Abstract
AP180, one of many assembly proteins and adaptors for clathrin, stimulates the assembly of clathrin lattices on membranes, but its unique contribution to clathrin function remains elusive. In this study we identified the Dictyostelium discoideum ortholog of the adaptor protein AP180 and characterized a mutant strain carrying a deletion in this gene. Imaging GFP-labeled AP180 showed that it localized to punctae at the plasma membrane, the contractile vacuole, and the cytoplasm and associated with clathrin. AP180 null cells did not display defects characteristic of clathrin mutants and continued to localize clathrin punctae on their plasma membrane and within the cytoplasm. However, like clathrin mutants, AP180 mutants, were osmosensitive. When immersed in water, AP180 null cells formed abnormally large contractile vacuoles. Furthermore, the cycle of expansion and contraction for contractile vacuoles in AP80 null cells was twice as long as that of wild-type cells. Taken together, our results suggest that AP180 plays a unique role as a regulator of contractile vacuole morphology and activity in Dictyostelium.
INTRODUCTION
Endocytosis via clathrin-coated vesicles is an essential process for the regulated uptake of nutrients and the trafficking of membrane receptors. Although clathrin is the principal structural player in building a coated vesicle, numerous adaptor and accessory proteins fine tune and regulate endocytosis (Lafer, 2002). Together they assemble clathrin triskelia onto membranes to construct a clathrin-coated pit (ter Haar et al., 2000; Brodsky et al., 2001). Four heterotetrameric adaptor proteins have been identified; each is thought to function at a different cellular location (Kirchhausen, 1999). At the plasma membrane the heterotetrameric protein AP-2 recruits clathrin and initiates the assembly of clathrin triskelia into lattices (Gallusser and Kirchhausen, 1993; Owen et al., 2000). Recently, monomeric assembly proteins have been identified at the plasma membrane that could also regulate clathrin traffic. The monomeric assembly protein, AP180, was first purified from coated vesicles of bovine brain (Ahle and Ungewickell, 1986), although a nonneuronal homologue of AP180, CALM, was identified more recently (Dreyling et al., 1996; Tebar et al., 1999). AP180 binds directly to clathrin and promotes assembly of clathrin triskelia into cages of uniform size (Ahle and Ungewickell, 1986; Prasad and Lippoldt, 1988; Ye and Lafer, 1995b).
Analysis of mutants of the AP180 orthologues in Drosophila and Caenorhabditis elegans shows that AP180 regulates synaptic vesicle size as well as the sorting of synaptic proteins such as synaptobrevin (Zhang et al., 1998; Nonet et al., 1999; Bao et al., 2005). In mammalian cells, reduction of AP180 results in irregular clathrin lattices, demonstrating an important role for AP180 in the assembly of clathrin into geometrically precise coated vesicles (Meyerholz et al., 2005). Furthermore, recent studies show that AP180 is involved in the internalization of some receptors like the EGF receptor, but not of others such as the transferrin receptor (Huang et al., 2004). In yeast, the role of AP180 in clathrin-mediated endocytosis is less clear. Deletion of both genes encoding the AP180 orthologues (yAP180a and yAP180b) show no defects in any clathrin-mediated processes (Huang et al., 1999).
In vitro experiments show that AP180 binds clathrin and phosphoinositides such as PIP2 and promotes clathrin assembly on lipid monolayers (Ford et al., 2001). Binding of AP180 to PIP2 is mediated by an NH2-terminal homology domain called the ANTH (AP180 N-terminal homology) domain that is conserved in all members of the AP180 family (Norris et al., 1995; Ye et al., 1995; Hao et al., 1997; Ford et al., 2001; Mao et al., 2001). Other endocytic proteins such as epsin have at their amino terminus a structurally similar ENTH domain (epsin N-terminal homology). Interestingly, binding of ENTH-domain–containing proteins such as epsin, to PIP2 induces curvature of a lipid monolayer, whereas AP180 fails to do so (Ford et al., 2002; Stahelin et al., 2003). This points to important mechanistic differences between various protein components of the endocytic machinery.
Here we examined the intracellular role of AP180 in the social amoeba, Dictyostelium discoideum. Although Dictyostelium AP180 colocalized with clathrin on the plasma membrane of wild-type cells, AP180 null cells displayed a normal distribution of clathrin on the plasma membrane. However AP180 knockouts were deficient in osmoregulation mediated by the contractile vacuole, a process where clathrin is also a key regulator. Collectively our results suggest that AP180 is a clathrin assembly protein with unique contributions to the regulation of contractile vacuole size.
MATERIALS AND METHODS
Strains and Cell Culture
Dictyostelium discoideum wild-type Ax2 cells were grown axenically in HL-5 medium (Damer and O'Halloran, 2000) supplemented with 0.6% penicillin-streptomycin (GIBCO BRL, Gaithersburg, MD) at 20°C on Petri dishes. Clathrin heavy-chain mutants were derived from Ax2 wild-type cells, and clathrin light-chain mutants were derived from the wild-type axenic strain NC4A2 (Niswonger and O'Halloran, 1997a; Wang et al., 2003). Both mutant strains were grown in HL-5 media supplemented with 5 μg/ml blasticidin (Calbiochem, EMD Biosciences, La Jolla, CA).
Cloning of clmA and GFP-AP180 Construct
The clmA gene encoding the AP180 gene product was identified from a Dictyostelium genome database (www.dictybase.org) using a BLAST search (tBLASTn) with the first 300 amino acids of the mammalian neuronal AP180. Predicted protein domains at GeneDB identified an ANTH domain in the first 300 amino acids. Alignment and analysis of the predicted Dictyostelium AP180 protein sequence with protein sequences from other members of the AP180 family were performed using the Megalign program (DNAStar, Madison, WI). The percent identity between the Dictyostelium AP180 and those of other species was determined using the ClustalV parameters. A cDNA for the clmA gene was amplified using the PCR with primers selected from the genomic sequence (DDB0218102), 5′GGATCCATGTCGACACCAT GGGGAAAAGC3′ and 5′CCCGGGCTCGAGTATTTAAAAGTAAATATTTTGAAC CTTTTGTTGTTG3′. The 2.1-kb amplified product was subcloned into the pTX-GFP expression vector (Levi et al., 2000) at the BamHI and XhoI sites. This plasmid, pTX-GFP-AP180, was then introduced into cells by electroporation and transformants were selected in HL-5 medium supplemented with 10 μg/ml G418 (geniticin; GIBCO BRL, Grand Island, NY).
Protein Expression and Generation of AP180 Polyclonal Antibody
The amplified AP180 cDNA was subcloned into the glutathione-S-transferase bacterial expression vector pGEX-2T (Smith and Johnson, 1988) using the BamHI and SmaI sites. GST-AP180 was transformed into Escherichia coli BL-21 cells, and the expressed protein was purified from bacteria lysates as previously described (O'Halloran and Anderson, 1992a). The purified protein was used to raise rabbit polyclonal antisera against AP180 (Cocalico Biologicals, Reamstown, PA).
Disruption of clmA by Gene Replacement
A 1.3-kb fragment from the 5′coding sequence of clmA was cloned into the pSP72-Bsr vector (Wang et al., 2002), a derivative of pBluescriptII that encodes a 1.4-kb gene for blasticidin resistance, using the BamHI and XbaI sites. Similarly, a 1.6-kb fragment from the 3′ coding sequence of clmA was cloned into the pSP72-Bsr vector using the HindIII and XhoI sites. The two clmA fragments flanking the blasticidin (Bsr)-resistant gene cassette had 20 nucleotides missing from the clmA coding sequence, which were replaced by the Bsr gene. The resulting vector, pSP72-Bsr-AP180 was linearized with BamHI and XhoI and transformed into wild-type Ax2 cells via electroporation. Transformed cells were diluted in HL-5 media supplemented with 5 μg/ml blasticidin and plated in 96-well plates. Resulting clones were screened for the absence of clmA gene by PCR and verified for the absence of the AP180 protein by Western blot analysis.
