Abstract
Chromatin assembly factor 1 (CAF-1) is involved in nucleo some assembly following DNA replication and nucleotide excision repair. In Arabidopsis thaliana, the three CAF-1 subunits are encoded by FAS1, FAS2 and, most likely, MSI1, respectively. In this study, we asked whether genomic stability is altered in fas1 and fas2 mutants that are lacking CAF-1 activity. Depletion of either subunit increased the frequency of somatic homologous recombination (HR) in planta ∼40-fold. The frequency of transferred DNA (T-DNA) integration was also elevated. A delay in loading histones onto newly replicated or repaired DNA might make these DNA stretches more accessible, both to repair enzymes and to foreign DNA. Furthermore, fas mutants exhibited increased levels of DNA double-strand breaks, a G2-phase retardation that accelerates endoreduplication, and elevated levels of mRNAs coding for proteins involved in HR—all factors that could also contribute to upregulation of HR frequency in fas mutants.
Keywords: Arabidopsis , CAF-1, cell cycle, DNA damage, homologous recombination
Introduction
In eukaryotic cells, the genomic DNA is highly compacted into chromatin through assembly with histone and nonhistone proteins. In proliferating cells, the bulk of the chromatin is assembled during DNA replication in the S phase of the cell cycle (Krude and Keller, 2001; Tyler, 2002; Verreault, 2003). Replication-specific nucleosome assembly is mediated by histone chaperones such as chromatin assembly factor 1 (CAF-1). CAF-1 was originally purified from nuclear extracts of human cells as a factor that supports the assembly of nucleosomes specifically onto replicating DNA in vitro (Smith and Stillman, 1989). CAF-1 mediates the first step of nucleosome assembly, that is, the deposition of H3/H4 histones onto replicating DNA (Smith and Stillman, 1989, 1991; Shibahara and Stillman, 1999; Tagami et al, 2004). CAF-1 is also involved in nucleosome assembly after nucleotide excision repair (NER; Ridgeway and Almouzni, 2000). CAF-1 is evolutionarily conserved, and homologs have been described in yeast, insects, plants, and vertebrates. In yeast, CAC1, CAC2, and CAC3 are counterparts of the p150, p60, and p48 subunits of human CAF-1. Despite the important role played by CAF-1 in nucleosome assembly during DNA synthesis, yeast cac mutants did not yield a lethal phenotype. However, increased UV sensitivity (Kaufman et al, 1997; Game and Kaufman, 1999), impaired gene silencing at telomeres (Monson et al, 1997; Enomoto and Berman, 1998) and at mating loci (Enomoto et al, 1997), and gross chromosomal rearrangements (Myung et al, 2003) were reported in such mutants. In higher eukaryotes, on the other hand, evidence for a more essential in vivo function of CAF-1 is accumulating. Ectopic expression of a dominant-negative form of the p150 subunit of CAF-1 caused severe early developmental defects in Xenopus laevis (Quivy et al, 2001), and induced S-phase arrest, accompanied by DNA damage and S-phase checkpoint activation, in human cells (Ye et al, 2003). Knockdown of the p60 subunit of CAF-1 by RNAi in human cells led to induction of cell death in proliferating, but not in quiescent, cells (Nabatiyan and Krude, 2004).
In Arabidopsis thaliana, the CAF-1 subunits corresponding to the human subunits p150, p60, and p48 are encoded by FAS1, FAS2, and, most likely, MSI1, respectively (Kaya et al, 2001; Henning et al, 2003). fas1 and fas2 mutants of Arabidopsis were originally described as mutations causing stem fasciation, and abnormal phyllotaxy, leaf shape, root growth, and flower organ number (Reinholz, 1966; Leyser and Furner, 1992). These mutants display severely disturbed cellular and functional organization of both shoot apical meristem (SAM) and root apical meristem (RAM). They also show a varied pattern of distorted expression of both WUSCHEL and SCARECROW, which play key roles in the organization of SAM and RAM, respectively (Kaya et al, 2001). Thus, CAF-1 appears to be important for the maintenance of plant developmental gene expression patterns. Other than these alterations in postembryonic development, these mutants are viable.
We speculated that delayed assembly of histones, expected as a consequence of a lack of CAF-1 activity, might lead to enhanced genomic instability in fas mutants. In this study, we used two different assays to measure DNA instability in a chromatin context: somatic homologous recombination (HR) and integration of the transferred DNA (T-DNA) of Agrobacterium tumefaciens. HR is a process that is used for precise DNA repair in somatic plant cells, whereas in meiosis it is used to generate novel distribution of genetic material between maternal and paternal chromosomes (Schuermann et al, 2005). T-DNA is a widely used tool for genetic engineering and plant insertional mutagenesis (Galbiati et al, 2000). Although we currently have very limited knowledge of the roles played by plant genes and proteins during this process, T-DNA integration is considered to use a nonhomologous end-joining (NHEJ)-related mechanism (Zupan et al, 2000; van Attikum et al, 2001; Friesner and Britt, 2003). Analysis of flanking sequence tags (FSTs) revealed that integration events are progressively less frequently observed towards the centromere (Brunaud et al, 2002), and also that about 40% of integration events occur in genes. These data suggested that chromatin structure can prevent T-DNA integration.
In this study, we detected an ∼40-fold increase in the frequency of HR, as well as increased T-DNA integration, in fas mutants. To aid further understanding of these findings, we analyzed the transcription of DNA repair genes, the generation of DNA double-strand breaks (DSBs), and cell cycle progression in fas mutants. The results presented here suggested that delayed chromatin assembly could lead to prolonged exposure of not yet chromatinized DNA to enzymes capable of repairing DNA by either HR or NHEJ in plants. In addition, induced DNA DSBs and enhanced transcription of genes involved in HR at S phase could stimulate HR.
