose that it serves a novel role in the intra-S phase checkpoint response. The propensity of Tif1p for single-stranded DNA raises the possibility that it might act in concert with persistent chromatin-bound replication protein A to recruit ATR to sites of DNA damage (Figure 7, TIF1, top arrow) (Zou and Elledge, 2003). Furthermore, because Tif1p represses early initiation from the rDNA origin during normal cell cycles (Morrison et al., 2005), this repressive function might be extended to other origins during normal cell cycles and/or in response to genotoxic stress.
Supplementary Material
ACKNOWLEDGMENTS
We thank Linda Sperling for BLAST analysis of the P. tetraurelia genome database. We thank Dorothy Shippen and Aaron Smith for insightful comments on the manuscript. This work was supported by National Institutes of Health Grant GM-53572 and National Science Foundation Grant MCB-0132675 (to G.M.K.).
Abbreviations used:
- ATM
Ataxia Telangectasia Mutated;
- ATR
Ataxia Telangectasia and Rad3-related;
- BrdU
5′-bromo-2′-deoxyuridine;
- DAPI
4′,6′-diamidino-2-phenylindole;
- DSB
double-strand break;
- HU
hydroxyurea;
- MMS
methylmethanesulphonate;
- WM
wortmannin.
Footnotes
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-05-0469) on September 27, 2006.
REFERENCES
- Abraham R. T. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- Allen S. L. Genomic exclusion–a rapid means for inducing homozygous diploid lines in Tetrahymena pyriformis syngen I. Science. 1967;155:575–577. doi: 10.1126/science.155.3762.575. [DOI] [PubMed] [Google Scholar]
- Bodenbender J., Prohaska A., Jauker F., Hipke H., Cleffmann G. DNA elimination and its relation to quantities in the macronucleus of Tetrahymena. Dev. Genet. 1992;13:103–110. doi: 10.1002/dvg.1020130203. [DOI] [PubMed] [Google Scholar]
- Brown J. M., Marsala C., Kosoy R., Gaertig J. Kinesin-II is preferentially targeted to assembling cilia and is required for ciliogenesis and normal cytokinesis in Tetrahymena. Mol. Biol. Cell. 1999;10:3081–3096. doi: 10.1091/mbc.10.10.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C., Romero D. P. Identification and characterization of the RAD51 gene from the ciliate Tetrahymena thermophila. Nucleic Acids Res. 1998;26:3165–3172. doi: 10.1093/nar/26.13.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy-Hanley D., Bowen J., Lee J. H., Cole E., VerPlank L. A., Gaertig J., Gorovsky M. A., Bruns P. J. Germline and somatic transformation of mating Tetrahymena thermophila by particle bombardment. Genetics. 1997;146:135–147. doi: 10.1093/genetics/146.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M., Bellaoui M., Boone C., Brown G. W. A genome-wide screen for methyl methanesulfonate-sensitive mutants reveals genes required for S phase progression in the presence of DNA damage. Proc. Natl. Acad. Sci. USA. 2002;99:16934–16939. doi: 10.1073/pnas.262669299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleffmann G. Regulation of the amount of DNA in the macronucleus of Tetrahymena. Exp. Cell Res. 1968;50:193–207. doi: 10.1016/0014-4827(68)90407-2. [DOI] [PubMed] [Google Scholar]
- Desveaux D., Allard J., Brisson N., Sygusch J. Crystallization and preliminary X-ray crystallographic analysis of p24, a component of the potato nuclear factor PBF-2. Acta Crystallogr. D Biol. Crystallogr. 2002;58:296–298. doi: 10.1107/s0907444901018741. [DOI] [PubMed] [Google Scholar]
- Desveaux D., Despres C., Joyeux A., Subramaniam R., Brisson N. PBF-2 is a novel single-stranded DNA binding factor implicated in PR-10a gene activation in potato. Plant Cell. 2000;12:1477–1489. doi: 10.1105/tpc.12.8.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs D. L., Shaiu W. L., Benbow R. M. Modular sequence elements associated with origin regions in eukaryotic chromosomal DNA. Nucleic Acids Res. 1994;22:2479–2489. doi: 10.1093/nar/22.13.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerder F. P., Debault L. E. Cytofluorimetric analysis of nuclear DNA during meiosis, fertilization and macronuclear development in the ciliate Tetrahymena pyriformis, syngen 1. J. Cell Sci. 1975;17:471–493. doi: 10.1242/jcs.17.3.471. [DOI] [PubMed] [Google Scholar]
- Foss E. Tof1p regulates DNA damage responses during S phase in Saccharomyces cerevisiae. Genetics. 2001;157:567–577. doi: 10.1093/genetics/157.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. S., Green C. M., Lowndes N. F. Budding yeast Rad9 is an ATP-dependent Rad53 activating machine. Mol. Cell. 2001;8:129–136. doi: 10.1016/s1097-2765(01)00267-2. [DOI] [PubMed] [Google Scholar]