Western Blot Analysis, Endocytosis Assay, and Differential Fractionation
Samples for Western blotting were prepared by resuspending cells in hot sample buffer and running 1 × 106 cells/lane on a 10% SDS polyacrylamide gel. The gel was transferred onto a nitrocellulose membrane (0.2 μm, Bio-Rad, Hercules, CA) and probed with a 1:2000 dilution of our rabbit anti-AP180 polyclonal antibody followed by a goat anti-rabbit Ig-HRP. Signal was detected using an ECL kit (Pierce Biotechnology, Rockford, IL).
For the fluid-phase uptake assay, 2 mg/ml FITC-Dextran (mw 70 kDa, Sigma-Aldrich, St. Louis, MO) was added to 3 × 106 cells/ml growing in HL-5 suspension cultures. Sodium azide (0.02%) was added to a control flask. To stop uptake of FITC-Dextran, cells were chilled on ice. Samples were taken at 0-, 15-, 30-, 60-, 90-, and 120-min time points and centrifuged at 1100 rpm at 4°C for 5 min. Cells were washed twice and resuspended in HL-5 containing 0.02% sodium azide and kept on ice until all samples were collected. All samples were centrifuged at 1100 rpm at 4°C for 5 min, and the pellet was resuspended in cold Na2HPO4 buffer. The cells were lysed with 20% Triton X-100, and fluorescence uptake was analyzed immediately using a Bio-Rad VersaFluor fluorometer. A sample of the lysate was taken after the addition of Triton X-100 and assessed for protein concentration using Bio-Rad protein Assay (Bio-Rad, Hercules, CA).
Differential centrifugation experiments were performed according to Wang et al. (2003). Briefly, cells were collected and washed in isolation buffer [(10 mM MES, pH 6.5, 50 mM KC2H3O2, pH 6.5, 0.5 mM MgCl2, 1 mM EGTA, 1 mM DTT, and 0.02% NaN3) with 1% protease inhibitors (Fungal Protease Inhibitor cocktail, Sigma-Aldrich, St. Louis, MO) and then lysed through a 0.5 μim polycarbonate membrane (GE Osmonics, Trevose, PA) fitted in a Gelman Luer-Lock-style filter (Gelman Sciences, Ann Arbor, MI). The cell lysates were then centrifuged at 3000 × g for 10 min at 4°C, and the resulting post-nuclear supernatant (PNS) was subjected to 100,000 × g ultracentrifugation for 60 min at 4°C to produce a high-speed supernatant (HSS) and a high-speed pellet (HSP; Wang et al., 2003).
Fluorescence Microscopy
Cells expressing GFP-AP180 (2 × 106 cells/ml) were allowed to attach on coverslips for 15 min at room temperature and washed briefly with PDF buffer (2 mM KCl, 1.1 mM K2HPO4, 1.32 mM KH2PO4, 0.1 mM CaCl2, 0.25 mM MgSO4, pH 6.7) and then overlaid with thin layer of 2% agar NA (Amersham Biosciences, Uppsala, Sweden; Fukui et al., 1987). For imaging the contractile vacuole, the agar layers were incubated in water. Cells were then fixed in 1% formaldehyde in methanol for 5 min at −20°C followed by two washes with phosphate-buffered saline (PBS), rinsed briefly with distilled water, and mounted on microscope slides with mounting media (MOWIOL, Calbiochem, EMD Biosciences, La Jolla, CA). The slides were allowed to dry overnight in the dark and analyzed the following day. For imaging clathrin on the contractile vacuole, we filmed live wild-type and AP180 null cells expressing clathrin light chain tagged with GFP in water. For colocalization studies, clathrin light-chain antibody (Wang et al., 2003) was prepared for immunofluorescence microscopy by preabsorption as follows. Clathrin light-chain mutant cells were grown to a density of 2 × 108 cells/ml and centrifuged at 1500 rpm for 5 min, and the cell pellet was resuspended in 2% formaldehyde in PBS. The cells suspension was incubated for 5 min at room temperature and then centrifuged at 2000 rpm for 5 min. The cell pellet was resuspended in 1% formaldehyde in methanol, incubated at −20°C for 5 min and then centrifuged at 2000 rpm for 5 min. The cell pellet was resuspended in 1.5 ml of 3% bovine serum albumin (Fisher Scientific, Fair Lawn, NJ) in PBS with 0.02% NaN3. Anti-clathrin light-chain serum was added to prepared cells at a 1:5 dilution and incubated at 4°C overnight. The antibody-cell suspension was centrifuged for 10 min at 2000 rpm, and the supernatant was added to the pelleted clathrin light-chain mutant cells and incubated at 4°C overnight for another round of preabsorption. This was repeated at least five times to ensure efficient absorption of nonspecific antibodies from the clathrin light-chain antibody serum. Preabsorbed clathrin light-chain antibody was added to the fixed cells and incubated for 1 h at 37°C in the dark. Cells were washed four times with PBS and incubated with Texas Red–conjugated goat anti-rabbit IgG antibody (30 μg/ml; Molecular Probes, Eugene, OR) for 1 h at 37°C in the dark. Cells were washed four times with PBS, rinsed briefly in water, and mounted on microscope slides as described above. To stain the actin cytoskeleton, wild-type Ax2 and AP180 null cells were allowed to attach to coverslips for 10 min at room temperature and then were fixed in 3.7% formaldehyde in PBS for 20 min at room temperature followed by permeabilization with 0.2% Triton X-100 in PBS for 5 min at room temperature. The cells were then incubated with Texas Red phalloidin (1 U/ml; Molecular Probes) in PBS for 20 min at room temperature. The cells were then washed twice with PBS and mounted on slides as described above.
Microscopy and Confocal Imaging
Cells were imaged using differential interference contrast microscopy and fluorescence microscopy on a Nikon Eclipse TE 200 microscope (Dallas, TX). GFP and Texas Red filters were used. Images were acquired on a Photometrics cooled CCD camera (Tucson, AZ), processed using Metamorph 5.0 software (Universal Imaging, West Chester, PA), and adjusted for better contrast using Photoshop 7.0 (Adobe Photosystems, San Jose, CA). When visualizing the contractile vacuole, exposure times for differential interference contrast (DIC) and GFP fluorescence were kept to a minimum because the activity of the contractile vacuole is sensitive to light. Fluorescent images from Nikon compiled into QuickTime movies (Apple, Cupertino, CA) were taken at 3-s intervals and played at 6 frames/s. Images of fruiting bodies during development were captured on a Zeiss SemiSR microscope (Thornwood, NY) with a 2.0× objective and using NIH image software. Confocal Z-series images (0.4 μm sections) of Ax2 cells expressing GFP-AP180 were obtained from Leica scanning laser confocal microscope (TCS-SP2; Deerfield, IL) and processed using Leica software.
For the quantification of AP180 association with the contractile vacuole, wild-type cells and clathrin light-chain mutant cells expressing GFP-AP180 were analyzed. Cells were fixed and flattened as described above and imaged under DIC and fluorescence optics. Contractile vacuoles were identified using the DIC images, and the presence of GFP-AP180 outlining most of the contractile vacuole was scored using fluorescent images.