Results
The frequency of HR is strongly elevated in fas mutants
We used an HR repair assay that allows recombination events to be visualized and scored by histochemical staining for a reconstituted recombination substrate locus (Swoboda et al, 1994; Schuermann et al, 2005). The constructs used as substrates for HR contain parts of the β-glucuronidase (GUS) gene in direct or inverted orientation (Figure 1A), which can recombine to reconstitute a functional GUS gene. fas1-2 (ecotype Nossen) and fas2-1 (ecotype Landsberg erecta (Ler)) plants were crossed to Arabidopsis lines (ecotype Columbia (Col)) carrying such recombination substrates, and F3 progeny plants were monitored for lines homozygous for the recombination reporter construct as well as for the fas1 and fas2 mutation. Mutants in either fas1 or fas2 resulted in around 40-fold more GUS recombination spots than in wild-type plants (Figure 1B–E), independent of the relative orientation of the truncated recombination target sequences, and regardless of which CAF-1 subunit was mutated. To confirm that the difference in HR frequency was not due simply to the heterogeneous genetic background of the parent plants, we analyzed HR frequency of FAS2 RNAi knockdown plants as well as a T-DNA tagging mutant of FAS2 (fas2-4), both of which are in a Col background. Both these plant lines also showed enhanced HR frequency compared to wild-type Columbia (Supplementary Figure S1).
Figure 1.
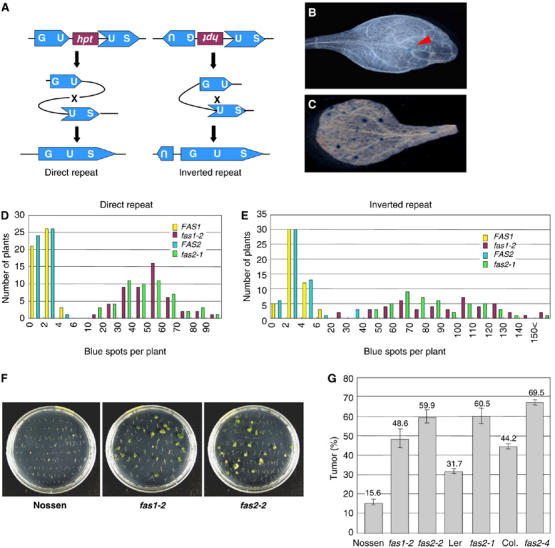
Genomic flexibility, as measured by intrachromosomal HR and T-DNA integration, is increased in fas mutants. (A) Recombination marker constructs. The β-glucuronidase (GUS; uidA) sequences have an overlap (indicated by ‘U') either in the direct (left) or inverted (right) orientation. Recombination (indicated by ‘x') between the two overlapping sequences produces a functional GUS gene. (B, C) Visualization of recombination events by histochemical GUS staining of leaves from a FAS1 control (B), and a fas1-2 plant homozygous for the inverted repeat-type recombination reporter (GU-US/GU-US) (C). An arrowhead in (B) indicates GUS-positive cells. (D, E) Frequency distribution histograms showing the proportions of plants with a given number of blue GUS spots in the direct repeat (D) and inverted repeat (E) populations. (F, G) Mutants fas1-2 and fas2-2 (ecotype Nossen), fas2-1 (ecotype Ler), and fas2-4 (ecotype Col), and the corresponding wild-type plants were inoculated with A. tumefaciens. (F) Plates showing growth of roots and tumors of Nossen, and its mutants fas1-2 and fas2-2, photographed 1 month after infection. (G) Efficiency of T-DNA integration as represented by the percentage of root segments that produced tumors. Error bars indicate standard error (s.e.). Data (means±s.e.) were taken from 10 plants of each type.
T-DNA integration is enhanced in fas mutants
The mechanism of in vivo integration of T-DNA into plant DNA represents a special case of a NHEJ process. NHEJ is the main pathway used by higher eukaryotic organisms to repair DSBs in DNA. This repair mechanism is usually accomplished with concomitant changes at the junction sequence, and is thus error prone (Lees-Miller and Meek, 2003).
The efficiency of T-DNA integration can be assessed using a root tumorigenesis assay (Nam et al, 1999). Root transformation with Agrobacterium A208 results in large green tumors on the roots. Indeed, increased numbers of tumors were observed on roots of fas1 and fas2 mutants infected with Agrobacterium compared to roots of the respective wild-type ecotypes (Figure 1F and G). When we analyzed transient expression of GFP following Agrobacterium-mediated root transformation, no difference in GFP expression between wild-type and fas mutants was observed (data not shown), suggesting that CAF-1 depletion does not increase T-DNA transmission from Agrobacterium to plant nuclei.
It is interesting to note that ecotypes naturally more refractory to T-DNA integration, such as Ler and, especially, Nossen, reacted more strongly to the mutation than Col, which is already very sensitive to T-DNA integration in the wild-type context.
Enhanced transcription of genes involved in HR in fas mutants
The results of the two experiments described above could be linked to defects in nucleosome assembly according to various, not necessarily mutually exclusive, scenarios: (i) the expression of repair enzymes is upregulated, (ii) there is an increased level of breaks in the DNA that can be repaired by HR and NHEJ activities, and (iii) repair enzymes, due to a lack, or delayed assembly, of nucleosomes, could have easier access to breaks in DNA in need of repair.
To test the hypothesis that an increased level of transcription of repair enzymes might contribute to enhanced rates of HR and T-DNA integration, we measured the transcription levels of several such genes by real-time PCR. As shown in Figure 2A, AtRAD51, which plays a central role in HR repair (Doutriaux et al, 1998), was found to be upregulated 4–5-fold in fas mutants. Transcription of AtRAD54 (a homolog of the yeast RAD54 gene, K Osakabe et al, 2006) was slightly induced in the mutants, whereas transcription of genes commonly grouped in the NHEJ pathway (AtKU70, AtKU80, and AtLIG IV) was unchanged, as was transcription of AtLIG I, which encodes a DNA replication factor. The results of Western blot analysis also indicated that the amount of AtKu70 protein remained constant in fas mutants (Supplementary Figure S2).
Figure 2.