- Hamilton E. P., Bruns P. J., Lin C., Merriam V., Orias E., Vong L., Cassidy-Hanley D. Genome-wide characterization of Tetrahymena thermophila chromosome breakage sites. I. Cloning and identification of functional sites. Genetics. 2005;170:1611–1621. doi: 10.1534/genetics.104.031401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer K. M. Tetrahymena genetics: two nuclei are better than one. Methods Cell Biol. 2000;62:127–186. doi: 10.1016/s0091-679x(08)61529-0. [DOI] [PubMed] [Google Scholar]
- Lambert S., Carr A. M. Checkpoint responses to replication fork barriers. Biochimie. 2005;87:591–602. doi: 10.1016/j.biochi.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Liu J. S., Kuo S. R., Melendy T. Comparison of checkpoint responses triggered by DNA polymerase inhibition versus DNA damaging agents. Mutat. Res. 2003;532:215–226. doi: 10.1016/j.mrfmmm.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Loidl J., Scherthan H. Organization and pairing of meiotic chromosomes in the ciliate Tetrahymena thermophila. J. Cell Sci. 2004;117:5791–5801. doi: 10.1242/jcs.01504. [DOI] [PubMed] [Google Scholar]
- Lupardus P. J., Byun T., Yee M. C., Hekmat-Nejad M., Cimprich K. A. A requirement for replication in activation of the ATR-dependent DNA damage checkpoint. Genes Dev. 2002;16:2327–2332. doi: 10.1101/gad.1013502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad M., Saha S., Kapler G. M. Three different proteins recognize a multifunctional determinant that controls replication initiation, fork arrest and transcription in Tetrahymena. Nucleic Acids Res. 2000;28:843–851. doi: 10.1093/nar/28.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad M., York R. D., Hommel J., Kapler G. M. Characterization of a novel origin recognition complex-like complex: implications for DNA recognition, cell cycle control, and locus-specific gene amplification. Mol. Cell. Biol. 2003;23:5005–5017. doi: 10.1128/MCB.23.14.5005-5017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. L., Yakisich J. S., Cassidy-Hanley D., Kapler G. M. TIF1 represses rDNA replication initiation, but promotes normal S phase progression and chromosome transmission in Tetrahymena. Mol. Biol. Cell. 2005;16:2624–2635. doi: 10.1091/mbc.E05-02-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orias E., Bruns P. J. Induction and isolation of mutants in Tetrahymena. Methods Cell Biol. 1976;13:247–282. [PubMed] [Google Scholar]
- Saha S., Kapler G. M. Allele-specific protein-DNA interactions between the single-stranded DNA-binding protein, ssA-TIBF, and DNA replication determinants in Tetrahymena. J. Mol. Biol. 2000;295:423–439. doi: 10.1006/jmbi.1999.3365. [DOI] [PubMed] [Google Scholar]
- Saha S., Nicholson A., Kapler G. M. Cloning and biochemical analysis of the Tetrahymena origin binding protein TIF1: competitive DNA binding in vitro and in vivo to critical rDNA replication determinants. J. Biol. Chem. 2001;276:45417–45426. doi: 10.1074/jbc.M106162200. [DOI] [PubMed] [Google Scholar]
- Shechter D., Costanzo V., Gautier J. ATR and ATM regulate the timing of DNA replication origin firing. Nat. Cell. Biol. 2004;6:648–655. doi: 10.1038/ncb1145. [DOI] [PubMed] [Google Scholar]
- Smith J. J., Cole E. S., Romero D. P. Transcriptional control of RAD51 expression in the ciliate Tetrahymena thermophila. Nucleic Acids Res. 2004a;32:4313–4321. doi: 10.1093/nar/gkh771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. J., Yakisich J. S., Kapler G. M., Cole E. S., Romero D. P. A β-tubulin mutation selectively uncouples nuclear division and cytokinesis in Tetrahymena thermophila. Eukaryotic Cell. 2004b;3:1217–1226. doi: 10.1128/EC.3.5.1217-1226.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyjka S. J., Viggiani C. J., Aparicio O. M. Mrc1 is required for normal progression of replication forks throughout chromatin in S. cerevisiae. Mol. Cell. 2005;19:691–697. doi: 10.1016/j.molcel.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Russell P. Mrc1 channels the DNA replication arrest signal to checkpoint kinase Cds1. Nat. Cell Biol. 2001;3:966–972. doi: 10.1038/ncb1101-966. [DOI] [PubMed] [Google Scholar]
- Traven A., Heierhorst J. SQ/TQ cluster domains: concentrated ATM/ATR kinase phosphorylation site regions in DNA-damage-response proteins. Bioessays. 2005;27:397–407. doi: 10.1002/bies.20204. [DOI] [PubMed] [Google Scholar]
- Yakisich J. S., Kapler G. M. The effect of phosphoinositide 3-kinase inhibitors on programmed nuclear degradation in Tetrahymena and fate of surviving nuclei. Cell. Death. Differ. 2004;11:1146–1149. doi: 10.1038/sj.cdd.4401473. [DOI] [PubMed] [Google Scholar]
- Yakisich J. S., Kapler G. M. Deletion of the Tetrahymena thermophila rDNA replication fork barrier region disrupts macronuclear rDNA excision and creates a fragile site in the micronuclear genome. Nucleic Acids Res. 2006;34:620–634. doi: 10.1093/nar/gkj466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Elledge S. J. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.