RESULTS
Dictyostelium AP180 Contains Membrane-binding and Clathrin Adaptor Signature Motifs
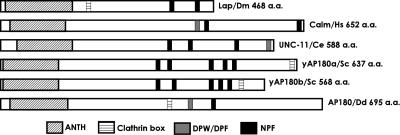
Members of the AP180 family have at their amino-terminus a signature domain called the ANTH domain. This domain has been extensively studied and established as a PtdIns(4,5)P2 binding domain (Norris et al., 1995; Ye et al., 1995; Hao et al., 1997). Using the amino acid sequence for the ANTH domain of the mammalian neuronal AP180 protein (Ahle and Ungewickell, 1986), we searched the database of the Dictyostelium genome and found a single gene that showed high homology to this sequence. Analysis of the retrieved sequence showed a conserved lysine-rich motif 28KATx6PKxKH at the amino-terminus, which agreed with the ANTH domain consensus sequence (K/G)A(T/I)x6(P/L/V)KxK(H/Y) (Kay et al., 1999; Ford et al., 2001). The first 300 amino acids at the amino-terminus shared significant homology with the ANTH domain of the AP180 orthologues from D. melanogaster (Lap; 32.3%; Zhang et al., 1998); C. elegans (UNC-11; 28.7%; Nonet et al., 1999); H. sapiens (CALM; 32.7%; Tebar et al., 1999); the neuronal isoform from Bos taurus (AP180; 34%; Ahle and Ungewickell, 1986; Kohtz and Puszkin, 1988); and Saccharomyces cerevisiae (YAP180; 20%; Wendland and Emr, 1998). Analysis of the amino acid sequence carboxyl-terminal to the ANTH domain revealed a clathrin box, LINFD (amino acids 360–364; Morgan et al., 2000), closely followed by an AP-2 binding motif, DPF (amino acids 407–409; Hao et al., 1999; Owen et al., 1999, 2000; Traub et al., 1999) and an NPF motif (amino acids 459–461), which confers binding to EH-domain–containing proteins such as Eps15 (Iannolo et al., 1997; de Beer et al., 1998; Figure 1). Because of the amino acid sequence homology and the presence of all signature motifs common to AP180 family members, including the nonneuronal mammalian AP180 ortholog, CALM, we named this gene clmA. ClmA encoded a protein predicted to contain 695 amino acids with a molecular mass of 79.7 kDa. A BLAST search of the Dictyostelium genome database located clmA to chromosome 3.
Figure 1.
Dictyostelium AP180 belongs to the AP180 family. All members of the AP180 family have an amino- terminal ANTH signature domain that confers binding to phospholipids at the plasma membrane. Most family members also contain the amino acid sequence DLL or L(L/I)(D/E/N)(L/F)(D/E), a short motif that binds to clathrin; a DPW/DPF or FXDXF motif that confers binding to the major adaptor protein AP-2, and NPF/W, a motif that binds to EH domain-containing proteins. Dm, D. melanogaster; Hs, H. sapiens; Ce, C. elegans; Sc, S. cerevisiae; Dd, Dictyostelium discoideum.
AP180 Colocalizes with Clathrin on the Plasma Membrane
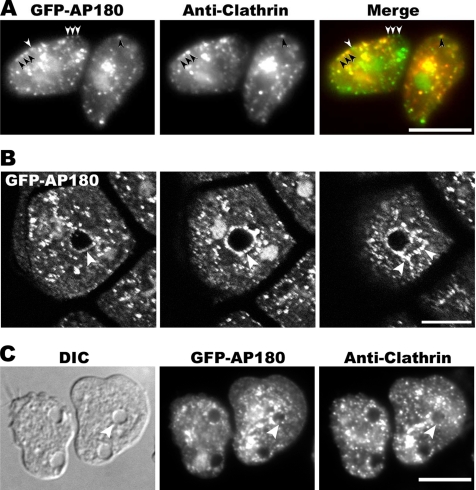
As monomeric adaptors for clathrin, members of the AP180 family associate with clathrin at the plasma membrane, and the clathrin box in the Dictyostelium AP180 sequence suggested that it might also associate with clathrin. To examine the intracellular location of the Dictyostelium ortholog, we made a plasmid that expressed the AP180 protein tagged with Green Fluorescent Protein (GFP). Growing Dictyostelium cells that expressed GFP-AP180 were gently flattened, fixed, and stained with an antibody against clathrin light chain followed by a secondary antibody conjugated to Texas Red (Figure 2A). GFP-AP180 localized as discrete punctae at the plasma membrane and in the cytoplasm. In addition, GFP-AP180 frequently labeled the nucleus and colocalized with the nuclear stain 4′-6-diamidino-2-phenylindole (DAPI; data not shown). Labeling cells with an antibody against clathrin showed that AP180 and clathrin colocalized in many punctae on the plasma membrane and in the cytoplasm. However AP180 punctae that lacked clathrin were also found on the plasma membrane. Clathrin punctae that lacked GFP-AP180 were more frequently observed in the cytoplasm, whereas the majority of clathrin punctae on the plasma membrane contained AP180.
Figure 2.
Localization of clathrin and AP180. (A) AP180 and clathrin colocalize. Cells expressing GFP-AP180 were fixed and stained with an anti-clathrin light-chain antibody detected with a Texas Red–conjugated secondary antibody. The majority of AP180 punctae at the plasma membrane colocalized with clathrin (black arrows) although some AP180 punctae lacked clathrin (white arrows). Merge shows GFP-AP180 in green and clathrin in red. Scale bar, 10 μm. (B) Z-series of cells expressing GFP-AP180 fixed and imaged by confocal microscopy. GFP-AP180 punctae decorated the contractile vacuole bladder (arrow, left and middle panels) as well as the tubules radiating from it (arrows, right panel). The two bright signals adjacent to the central bladder represent two nuclei. Each panel is 0.8 μm apart. Scale bar, 10 μm. (C) Cells expressing GFP-AP180 were fixed and stained with an anti-clathrin light-chain antibody followed by Texas Red–conjugated secondary antibody. Punctae of GFP-AP180 and clathrin outlined the contractile vacuole (arrow). Scale bar, 10 μm.
AP180 Associates with the Contractile Vacuole
Close examination of fixed or living cells expressing GFP-AP180 revealed that the tagged protein localized as punctae not only at the plasma membrane and within the cytoplasm, but also to ring-like structures. These structures were identified as contractile vacuoles when visualized under DIC in living cells because they were vacuoles that expanded and contracted periodically (Heuser et al., 1993). In addition to the vacuoles, GFP-AP180 also decorated the tubules of the contractile network that fed into the main vacuole (Figure 2B). Costaining fixed cells with an anti-clathrin antibody revealed that these vacuoles contained punctae of clathrin and of GFP-AP180 (Figure 2C).
Clathrin Mutants Display an Altered Distribution of AP180
To test whether clathrin was required for the clustering of AP180 into punctae on the plasma membrane, we expressed GFP-tagged AP180 in cells that lacked either the clathrin heavy-chain gene or the clathrin light-chain gene (Ruscetti et al., 1994; Wang et al., 2003; Figure 3A). In clathrin heavy-chain null cells, the GFP-AP180 punctae at the plasma membrane remained visible; however, most of the cytoplasmic punctae were lost. In general, punctae on the membrane of clathrin heavy-chain null cells were not as bright and were more diffuse than those of wild-type cells. In most clathrin light-chain null cells, the GFP-AP180 remained associated with punctae on the plasma membrane and scattered throughout the cytoplasm, a distribution similar to wild-type cells. In ∼20% of cells that lacked clathrin light chain, GFP-AP180 accumulated on one side of the plasma membrane, a pattern never seen in wild-type cells (Figure 3A, inset).