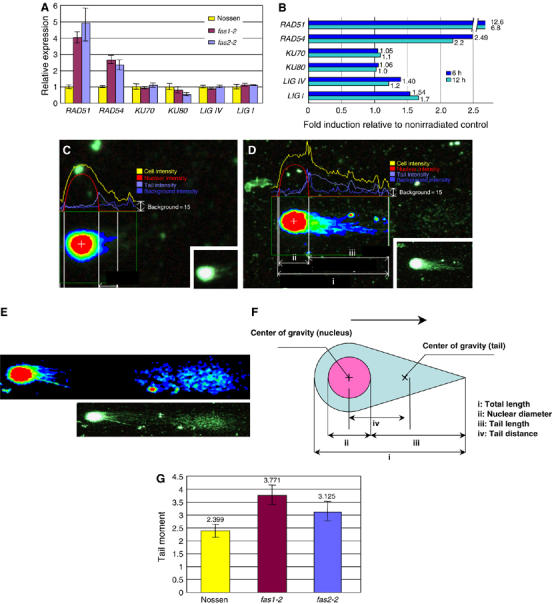
Elevated transcription of HR repair genes, and increased DNA damage in fas mutants. (A) Transcript levels of the repair genes indicated in fas1-2 and fas2-2 mutants relative to wild-type Nossen, as determined by real-time quantitative PCR. Error bars indicate standard error (s.e.). (B) Transcript levels of the repair genes indicated at 6 and 12 h after γ-irradiation in wild-type Nossen as determined by microarray analysis. (C–E) Comet images of intact and fragmented nuclear DNA from wild-type Nossen. (C) An almost intact nucleus (short tail). (D) A damaged nucleus (long tail). (E) A severely damaged nucleus (long fragmented tail). Original images are shown as white colored comets in the right corner panels of (C) and (D), and in the lower panel of (E). DNA intensity is indicated by gradation of color. (F) Schematic representation of a comet. (G) Statistical analysis of a comet assay. The level of DNA DSBs in the nucleus is represented as the tail moment, defined as the product of comet tail length and the fraction of total DNA in the tail (see Materials and methods). Error bars indicate the s.e. values of analyzed cells.
Interestingly, the same genes that were transcriptionally upregulated in the fas mutants (AtRAD51 and AtRAD54; Figure 2A) were also found to have a higher steady-state level of transcription in wild-type Arabidopsis plants exposed to γ-irradiation (Figure 2B).
Increased level of DNA DSBs in fas mutants
The experiments described above suggested the increased presence of DNA DSBs in fas mutants. To directly monitor the extent of DSBs in the DNA of wild-type and fas mutants, we attempted to quantify DNA DSBs by comet assay (Menke et al, 2001), which can indicate DNA damage in individual cells. A schematic representation of a comet assay is shown in Figure 2F. The amount of DNA in the comet tail separated from intact nuclear DNA in an electric field correlates with the number of breaks in the nuclear DNA (Menke et al, 2001). Figures 2C–E show typical comet assays of wild-type Arabidopsis nuclei with different amounts of DNA damage. As shown in Figure 2G, a small but significant increase in the number of DSBs was observed in fas mutants compared to wild-type plants. Severely damaged and fragmented nuclei (Figure 2E) were found more frequently in fas mutants (12.8 and 9.1% of the total number of cells counted in fas1-2 and fas2-2) than in wild-type (1.7% of total cells counted) but such nuclei were not included in the statistical analysis. Therefore, the relative level of DNA DSBs in fas mutants should be greater than that shown in Figure 2G.
One of the earliest known responses to DSB induction is the phosphorylation of thousands of molecules of the histone variant H2AX at the site of the break (Rogakou et al, 1998). In Arabidopsis, induction of phosphorylated H2AX (known as γ-H2AX) in an irradiation-dose-dependent manner, and its subsequent disappearance through DNA DSB repair have been demonstrated (Friesner et al, 2005). Quantification of γ-H2AX in wild-type and fas mutants by Western blot analysis revealed a small induction of γ-H2AX in fas mutants compared to wild-type (1.3-fold in fas1-2 and 2.1-fold in fas2-2) (Supplementary Figure S3), thus supporting the comet assay data.
fas mutants show increased sensitivity to DNA-damaging treatments
fas mutants were expected to be more sensitive to DNA-damaging stresses compared to wild-type plants due to the intrinsically higher level of DNA DSBs. Thus, we next investigated the γ-ray sensitivity of fas1 and fas2 mutants. As shown in Figures 3A–C, root growth of Arabidopsis seedlings was inhibited by γ-irradiation in a dose-dependent manner. This inhibition was greater in fas mutants than in wild-type plants, regardless of ecotype. We also assessed the development of true leaves in γ-irradiated plants (Figure 3D). fas1 and fas2 mutants exhibited increased yellowing of the cotyledons, resulting in death, following 600 Gy irradiation. In contrast, true leaves from wild-type plants did emerge. In fas1-2, 60, 20, 20, 10, and 0% of plants produced true leaves after 200, 300, 400, 500, and 600 Gy of γ-irradiation. In fas2-2, 70, 60, 50, 30, and 20% of plants produced true leaves after 200, 300, 400, 500, and 600 Gy of γ-irradiation. Under our experimental conditions, all plants could produce true leaves after 200–600 Gy of γ-irradiation in wild-type Nossen. Similarly, fas1 and fas2 mutants are more sensitive to UV-C irradiation than wild-type plants (see Supplementary Figure S4).
Figure 3.
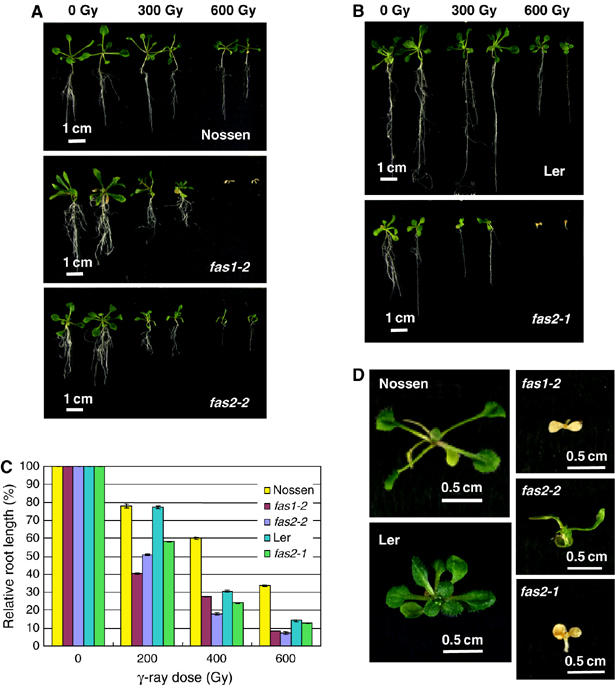
fas mutants show increased sensitivity to DNA-damaging treatments. (A, B) Phenotype of seedlings following exposure to the doses of γ-irradiation is indicated. (A) Wild-type Nossen (top), Nossen background mutants fas1-2 (middle) and fas2-2 (bottom). (B) Wild-type Ler (top) and Ler background mutant fas2-1 (bottom). (C) Relative root growth of fas1-2, fas2-2, and fas2-1 mutants and wild-type plants after exposure to the doses of γ-irradiation indicated. The average root length of nonirradiated plants in each case was taken as 100%. Error bars indicate s.e. Data are means±s.e., and represent the results of three independent experiments. (D) Development of true leaves of fas1-2, fas2-2, and fas2-1 mutants and the corresponding wild-type plants after γ-irradiation (600 Gy).
Cell cycle regulation in fas mutants
An essential step in the completion of S phase of the cell cycle is the reassembly of histone onto newly replicated DNA. CAF-1 is involved in this process. To analyze the effect of CAF-1 depletion on cell cycle progression, we analyzed the proportion of cells in each phase of the cell cycle by flow cytometry. Nuclei from the true leaves of 9-day-old seedlings showed an increase in 4C and a decrease in 2C cells in fas1-2 and fas2-2 mutants (Figure 4A and B). These results suggest an increased frequency of nuclei in G2/M phase in fas mutants.
Figure 4.
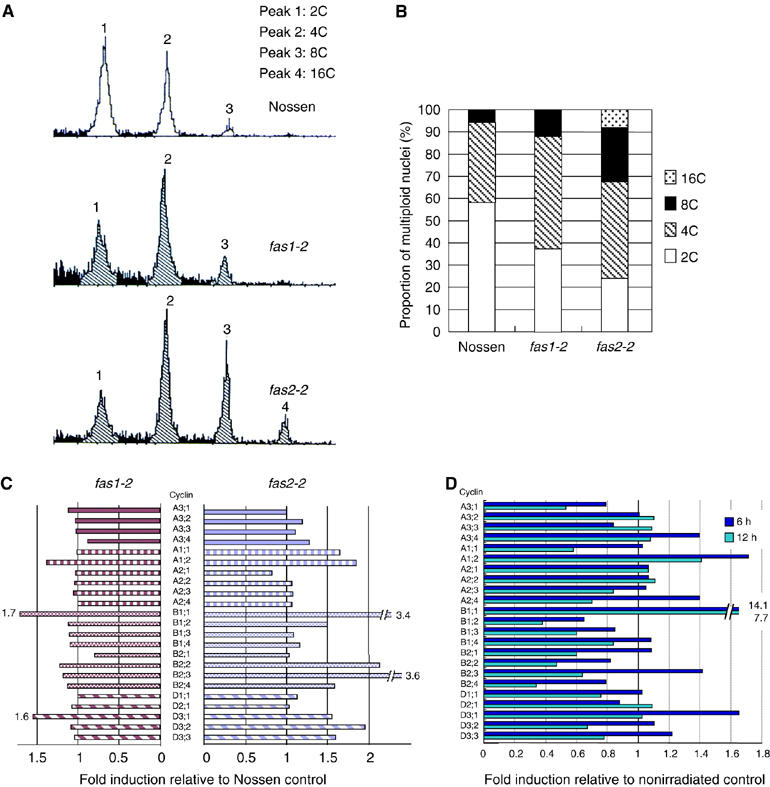
Aberrant cell cycle regulation in fas mutants. (A) Flow cytometric analysis of true leaves in 9-day-old Nossen, and Nossen background fas1-2 and fas2-2 mutants. (B) Calculated proportion of multiploid cells in 9-day-old Nossen, and Nossen background fas1-2 and fas2-2 mutants. (C) Transcription of cyclin genes in fas1-2 and fas2-2 mutants as determined by microarray analysis. (D) Transcript levels of cyclin genes 6 and 12 h after γ-irradiation in wild-type Nossen as determined by microarray analysis.
To further investigate cell cycle progression in fas mutants, we analyzed transcription of cyclin genes using microarrays (Figure 4C). In this assay, transcription of the mitotic cyclin AtCYCB1;1 (At4g37490, B-type cyclin gene) was drastically increased in fas mutants. The Arabidopsis mitotic cyclin CYCB1;1 product is reported to accumulate only around the time of the G2/M transition (Doerner et al, 1996; Shaul et al, 1996). Interestingly, expression of this cyclin was also strongly induced by γ-irradiation (Figure 4D), suggesting the crucial role of a G2 retardation for DNA DSB repair following γ-irradiation.
To confirm the results of microarray analysis and to further investigate the effects of CAF-1 depletion on cell cycle regulation, especially tissue-specific effects, we examined the expression of AtCYCB1;1∷GUS. Mitotic cyclin turnover requires a short peptide motif known as the ‘destruction box' (King et al, 1996). Transcriptional and post-translational regulation together restricts the accumulation of mitotic cyclins to G2 and M phase of the cell cycle. Arabidopsis Col plants transformed with AtCYCB1;1∷GUS (Colon-Carmona et al, 1999) were crossed with fas mutants. Figure 5 shows an example of AtCYCB1;1∷GUS expression in fas2-4 and wild-type Col. As shown in Figures 5A–C, cells expressing GUS activity appeared sporadically only in the root tip of wild-type Col. In contrast, significantly large numbers of cells in the stems, flower buds, leaves, and root tips of the fas2-4 mutant showed strong GUS activity (Figure 5D–F). Enhanced expression of AtCYCB1;1∷GUS was also detected regardless of ecotype and of which CAF-1 subunit was mutated (Supplementary Figure S5).
Figure 5.