Figure 3.
Localization of GFP-AP180. (A) Wild-type cells (Ax2), clathrin heavy-chain null cells (CHC null), and clathrin light-chain null cells (CLC null) expressing GFP-AP180 were fixed and imaged with fluorescence microscopy. In CHC null cells, GFP-AP180 punctae in the cytoplasm were absent. In most CLC null cells, GFP-AP180 retained punctae on the plasma membrane and cytoplasm. In some CLC null cells, GFP-AP180 formed a cap on the plasma membrane (inset). Scale bar, 10 μm. Inset scale bar, 5 μm. (B) AP180 associates less frequently with the contractile vacuoles of CLC null cells. Wild-type Ax2 cells (n = 43) and CLC null cells (n = 59) expressing GFP-AP180 were incubated in water, fixed, and scored for AP180 localization at the contractile vacuole.
In addition, clathrin light-chain null cells had a pronounced difference in the association of AP180 with the contractile vacuole. Examination of clathrin light-chain null cells revealed contractile vacuoles that were occasionally labeled with GFP-tagged AP180. However this association was strikingly less frequent than in wild-type cells expressing GFP-tagged AP180. Although 65% of the contractile vacuoles in wild-type cells were labeled with AP180, only 25% of contractile vacuoles in clathrin light-chain null cells were labeled with AP180 (Figure 3B).
Clathrin Localization on the Plasma Membrane Is Not Affected in Cells That Lack AP180
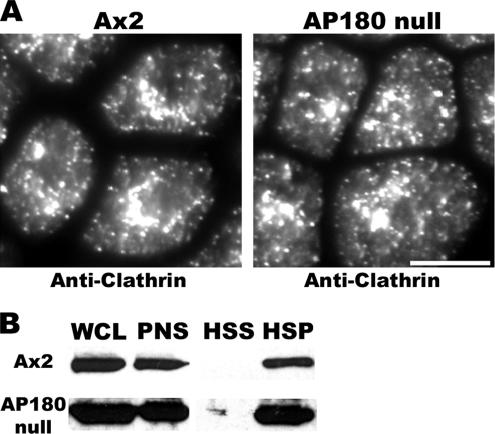
Members of the AP180 family associate with clathrin and assemble clathrin triskelia into cages in vitro (Ahle and Ungewickell, 1986; Ye and Lafer, 1995a). To test whether Dictyostelium AP180 is required for the association of clathrin with cellular membranes, we constructed AP180 null cells using homologous recombination to replace a portion of the coding sequence of the clmA gene with a blasticidin marker. Replacement within the clmA gene was confirmed by PCR and the absence of the AP180 protein in AP180 null mutants was verified by Western blot analysis (data not shown). To test whether Dictyostelium AP180 is required for the association of clathrin with cellular membranes, we assessed the distribution of clathrin in wild-type and AP180 null cells using an antibody against clathrin light chain and immunofluorescence microscopy. As shown previously in wild-type cells, clathrin localized as punctae at the plasma membrane, cytoplasm, and perinuclear region (Damer and O'Halloran, 2000). This localization pattern was unchanged in cells that lacked AP180 (Figure 4A). To examine directly the association of clathrin with intracellular membranes, we performed differential cell fractionation. In both wild-type cells and AP180 null cells, clathrin fractionated into the high speed (100,000 × g) pellet that contains membranes (Figure 4B). These results suggested that clathrin retained its ability to associate with intracellular membranes even in the absence of AP180.
Figure 4.
Membrane-associated clathrin punctae assemble in the absence of AP180. (A) Wild-type cells (Ax2) and AP180 null cells (AP180 null) fixed and stained with an anti-clathrin light-chain antibody. Scale bar, 10 μm. (B) Differential fractionation of wild-type cells (Ax2) and AP180 mutants (AP180 null) analyzed in immunoblots stained with anti-clathrin heavy chain show similar distribution of clathrin. WCL, whole cell lysate; PNS, post-nuclear supernatant; HSS, high-speed supernatant; and HSP, high-speed pellet.
Clathrin Dynamics at the Contractile Vacuole Are Altered in the Absence of AP180
Both clathrin light-chain and clathrin heavy-chain null cells display an osmoregulation defect. To examine the relationship between the contractile vacuole, clathrin, and AP180, we utilized a GFP-tagged construct of clathrin light chain and examined its behavior in wild-type cells and AP180 null cells. Although the immunofluorescence and fractionation data showed that most clathrin remained associated with membranes in the absence of AP180, examination of the contractile vacuole revealed an important difference. Wild-type and AP180 null cells expressing GFP-clc were shifted into water and filmed under low-light conditions to maintain contractile vacuole activity. In both wild-type cells and AP180 null cells, clathrin associated with the contractile vacuole (Figure 5A and Supplementary Movies 1 and 2). In a hypotonic environment, cells showed a transient association of clathrin with the bladder and the tubules of the contractile vacuole during all stages of contractile vacuole activity. Clathrin punctae on the contractile vacuole became most evident when the bladder was fully expanded before fusion. These punctae remained visible until the complete discharge of the contractile vacuole. After the collapse of the bladder into the tubular network, clathrin punctae dispersed into the cytoplasm. This pattern of association of clathrin with the contractile vacuole was also seen in AP180 null cells. However, in the mutants, clathrin punctae were more frequently associated with the contractile vacuole than in wild-type cells. In wild-type cells, about half of the contractile vacuoles were labeled with clathrin (47%, n = 19 contractile vacuoles) for at least a portion of the contractile vacuole cycle (Figure 5B). Strikingly, AP180 null cells showed more contractile vacuoles labeled with clathrin (90%, n = 20) during the contractile vacuole cycle (Figure 5B).
Figure 5.
(A) Time lapse of living wild-type (Ax2) and AP180 null cells expressing GFP-CLC after shifting from media to water. Clathrin localizes to the expanding and discharging contractile vacuole (arrow) in wild-type and AP180 null cells. Scale bar, 5 μm. See accompanying videos, Supplementary Movies 1 and 2. (B) An increased number of contractile vacuoles associate with clathrin in AP180 null cells. Wild-type Ax2 cells (n = 19) and AP180 null cells (n = 20) expressing GFP-CLC were incubated in water and scored for the presence of clathrin on the contractile vacuole.