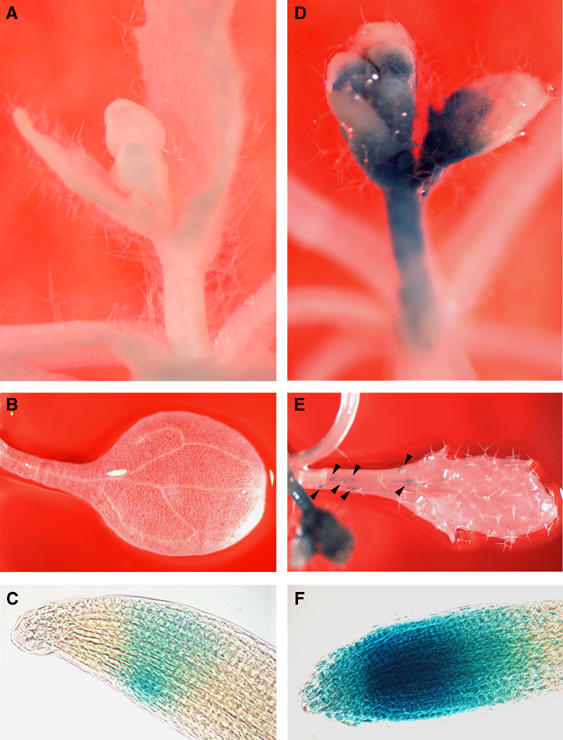
Histochemical assay of the AtCYCB1;1∷GUS reporter. GUS staining patterns of Col (A–C) and Col background fas2-4 mutant (D–F) are shown. (A, D) Close-up of flower buds. (B, E) GUS staining of true leaves. Arrowheads in (E) indicate GUS-positive cells in a fas2-4 leaf. (C, F) Close-up of root tips.
Discussion
Hyper-recombination of genomic DNA in fas mutants
In the present study, mutants in either fas1 or fas2 exhibited around 40-fold more recombination events than observed in wild-type plants (Figure 1A–E). We interpret this to mean that it is the complete CAF-1 complex, and not any one individual subunit, that maintains HR at a low level in wild-type plants. In this context, stimulated intrachromosomal recombination was reported in a yeast cac1 mutant (Prado et al, 2004), but in this case the increase was only 2–3-fold. These differences could be connected to the fact that HR efficiency in yeast is already high under normal conditions. In vertebrate systems, histone H3 comes in two major forms, histone H3.1 and histone H3.3. The former is loaded onto replicated DNA via CAF-1, whereas H3.3 replaces H3.1 in a postreplicative pathway via HIRA (Tagami et al, 2004). Interestingly, yeast has only the H3.3 version of histone H3 (Ahmad and Henikoff, 2002), hence CAF-1 might not be essential in yeast. The relative importance of CAF-1 between plants and yeast could thus result in a difference in the ratio of HR enhancement. It will be of interest to investigate HR in vertebrates with a similar assay.
CAF-1 was reported to be involved in the maintenance of epigenetic state in yeast (Monson et al, 1997; Enomoto and Berman, 1998). Therefore, the increased number of GUS spots observed in fas mutants could be explained by the release of transcriptional gene silencing (TGS) of the GUS gene. However, based on the following arguments, we judge the increased number of GUS spots observed in fas mutants to be due mainly to hyper recombination of the recombination substrate: we tested two different loci for indication of the hyper-recombination phenotype and both showed the same behavior. A hygromycin-resistance gene (hpt) was located between the two disrupted (but partially overlapping) GUS gene fragments (see Figure 1A) and the plants used in this assay showed stable hygromycin resistance. Hence, silencing of the GUS locus is unlikely. Furthermore, recently it has been reported that TGS of a silent GUS transgene was partially, but not totally de-repressed in fas mutants (Ono et al, 2006). These data thus concur with our prediction that fas mutants indeed exhibit an increased level of genome instability, as measured by intrachromosomal HR. A mutation in the yeast linker histone HHO1 led to an increased frequency of HR (Downs et al, 2003); partial depletion of histone H4 gave a similar phenotype (Prado and Aguilera, 2005). As CAF-1 assembles histones H3 and H4 following DNA replication (Smith and Stillman, 1989; Shibahara and Stillman, 1999; Tagami et al, 2004), depletion of CAF-1 and partial loss of histone H4 could enhance HR by similar mechanisms.
Increased accessibility of the T-DNA/protein complex to genomic DNA in fas mutants
Using a root tumorigenesis assay, we detected increased T-DNA integration in fas mutants (Figure 1F and G). As T-DNA integration occurs mainly through non-HR mechanisms, it is most likely that NHEJ proteins are required for the process of T-DNA integration. In fact, ku80-mutant Arabidopsis plants are defective in T-DNA integration in somatic cells, whereas Ku80-overexpressing plants exhibit increased susceptibility to Agrobacterium infection (Li et al, 2005). However, real-time PCR analysis failed to detect increased transcription of NHEJ pathway genes (AtKU70, AtKU80, and AtLIG IV) in fas mutants (Figure 2A). Furthermore, the protein level of Ku70 was unchanged in wild-type and fas mutants (Supplementary Figure S2). Therefore, the increased levels of T-DNA integration observed in fas mutants might reflect an increased level of genome instability rather than enhanced expression of NHEJ pathway genes.
The root tumorigenesis assay has been successfully used for the isolation of mutants resistant to Agrobacterium-mediated transformation in a population of T-DNA-mutagenized Arabidopsis plants (Mysore et al, 2000; Zhu et al, 2003). In this assay, loss-of-function mutants of histone H2A, histone deacetylases, histone acetyl transferase, and other chromatin-modifying proteins showed resistance to Agrobacterium-mediated transformation. These results indirectly suggest the importance of chromatin structure in T-DNA integration.
Taken together, the results of enhanced HR and T-DNA integration in fas mutants suggest that the condensed chromatin state in wild-type plants could maintain HR and T-DNA integration at a low level by preventing easy access of HR repair proteins and T-DNA to genomic DNA. CAF-1 is known to be involved in nucleosome assembly (Verreault, 2000; Krude and Keller, 2001; Mello and Almouzni, 2001). We postulated that rapid nucleosome formation would be disturbed, at least locally, in the absence of CAF-1. This would leave replicated DNA naked and easily accessible for a longer period of time. Recently, Schonrock et al (2006) reported reduced heterochromatin in fas mutants. Furthermore, Costa and Shaw (2006) showed, via three-dimensional fluorescence in situ hybridization on intact root epidermal tissue, that most nuclei of root epidermal cells in fas2 mutants were in an open chromatin state.