AP180 Null Cells Display Wild-type Endocytosis, Development, and Cytokinesis
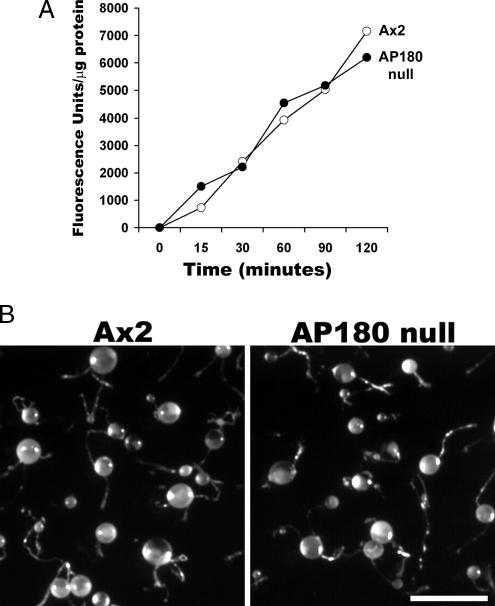
To understand how AP180 might contribute to development and cellular function in Dictyostelium, we examined the phenotype of AP180 null cells. We assessed these mutant cells for deficiencies associated with Dictyostelium clathrin mutants, including fluid-phase endocytosis, development, cytokinesis, and osmoregulation (Niswonger and O'Halloran, 1997b, 1997a; Wang et al., 2003). To examine fluid-phase endocytosis, we compared the ability of wild-type cells and AP180 null cells to internalize a fluid-phase marker, FITC-Dextran. We found that AP180 null cells were able to internalize FITC-Dextran as well as wild-type cells (Figure 6A). Both clathrin heavy-chain and clathrin light-chain mutants also display defects in development as shown by their inability to form fruiting bodies (Niswonger and O'Halloran, 1997a; Wang et al., 2003). In contrast, AP180 mutants formed robust fruiting bodies at the same rate as wild-type cells (Figure 6B). AP180 null cells grew well in suspension cultures without forming multinucleated cells, indicating that cytokinesis was also normal in these mutants (data not shown). In addition, imaging cells stained with Texas-Red–labeled phalloidin showed that wild-type and AP180 null mutants shared a similar organization of the actin cytoskeleton (Supplementary Figure 1S).
Figure 6.
(A) Fluid phase endocytosis in wild-type cells and AP180 null cells. Cells were incubated with FITC-Dextran (2 mg/ml). At the indicated times, samples of cells were lysed and quantified for the amount of internalized FITC-Dextran using a fluorometer. AP180 null cells (○) internalized FITC-Dextran at the same rate as wild-type cells (•). (B) Development of wild-type cells (Ax2) and AP180 null cells. Cells were plated on a lawn of E. coli. Following depletion of bacteria, wild-type (Ax2) and AP180 null cells differentiated into fruiting bodies consisting of a robust stalk topped by a round sorus. Scale bar, 0.5 mm.
AP180 Null Cells Are Osmosensitive
To investigate the role of AP180 in osmoregulation, we examined the behavior of AP180 null cells in a hypo-osmotic environment. We transferred wild-type and AP180 mutant cells from their culture media to water and imaged them using DIC microscopy (Figure 7). Wild-type cells adapted quickly to the hypo-osmotic environment by increasing the number and activity of their contractile vacuoles. In contrast, AP180 null cells were osmosensitive. When placed in a hypo-osmotic environment, AP180 null cells initially formed blebs on their membrane and then formed large contractile vacuoles (Figure 7 and Supplementary Movies 3 and 4). Although the enlarged contractile vacuoles persisted after hours in water, the effect was not completely deleterious. Even after several hours in water, AP180 null cells remained viable when they were returned to nutrient growth. The contractile vacuole defect was specific for the absence of AP180 because expressing GFP-AP180 in the null cells rescued this defect (data not shown). The osmosensitive phenotype was reminiscent of a similar defect exhibited by clathrin light-chain null cells (Wang et al., 2003). Similar to the response of AP180 null cells, clathrin light-chain mutants also formed enlarged contractile vacuoles when shifted to a hypo-osmotic environment. Like those of AP180 null cells, the contractile vacuoles in clathrin light-chain mutants were able to fill and discharge, but they grew to a much larger size than the contractile vacuoles of wild-type cells.
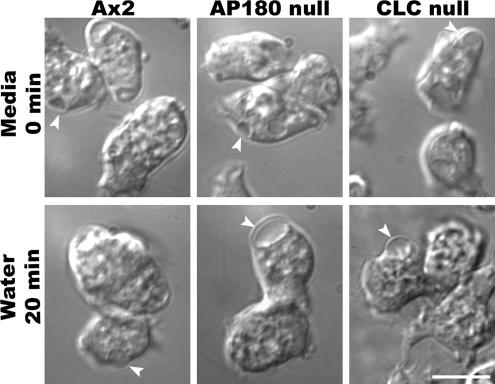
Figure 7.
AP180 null cells develop large contractile vacuoles in water. Wild-type cells (Ax2), clathrin light-null cells (CLC null), and AP180 null cells are shown in HL-5 media (Media, top row) and 20 min after shifting cells to water (bottom row). Contractile vacuoles are indicated with arrows. Scale bar, 10 μm. See accompanying videos, Supplementary Movies 3 and 4.
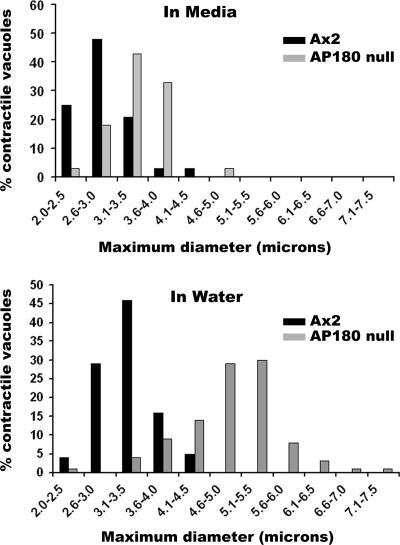
We compared the maximum size attained by contractile vacuoles in wild-type and AP180 null cells using time-lapse DIC images of cells in water. In nutrient medium (HL-5) the average maximum diameter of wild-type contractile vacuoles was 2.8 ± 0.49 μm (n = 27; Figure 8A). When placed in water, the contractile vacuoles of wild-type cells grew larger (3.2 ± 0.43 μm, n = 79) and then folded into the plasma membrane as they discharged their contents to the extracellular environment (Figure 8B). By comparison, AP180 null cells grown in nutrient medium had contractile vacuoles that were slightly larger than those in wild-type cells (3.4 ± 0.52 μm, n = 27; Figure 8A). When AP180 null cells were exposed to water, their contractile vacuoles grew substantially larger (Figure 8B). Their average size reached 4.9 ± 0.79 μm (n = 79) and a significant number of contractile vacuoles (>40%) expanded to a maximum diameter larger than 5 μm.
Figure 8.
Contractile vacuole size in wild-type and AP180 null cells. Living wild-type (Ax2) and AP180 null cells were imaged under DIC optics. The expansion and discharge cycle of contractile vacuoles in media (n = 27) and in water (n = 79) for each cell line was monitored and the maximum diameter of the vacuole was recorded. In media (top graph), the contractile vacuoles of AP180 null cells were slightly larger than those of wild-type cells. In water (bottom graph) the contractile vacuoles of AP180 null cells were much larger than those of wild-type cells.
The Contractile Vacuole Cycle Is Prolonged in AP180 Mutant Cells
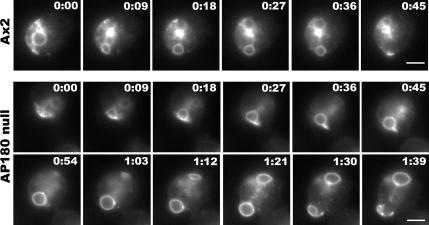
To monitor the dynamics of the contractile vacuole in living cells, we transfected wild-type and AP180 null cells with an expression plasmid for Dajumin-GFP, a marker for the contractile vacuole network in Dictyostelium (Gabriel et al., 1999). This marker allowed us to visualize the dynamics of the contractile vacuole in real time in a hypo-osmotic environment. We shifted wild-type cells and AP180 null cells expressing Dajumin-GFP from culture media to water and imaged active contractile vacuoles as they filled and discharged their contents. The contractile vacuole of wild-type cells filled quickly, reached its maximum size, and then discharged to the extracellular environment. Examination of contractile vacuole activity in wild-type cells showed an average cycle time of 44 ± 6.9 s (n = 16; Figure 9). In contrast, the activity of the contractile vacuoles of AP180 null cells persisted. After expanding to their maximum diameter, the enlarged contractile vacuoles in AP180 null cells lingered near the plasma membrane, stretched the membrane, and then fused and discharged their contents. Examination of contractile vacuole activity in AP180 mutants showed that these contractile vacuoles filled with kinetics similar to wild-type cells. However, because these contractile vacuoles became much larger and were delayed in fusion, the total cycle from filling to expulsion was 90 ± 15.6 s (n = 12), twice as long as the cycle displayed by wild-type cells (Figure 9).
Figure 9.
Contractile vacuole activity is prolonged in AP180 null cells. Time-lapse images of a wild-type cell (Ax2) and an AP180 null cell expressing a contractile vacuole marker, Dajumin-GFP, are shown after incubation in water and imaging with fluorescence optics. In the wild-type cell (Ax2), a contractile vacuole at the lower edge of the cell expands and discharges within 45 s. In contrast, the contractile vacuole at the lower edge of the AP180 null cell expands and discharges within 1 min and 39 s. Scale bar, 5 μm.
DISCUSSION
Identification of the Dictyostelium AP180 Ortholog Reveals a New Function for an Adaptor Protein
In this work, we identified the AP180 ortholog in Dictyostelium and examined the contribution of AP180 to clathrin-mediated functions. AP180 localized as punctae at the plasma membrane, cytoplasm, and the contractile vacuole and shared extensive colocalization with clathrin at these sites. In clathrin heavy-chain null cells, AP180 continued to localize in punctae on the plasma membrane, but not in cytoplasmic punctae. In AP180 null cells, the distribution of clathrin punctae was similar to wild-type cells on the plasma membrane and within the cytoplasm, but was increased on the contractile vacuole. AP180 cells null showed profound defects in osmoregulation. Like clathrin light-chain null cells, AP180 null cells exhibited enlarged contractile vacuoles with protracted expansion. These results suggest that AP180 is a specific adaptor for clathrin at the contractile vacuole and functions with clathrin in regulation of contractile vacuole size.
Relationship between Clathrin and AP180 on the Plasma Membrane
The assembly of clathrin triskelions into ordered lattices on membranes is thought to be promoted by assembly proteins that bind to the plasma membrane through their interactions with phosphoinositides and specific cargo. In vitro assembly studies clearly show that AP180 binds PIP2 and that AP180 can efficiently assemble clathrin triskelia into lattices (Lindner and Ungewickell, 1992; Morris et al., 1993; Ye et al., 1995; Ye and Lafer, 1995b; Ford et al., 2001). In Dictyostelium clathrin heavy-chain null cells, AP180 was clustered into punctae on the plasma membrane. This association of AP180 with the plasma membrane in the absence of clathrin heavy chain is likely driven by the interaction between the ANTH domain of AP180 and the phosphoinositides at the plasma membrane or through interactions between binding motifs within AP180 for other adaptor proteins that reside at the plasma membrane such as AP-2 or EH-domain-containing proteins. The punctae for AP180 suggests that these other proteins might cross-link AP180 even in the absence of clathrin.
Reconstitution of clathrin-budding reactions with lipids and purified AP180, epsin, and clathrin show that AP180 is essential for binding clathrin to the liposomes, whereas epsin drives curvature of the lattice (Ford et al., 2001, 2002). In contrast, our study of living Dictyostelium AP180 null cells showed that clathrin could assemble into punctae on the plasma membrane even in the absence of AP180. Because the Dictyostelium genome contains only a single gene for AP180, other clathrin assembly proteins must drive clathrin assembly on the plasma membrane of AP180 null cells.
AP180 Null Cells Are Osmosensitive
The contractile vacuole system functions in osmoregulation, a particularly important adaptation for protists exposed to continuous osmotic changes in their environment. This organelle consists of an interconnected meshwork of tubules and bladders that fill with water, fuse with the plasma membrane and then contract to discharge water to the extracellular milieu. Normally, wild-type cells growing in culture media contain a few moderately active contractile vacuoles that maintain the osmotic balance of the cell. When the extracellular environment changes from iso-osmotic to hypo-osmotic, the number of contractile vacuoles and their activity increase to cope with increased osmotic pressure and thus prevent the cell from swelling and bursting. Among the fascinating features of the contractile vacuole is its ability to fill the bladder to a discrete size every time it goes though the cycle of expanding and contracting. The mechanism for the control of size for the contractile vacuole bladder is not known. As the bladder fills with water, the tubules connected to the bladder shorten as they are incorporated into the expanding bladder. After the emptying of the bladder, the tubules elongate to regenerate the contractile vacuole system and repeat the cycle (Gerisch et al., 2002; Heuser, 2006).
Clathrin is required for a functional contractile vacuole because clathrin heavy-chain mutant cells contain a dispersed contractile vacuole system without tubules and are osmosensitive (O'Halloran and Anderson, 1992b). Similarly, clathrin light-chain null cells are also osmosensitive and display abnormally large contractile vacuoles (Wang et al., 2003). Our results suggest that AP180 and clathrin cooperate in the cycle of contractile vacuole activity because of two observations: 1) the contractile vacuole phenotype shared between AP180 null cells and clathrin light-chain null cells; and 2) AP180 and clathrin are each found at the contractile vacuole. Both clathrin light chain and AP180 null cells show enlarged contractile vacuole bladders and prolonged contractile vacuole cycles. These deficiencies could be caused by defective clathrin lattices assembled without AP180 on the contractile vacuole. AP180 null cells continued to target clathrin on the contractile vacuole; indeed, clathrin localized even more prominently on the contractile vacuoles of AP180 null cells. This increase in clathrin could reflect an increased time for clathrin assembly without AP180, in view of in vitro studies that AP180 increases the efficiency of clathrin assembly into lattices (Hao et al., 1999). It is also possible that clathrin forms imperfect lattices on the contractile vacuoles of AP180 null cells, as shown recently for mammalian cells (Meyerholz et al., 2005). If clathrin vesicles must form efficiently into structured lattices on the contractile vacuole for full function, then inefficient clathrin assembly could account for the delayed cycle of the contractile vacuole seen in AP180 null cells.
How do clathrin and AP180 contribute to contractile vacuole function? One possibility is that AP180 null cells fail to sort proteins that are important for fusion of the contractile vacuole. The mechanism for the fusion of the Dictyostelium contractile vacuole with the plasma membrane is not known, but conceivably the fusion of this organelle could be regulated by SNARE proteins. In synapses, AP180 is thought to selectively retrieve a synaptic vesicle v-SNARE, synaptobrevin, into clathrin-coated vesicles, and AP180 mutants do not localize this v-SNARE properly (Nonet et al., 1999; Bao et al., 2005). By analogy, AP180 could function in Dictyostelium by retrieving a v-SNARE important for contractile vacuole fusion. In the absence of AP180, the regenerating contractile vacuole might lack sufficient v-SNARES for efficient fusion and consequently would expand to an abnormally large size. Alternatively, the presence of clathrin and AP180 assembled into punctae on the contractile vacuole suggests that AP180-associated coated vesicles function on the contractile vacuole membrane itself, perhaps by remodeling and preparing the contractile vacuole membrane so that it can fuse with the plasma membrane and efficiently discharge its contents.