Factors enhancing HR in fas mutants
In addition to the enhanced accessibility of HR repair proteins to the site of DNA damage, various other scenarios can be envisaged to explain the increased frequency of HR in fas mutants. We found enhanced transcription of the HR genes AtRAD51 and AtRAD54 as well as the presence of increased numbers of DNA DSBs in fas mutants (Figure 2A and G). Schonrock et al (2006) also reported increased expression of several HR-related genes including AtRAD51 in fas mutants. In our experiment, the same set of genes that was transcriptionally upregulated in fas mutants was also found to have a higher steady-state level of transcription in wild-type Arabidopsis plants exposed to γ-irradiation, which induces DNA DSBs (Figure 2B). These results are consistent with the increased level of DNA DSBs observed in fas mutants (Figure 2G).
In fact, a dominant-negative human p150 mutant also induced S-phase arrest, accompanied by increased DNA damage (Ye et al, 2003). On the other hand, a robust transcriptional induction of HR genes has never been reported, even after γ-irradiation, in yeast and vertebrates (Haaf et al, 1995; Mercier et al, 2001). This fact suggests that if there is any correlation between the enhanced HR observed in yeast and induction of HR factors, it might involve translational or post-translational events. The enhanced γ-ray (Figure 3A–D) and UV-C sensitivity (Supplementary Figure S4) observed in fas mutants might also be due to intrinsic DSBs. Besides intrinsically induced DNA DSBs, a defect in NER repair activity in fas mutants could also contribute to this UV-sensitivity phenotype.
Furthermore, a small increment of phosphorylated H2AX (γ-H2AX) was also detected in fas mutants (Supplementary Figure S3). As induction of γ-H2AX following ionising radiation exposure—with protein levels increasing with irradiation dose—has been reported in Arabidopsis (Friesner et al, 2005), the increased amount of γ-H2AX found in fas mutants indicates an increased level of DSBs. Considering the function of CAF-1, which is involved in nucleosome assembly following DNA replication, increased DSBs in fas mutants were induced in the small proportion of the cells that are dividing. In mammals, γ-H2AX is induced in an ATM-dependent manner in response to DSBs, whereas γ-H2AX is induced in an ATR-dependent manner in situations where DNA synthesis is blocked. Interestingly, in Arabidopsis, both ATM and ATR contribute to ionizing radiation-induced γ-H2AX formation (Friesner et al, 2005). It will be of interest to investigate the roles of ATM and/or ATR on the enhanced induction of γ-H2AX in fas mutants.
As both enhanced expression of HR genes and induction of DNA DSBs have been reported to be involved in upregulation of HR (Reiss et al, 1996, 2000; Shalev et al, 1999; Molinier et al, 2005), the elevated levels of HR gene expression found in fas mutants could be explained by these factors. Therefore, the small increase in the number of DNA DSBs together with the relatively low enhancement of HR gene expression can partially explain the elevated levels of HR in fas mutants.
Flow cytometric analysis showed an increased proportion of 4C nuclei in fas mutants (Figure 4A and B). Our microarray analysis showed an increased transcription level of AtCYCB1;1, expressed only around the time of G2/M in fas mutants and in γ-irradiated wild-type plants (Figure 4C and D). Histochemical analysis of plants transformed with an AtCYCB1;1∷GUS construct confirmed that a large number of cells remain at the pre-M phase in proliferating cells of fas mutants compared to wild-type plants (Figure 5A–F, Supplementary Figure S5). Schonrock et al (2006) also showed enhanced expression of AtCYCB1;1∷GUS in fas mutants. These results indicate that the enhanced expression of AtCYCB1;1∷GUS observed in fas mutants was probably the result of a G2 retardation. Furthermore, γ-irradiation-induced G2 arrest was also reported in Arabidopsis using an AtCYCB1;1∷GUS reporter assay (Culligan et al, 2004).
The reported ploidy results could mean either that cells spend more time in G2 or that endoreduplication is accelerated. According to the expression data of mitotic cyclin, AtCYCB1;1, a severe delay in the cell cycle at the postreplicative (G2) phase in fas mutants might contribute to the ploidy results.
Several recent reports hint at the direct or indirect mediation of DNA repair by cell cycle regulators. It was reported that transcription and expression of cyclin A1 in mice was induced by γ-irradiation, and that cyclin A1 and A2 enhance DNA DSB repair by HR (Müller-Tidow et al, 2004). Moreover, a direct involvement of cyclin-dependent kinase (CDK) in HR has been reported. Esashi et al (2005) reported CDK-dependent phosphorylation of BRCA2, which interacts directly with the essential recombination protein Rad51 in cultured human cells. An involvement of CDK in the recruitment of Rad51 to the site of DNA DSBs has also been shown in Saccharomyces cerevisiae (Aylon et al, 2004; Ira et al, 2004). Further analysis of cell cycle regulation in fas mutants will reveal the cross-talk of cell cycle progression and regulation of HR in plants.
In the vertebrate system, CAF-1 depletion induces DNA DSBs at S phase, resulting in S-phase arrest and cell death (Ye et al, 2003; Nabatiyan and Krude, 2004). In contrast, fas mutants grow to maturity. This means that DNA DSBs generated in fas mutants, most probably during S phase, must be repaired before M phase. This fact also supports the presence of a G2 retardation in fas mutants. G2 retardation might make the chromatin structure of fas mutants relatively open, allowing enhanced HR and increased T-DNA integration. Interestingly, transcription of the AtRAD51 gene has been reported to be upregulated not only by DNA damage stress but also during S phase of the cell cycle (Doutriaux et al, 1998). Furthermore, it has been reported that DNA DSBs induced during late S–G2 phase are preferentially repaired by HR in vertebrate and yeast (Reski, 1998; Dronkert et al, 2000).