The finding that clathrin light-chain null cells showed a diminished association of AP180 with the contractile vacuole may seem paradoxical. The standard view is that assembly proteins bind to the plasma membrane first and then recruit clathrin. However the decrease in AP180 localization on the contractile vacuole of clathrin light-chain cells suggests that clathrin could also influence AP180 distribution on membranes. It is possible that coated pits are built by the dynamic interaction of clathrin triskelions and AP180 proteins, as each recruits the other to stabilize the growing clathrin lattice. Without light chain, clathrin triskelia are crippled in function (Wang et al., 2003), and perhaps these compromised triskelia are unable to stabilize AP180 on the contractile vacuole. Thus the interplay between AP180 and clathrin to build a stable and regular lattice on this membrane could be impaired in clathrin light-chain null cells. Clearly, our results support current views that different clathrin-mediated trafficking pathways require different adaptor proteins.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Gunther Gerisch for the gift of the dajumin-GFP plasmid and Tom Egelhoff for the pTX-GFP plasmid. We also thank members of the O'Halloran and De Lozanne labs for helpful discussions and Arturo De Lozanne for comments on our manuscript. This work is supported by National Institutes of Health Grant RO1 GM048625 to T.J.O.
Footnotes

 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-02-0144) on October 18, 2006.
REFERENCES
- Ahle S., Ungewickell E. Purification and properties of a new clathrin assembly protein. EMBO J. 1986;5:3143–3149. doi: 10.1002/j.1460-2075.1986.tb04621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao H., Daniels R. W., MacLeod G. T., Charlton M. P., Atwood H. L., Zhang B. AP180 maintains the distribution of synaptic and vesicle proteins in the nerve terminal and indirectly regulates the efficacy of Ca2+-triggered exocytosis. J. Neurophysiol. 2005;94:1888–1903. doi: 10.1152/jn.00080.2005. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Chen C. Y., Knuehl C., Towler M. C., Wakeham D. E. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 2001;17:517–568. doi: 10.1146/annurev.cellbio.17.1.517. [DOI] [PubMed] [Google Scholar]
- Damer C. K., O'Halloran T. J. Spatially regulated recruitment of clathrin to the plasma membrane during capping and cell translocation. Mol. Biol. Cell. 2000;11:2151–2159. doi: 10.1091/mbc.11.6.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beer T., Carter R. E., Lobel-Rice K. E., Sorkin A., Overduin M. Structure and Asn-Pro-Phe binding pocket of the Eps15 homology domain. Science. 1998;281:1357–1360. doi: 10.1126/science.281.5381.1357. [DOI] [PubMed] [Google Scholar]
- Dreyling M. H., Martinez-Climent J. A., Zheng M., Mao J., Rowley J. D., Bohlander S. K. The t(10;11)(p13;q14) in the U937 cell line results in the fusion of the AF10 gene and CALM, encoding a new member of the AP-3 clathrin assembly protein family. Proc. Natl. Acad. Sci. USA. 1996;93:4804–4809. doi: 10.1073/pnas.93.10.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford M. G., Mills I. G., Peter B. J., Vallis Y., Praefcke G. J., Evans P. R., McMahon H. T. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- Ford M. G., Pearse B. M., Higgins M. K., Vallis Y., Owen D. J., Gibson A., Hopkins C. R., Evans P. R., McMahon H. T. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science. 2001;291:1051–1055. doi: 10.1126/science.291.5506.1051. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Yumura S., Yumura T. K. Agar-overlay immunofluorescence: high-resolution studies of cytoskeletal components and their changes during chemotaxis. Methods Cell Biol. 1987;28:347–356. doi: 10.1016/s0091-679x(08)61655-6. [DOI] [PubMed] [Google Scholar]
- Gabriel D., Hacker U., Kohler J., Muller-Taubenberger A., Schwartz J. M., Westphal M., Gerisch G. The contractile vacuole network of Dictyostelium as a distinct organelle: its dynamics visualized by a GFP marker protein. J. Cell Sci. 1999;112(Pt 22):3995–4005. doi: 10.1242/jcs.112.22.3995. [DOI] [PubMed] [Google Scholar]
- Gallusser A., Kirchhausen T. The beta 1 and beta 2 subunits of the AP complexes are the clathrin coat assembly components. EMBO J. 1993;12:5237–5244. doi: 10.1002/j.1460-2075.1993.tb06219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch G., Heuser J., Clarke M. Tubular-vesicular transformation in the contractile vacuole system of Dictyostelium. Cell Biol. Int. 2002;26:845–852. doi: 10.1006/cbir.2002.0938. [DOI] [PubMed] [Google Scholar]
- Hao W., Luo Z., Zheng L., Prasad K., Lafer E. M. AP180 and AP-2 interact directly in a complex that cooperatively assembles clathrin. J. Biol. Chem. 1999;274:22785–22794. doi: 10.1074/jbc.274.32.22785. [DOI] [PubMed] [Google Scholar]
- Hao W., Tan Z., Prasad K., Reddy K. K., Chen J., Prestwich G. D., Falck J. R., Shears S. B., Lafer E. M. Regulation of AP-3 function by inositides. Identification of phosphatidylinositol 3,4,5–trisphosphate as a potent ligand. J. Biol. Chem. 1997;272:6393–6398. doi: 10.1074/jbc.272.10.6393. [DOI] [PubMed] [Google Scholar]
- Heuser J. Evidence for recycling of contractile vacuole membrane during osmoregulation in Dictyostelium amoebae—a tribute to Gunther Gerisch. Eur J. Cell Biol. 2006 doi: 10.1016/j.ejcb.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Heuser J., Zhu Q., Clarke M. Proton pumps populate the contractile vacuoles of Dictyostelium amoebae. J. Cell Biol. 1993;121:1311–1327. doi: 10.1083/jcb.121.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F., Khvorova A., Marshall W., Sorkin A. Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J. Biol. Chem. 2004;279:16657–16661. doi: 10.1074/jbc.C400046200. [DOI] [PubMed] [Google Scholar]
- Huang K. M., D'Hondt K., Riezman H., Lemmon S. K. Clathrin functions in the absence of heterotetrameric adaptors and AP180-related proteins in yeast. EMBO J. 1999;18:3897–3908. doi: 10.1093/emboj/18.14.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannolo G., Salcini A. E., Gaidarov I., Goodman O. B., Jr, Baulida J., Carpenter G., Pelicci P. G., Di Fiore P. P., Keen J. H. Mapping of the molecular determinants involved in the interaction between eps15 and AP-2. Cancer Res. 1997;57:240–245. [PubMed] [Google Scholar]
- Kay B. K., Yamabhai M., Wendland B., Emr S. D. Identification of a novel domain shared by putative components of the endocytic and cytoskeletal machinery. Protein Sci. 1999;8:435–438. doi: 10.1110/ps.8.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T. Adaptors for clathrin-mediated traffic. Annu. Rev. Cell Dev. Biol. 1999;15:705–732. doi: 10.1146/annurev.cellbio.15.1.705. [DOI] [PubMed] [Google Scholar]
- Kohtz D. S., Puszkin S. A neuronal protein (NP185) associated with clathrin-coated vesicles. Characterization of NP185 with monoclonal antibodies. J. Biol. Chem. 1988;263:7418–7425. [PubMed] [Google Scholar]
- Lafer E. M. Clathrin-protein interactions. Traffic. 2002;3:513–520. doi: 10.1034/j.1600-0854.2002.30801.x. [DOI] [PubMed] [Google Scholar]
- Levi S., Polyakov M., Egelhoff T. T. Green fluorescent protein and epitope tag fusion vectors for Dictyostelium discoideum. Plasmid. 2000;44:231–238. doi: 10.1006/plas.2000.1487. [DOI] [PubMed] [Google Scholar]
- Lindner R., Ungewickell E. Clathrin-associated proteins of bovine brain coated vesicles. An analysis of their number and assembly-promoting activity. J. Biol. Chem. 1992;267:16567–16573. [PubMed] [Google Scholar]
- Mao Y., Chen J., Maynard J. A., Zhang B., Quiocho F. A. A novel all helix fold of the AP180 amino-terminal domain for phosphoinositide binding and clathrin assembly in synaptic vesicle endocytosis. Cell. 2001;104:433–440. doi: 10.1016/s0092-8674(01)00230-6. [DOI] [PubMed] [Google Scholar]
- Meyerholz A., Hinrichsen L., Groos S., Esk P. C., Brandes G., Ungewickell E. J. Effect of clathrin assembly lymphoid myeloid leukemia protein depletion on clathrin coat formation. Traffic. 2005;6:1225–1234. doi: 10.1111/j.1600-0854.2005.00355.x. [DOI] [PubMed] [Google Scholar]
- Morgan J. R., Prasa K., Hao W., Augustine G. J., Lafer E. M. A conserved clathrin assembly motif essential for synaptic vesicle endocytosis. J. Neurosci. 2000;20:8667–8676. doi: 10.1523/JNEUROSCI.20-23-08667.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S. A., Schroder S., Plessmann U., Weber K., Ungewickell E. Clathrin assembly protein AP180, primary structure, domain organization and identification of a clathrin binding site. EMBO J. 1993;12:667–675. doi: 10.1002/j.1460-2075.1993.tb05700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswonger M. L., O'Halloran T. J. Clathrin heavy chain is required for spore cell but not stalk cell differentiation in Dictyostelium discoideum. Development. 1997a;124:443–451. doi: 10.1242/dev.124.2.443. [DOI] [PubMed] [Google Scholar]
- Niswonger M. L., O'Halloran T. J. A novel role for clathrin in cytokinesis. Proc. Natl. Acad. Sci. USA. 1997b;94:8575–8578. doi: 10.1073/pnas.94.16.8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet M. L., Holgado A. M., Brewer F., Serpe C. J., Norbeck B. A., Holleran J., Wei L., Hartwieg E., Jorgensen E. M., Alfonso A. UNC-11, a Caenorhabditis elegans AP180 homologue, regulates the size and protein composition of synaptic vesicles. Mol. Biol. Cell. 1999;10:2343–2360. doi: 10.1091/mbc.10.7.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris F. A., Ungewickell E., Majerus P. W. Inositol hexakisphosphate binds to clathrin assembly protein 3 (AP-3/AP180) and inhibits clathrin cage assembly in vitro. J. Biol. Chem. 1995;270:214–217. doi: 10.1074/jbc.270.1.214. [DOI] [PubMed] [Google Scholar]
- O'Halloran T. J., Anderson R. G. Characterization of the clathrin heavy chain from Dictyostelium discoideum. DNA Cell Biol. 1992a;11:321–330. doi: 10.1089/dna.1992.11.321. [DOI] [PubMed] [Google Scholar]
- O'Halloran T. J., Anderson R. G. Clathrin heavy chain is required for pinocytosis, the presence of large vacuoles, and development in Dictyostelium. J. Cell Biol. 1992b;118:1371–1377. doi: 10.1083/jcb.118.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D. J., Vallis Y., Noble M. E., Hunter J. B., Dafforn T. R., Evans P. R., McMahon H. T. A structural explanation for the binding of multiple ligands by the alpha-adaptin appendage domain. Cell. 1999;97:805–815. doi: 10.1016/s0092-8674(00)80791-6. [DOI] [PubMed] [Google Scholar]
- Owen D. J., Vallis Y., Pearse B. M., McMahon H. T., Evans P. R. The structure and function of the beta 2-adaptin appendage domain. EMBO J. 2000;19:4216–4227. doi: 10.1093/emboj/19.16.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K., Lippoldt R. E. Molecular characterization of the AP180 coated vesicle assembly protein. Biochemistry. 1988;27:6098–6104. doi: 10.1021/bi00416a040. [DOI] [PubMed] [Google Scholar]
- Ruscetti T., Cardelli J. A., Niswonger M. L., O'Halloran T. J. Clathrin heavy chain functions in sorting and secretion of lysosomal enzymes in Dictyostelium discoideum. J. Cell Biol. 1994;126:343–352. doi: 10.1083/jcb.126.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Stahelin R. V., Long F., Peter B. J., Murray D., De Camilli P., McMahon H. T., Cho W. Contrasting membrane interaction mechanisms of AP180 N-terminal homology (ANTH) and epsin N-terminal homology (ENTH) domains. J. Biol. Chem. 2003;278:28993–28999. doi: 10.1074/jbc.M302865200. [DOI] [PubMed] [Google Scholar]
- Tebar F., Bohlander S. K., Sorkin A. Clathrin assembly lymphoid myeloid leukemia (CALM) protein: localization in endocytic-coated pits, interactions with clathrin, and the impact of overexpression on clathrin-mediated traffic. Mol. Biol. Cell. 1999;10:2687–2702. doi: 10.1091/mbc.10.8.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Haar E., Harrison S. C., Kirchhausen T. Peptide-in-groove interactions link target proteins to the beta-propeller of clathrin. Proc. Natl. Acad. Sci. USA. 2000;97:1096–1100. doi: 10.1073/pnas.97.3.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub L. M., Downs M. A., Westrich J. L., Fremont D. H. Crystal structure of the alpha appendage of AP-2 reveals a recruitment platform for clathrin-coat assembly. Proc. Natl. Acad. Sci. USA. 1999;96:8907–8912. doi: 10.1073/pnas.96.16.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Virta V. C., Riddelle-Spencer K., O'Halloran T. J. Compromise of clathrin function and membrane association by clathrin light chain deletion. Traffic. 2003;4:891–901. doi: 10.1046/j.1600-0854.2003.00144.x. [DOI] [PubMed] [Google Scholar]
- Wang N., Wu W. I., De Lozanne A. BEACH family of proteins: phylogenetic and functional analysis of six Dictyostelium BEACH proteins. J. Cell Biochem. 2002;86:561–570. doi: 10.1002/jcb.10254. [DOI] [PubMed] [Google Scholar]
- Wendland B., Emr S. D. Pan1p, yeast eps15, functions as a multivalent adaptor that coordinates protein-protein interactions essential for endocytosis. J. Cell Biol. 1998;141:71–84. doi: 10.1083/jcb.141.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W., Ali N., Bembenek M. E., Shears S. B., Lafer E. M. Inhibition of clathrin assembly by high affinity binding of specific inositol polyphosphates to the synapse-specific clathrin assembly protein AP-3. J. Biol. Chem. 1995;270:1564–1568. [PubMed] [Google Scholar]
- Ye W., Lafer E. M. Bacterially expressed F1-20/AP-3 assembles clathrin into cages with a narrow size distribution: implications for the regulation of quantal size during neurotransmission. J. Neurosci. Res. 1995a;41:15–26. doi: 10.1002/jnr.490410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W., Lafer E. M. Clathrin binding and assembly activities of expressed domains of the synapse-specific clathrin assembly protein AP-3. J. Biol. Chem. 1995b;270:10933–10939. doi: 10.1074/jbc.270.18.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Koh Y. H., Beckstead R. B., Budnik V., Ganetzky B., Bellen H. J. Synaptic vesicle size and number are regulated by a clathrin adaptor protein required for endocytosis. Neuron. 1998;21:1465–1475. doi: 10.1016/s0896-6273(00)80664-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.