Summarizing the above discussion, in fas mutants, delayed chromatin assembly at S phase could lead to prolonged exposure of not yet chromatinized DNA to enzymes capable of repairing DNA by HR. In addition, induction of DNA DSBs and enhanced transcription of genes involved in HR might occur during S phase and stimulate HR in fas mutants. In this communication, we have discussed the effects of CAF-1 depletion in plants on nucleosome assembly following DNA replication. However, CAF-1 is also known to be involved in coupling of nucleosome assembly to NER (Ridgeway and Almouzni, 2000). With respect to the connection between NER and HR in Arabidopsis, a T-DNA-mediated knockout of the Centrin2 gene involved in the NER pathway led to a hyper-recombinogenic mutant (Molinier et al, 2004). Thus, a direct or indirect involvement of the NER pathway in the upregulation of HR and T-DNA integration remains to be investigated.
A delay in, or downregulation of, chromatin assembly seems to enable improved recruitment of repair enzymes to sites of damage as well as easy access of T-DNA to the plant genomic DNA. These studies thus demonstrate that nucleosome structures maintain recombination repair activities at a low level, necessitating chromatin remodelling functions such as that proposed for the AtINO80 protein involved in controlling HR (Fritsch et al, 2004). Also in yeast, remodelling and decompaction of chromatin is essential for efficient DNA repair (van Attikum et al, 2004; Morrison et al, 2004; Prado et al, 2004; Tsukuda et al, 2005).
During submission of our manuscript, Kirik et al (2006) reported enhanced intrachromosomal HR and increased ploidy level in a C24 background fas1 mutant, fas1-4. However, in contrast to our data and those of Schonrock et al (2006), nearly normal transcription levels (1.5-fold) of AtRAD51 were observed in fas1-4. This difference might be attributed to different experimental conditions, differences in the mutants, or differences in the ecotypes used.
Materials and methods
Plants
fas1-2 (Kaya et al, 2001) and fas2-2 (Kaya et al, 2001) from ecotype Nossen, fas2-1 (Leyser and Furner, 1992) from ecotype Landsberg erecta (Ler), and fas2-4 (H Kaya and T Araki, unpublished data) from ecotype Columbia (Col) were used in this study. fas2-4 was found as SALK_033228 in the searchable database of T-DNA insertion sequences established by the Salk Institute Genome Analysis Laboratory. fas2-4 also shows some morphological changes seen in other fas mutants. The position of insertion of the T-DNA in fas2-4 is shown in Supplementary Figure S1 (A).
Recombination assays
Recombination reporter lines (Gherbi et al, 2001) 1406 (direct repeat-type line), 1415 (inverted repeat-type line); ecotype Col were used in this study. The two GUS reporter lines were crossed with fas1-2 (ecotype Nossen) and fas2-1 (ecotype Ler), respectively. F3 seeds from plants homozygous for the GUS recombination reporter and homozygous for the mutant fas1-2 (GU-US/GU-US, fas1-2/fas1-2) or fas2-1 (GU-US/GU-US, fas2-1/fas2-1) allele were stained for GUS activity. Plants homozygous for the GUS recombination reporter and either homozygous or heterozygous for FAS1 (GU-US/GU-US, FAS1/FAS1 or FAS1/fas1-2) or FAS2 (GU-US/GU-US, FAS2/FAS2 or FAS2/fas2-1) were used as controls. Histochemical GUS staining was performed as described previously (Schuermann et al, 2005). The number of spots, each indicating a recombination event, on each seedling was then determined visually under a dissecting microscope. For each line, 50 seedlings were analyzed. Initial visual identification of fas1-2/fas1-2 and fas2-1/fas2-1 homozygote seedlings was based on the fact that they had unusual shoots (Kaya et al, 2001). The FAS1 and FAS2 genotypes of individual plants were then confirmed by DNA sequencing.
T-DNA integration assay
For the root tumorigenesis assay, A. tumefaciens A208 (Sciaky et al, 1978) was grown at 28°C in YEB medium supplemented with 10 mg/l Rifampicin. A. thaliana seeds were surface sterilized and germinated on solidified MS medium. Roots of 3-week-old plants were cut and infected with A. tumefaciens as previously described (Nam et al, 1999). After 2 days co-cultivation, root segments were transferred to solidified MS medium containing 100 mg/l Timentin to remove agrobacteria. The number of tumors was counted 1 month after infection. The number of root segments analyzed is as follows: Nossen, 551; fas1-2, 715; fas2-2, 833; Ler, 631; fas2-1, 718; Col., 1115; fas2-4, 857. We repeated this experiment more than three times. Methods of transient GFP expression are described in Supplementary Materials and methods.
RNA isolation
Total RNA prepared from 4-week-old seedlings (without the roots, i.e. true leaves with cotyledon and hypocotyls) using an RNeasy Plant Mini Kit (Qiagen, Hilden, Germany) was used for microarray and real-time PCR analysis. Four-week-old sterile seedlings of wild-type plants (ecotype Nossen) were irradiated with 100 Gy of γ-rays at a dose rate of 300 Gy/h. At 6 and 12 h after irradiation, samples were frozen in liquid nitrogen and immediately ground for total RNA isolation (RNeasy Plant Mini Kit).
Real-time PCR
Reverse transcription–PCR was performed as described previously (Osakabe et al, 2002). Detailed condition of real-time PCR and primer sequences used in this experiment are described in Supplementary Materials and methods.
Microarray analysis
An Arabidopsis 2 Oligo Microarray kit (Agilent Technology, CA, USA) was used in this study. Detailed conditions for these experiments are described in Supplementary Materials and methods.
Comet assay
Twelve-day-old seedlings (as defined above) were used for the comet assay. The experimental conditions used were described previously as N/N protocol (Menke et al, 2001). A CCD camera was used to capture images of SYBR green-stained comets. Signal quantification was performed using Comet analyzer Software (YOUWORKS, Japan) under conditions excluding severely damaged nuclei. Intensity of DNA is shown in graded colors. DNA DSBs are represented as the ‘tail moment' (Olive et al, 1990), which is defined as tail distance × (sum of tail intensity/sum of cell intensity). For individual parameters, see Figure 2D and F. We analyzed 40–70 nuclei/line and the experiment was repeated at least three times.
γ-Ray sensitivity tests
Relative root growth after γ-irradiation was analyzed as described by Harlow et al (1994) with minor modifications. Detailed condition for γ-ray sensitivity tests are described in Supplementary Materials and methods.
More than 20 plants each were used in the DNA damaging, γ-ray sensitivity, UV sensitivity, and comet assays. These experiments were repeated at least three times.
Flow cytometric analysis
True leaves of 9-day-old seedlings were chopped in an extraction buffer (Galbraith et al, 1983) with 2.5 mg/ml DAPI. The filtered nuclei (filter, 30 μm) were subjected to flow analysis with laser excitation at 357 nm.
Histochemical assay of AtCYCB1;1∷GUS reporter
Arabidopsis Col plants transformed with AtCYCB1;1∷GUS (Colon-Carmona et al, 1999) were crossed with fas mutants and wild-type plants of corresponding ecotypes. Sterile 4-week-old plants containing the AtCYCB1;1∷GUS reporter gene and homozygous for the fas1 and fas2 mutation (fas1/fas1 and fas2/fas2) and either homozygous or heterozygous for FAS1 (FAS1/FAS1 or FAS1/fas1), or FAS2 (FAS2/FAS2 or FAS2/fas2), were used for histochemical GUS staining.
Accession number
The microarray data comparing wild-type plants versus fas mutants and nonirradiated wild-type plants versus γ-irradiated wild-type plants are available at GEO (http://www.ncbi.nlm.nih.gov/geo/) with the accession numbers GSM112793, GSM112794, GSM112815, and GSM112816.
Supplementary Material
Supplementary Figure S1
Supplementary Figure S1
Supplementary Figure S1
Supplementary Figure S1
Supplementary Figure S1
Supplementary Materials and Methods
Acknowledgments
We thank Y Ito for γ-ray irradiation, H Ezura for FACS analysis, Y Nagamura and R Motoyama for microarray analysis, JD Friesner and AB Britt for anti γ-H2AX antibody, P Doerner for CycB1;1:GUS, S Takeda, K Sugimoto-Shirasu, M Umeda, and H Rothnie for critical reading of the manuscript, B Reiss for providing us with unpublished information on CAF-1-depleted plants. This work was supported by a PROBRAIN (Program for Promotion of Basic Research Activities for Innovative Biosciences) grant to HI and ST from the Bio-Oriented Technology Research Advancement Institution (BRAIN) of Japan. ST was supported by grants from the Ministry of Agriculture, Forestry and Fishery of Japan, and budget for Nuclear Research from the Ministry of Education, Culture, Sports, and Technology of Japan. ME was supported by a fellowship from Grant-in-Aid for JSPS (Japan Society for the Promotion of Science). KS was supported from Japan Science and Technology Agency. BH and LV acknowledge the support of the Novartis Research Foundation.
References
- Ahmad K, Henikoff S (2002) Histone H3 variants specify modes of chromatin assembly. Proc Natl Acad Sci USA 99: 16477–16484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylon Y, Liefshitz B, Kupiec M (2004) The CDK regulates repair of double-strand breaks by homologous recombination during the cell cycle. EMBO J 23: 4868–4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunaud V, Balzergue S, Dubreucq B, Aubourg S, Samson F, Chauvin S, Bechtold N, Cruaud C, DeRose R, Pelletier G, Lepiniec L, Caboche M, Lecharny A (2002) T-DNA integration into the Arabidopsis genome depends on sequences of pre-insertion sites. EMBO Rep 3: 1152–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon-Carmona A, You R, Haimovitch-Gal T, Doerner P (1999) Spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J 20: 503–508 [DOI] [PubMed] [Google Scholar]
- Costa S, Shaw P (2006) Chromatin organization and cell fate switch respond to positional information in Arabidopsis. Nature 439: 493–496 [DOI] [PubMed] [Google Scholar]
- Culligan K, Tissier A, Britt A (2004) ATR regulates a G2-phase cell-cycle checkpoint in Arabidopsis thaliana. Plant Cell 16: 1091–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerner P, Jorgensen JE, You R, Steppuhn J, Lamb C (1996) Control of root growth and development by cyclin expression. Nature 380: 520–523 [DOI] [PubMed] [Google Scholar]
- Doutriaux MP, Couteau F, Bergounioux C, White C (1998) Isolation and characterisation of the RAD51 and DMC1 homologs from Arabidopsis thaliana. Mol Gen Genet 257: 283–291 [DOI] [PubMed] [Google Scholar]
- Downs JA, Kosmidou E, Morgan A, Jackson SP (2003) Suppression of homologous recombination by the Saccharomyces cerevisiae linker histone. Mol Cell 11: 1685–1692 [DOI] [PubMed] [Google Scholar]
- Dronkert ML, Beverloo HB, Johnson RD, Hoeijmakers JH, Jasin M, Kanaar R (2000) Mouse RAD54 affects DNA double-strand break repair and sister chromatid exchange. Mol Cell Biol 20: 3147–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto S, Berman J (1998) Chromatin assembly factor I contributes to the maintenance, but not the re-establishment, of silencing at the yeast silent mating loci. Genes Dev 12: 219–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto S, McCune-Zierath PD, Gerami-Nejad M, Sanders MA, Berman J (1997) RLF2, a subunit of yeast chromatin assembly factor-I, is required for telomeric chromatin function in vivo. Genes Dev 11: 358–370 [DOI] [PubMed] [Google Scholar]
- Esashi F, Christ N, Gannon J, Liu Y, Hunt T, Jasin M, West JS (2005) CDK-dependent phosphorylation of BRCA2 as a regulatory mechanism for recombinational repair. Nature 434: 598–604 [DOI] [PubMed] [Google Scholar]
- Friesner J, Britt AB (2003) Ku80- and DNA ligase IV-deficient plants are sensitive to ionizing radiation and defective in T-DNA integration. Plant J 34: 427–440 [DOI] [PubMed] [Google Scholar]
- Friesner JD, Liu B, Culligan K, Britt AB (2005) Ionizing radiation-dependent gamma-H2AX focus formation requires ataxia telangiectasia mutated and ataxia telangiectasia mutated and Rad3-related. Mol Biol Cell 16: 2566–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch O, Benvenuto G, Bowler C, Molinier J, Hohn B (2004) The INO80 protein controls homologous recombination in Arabidopsis thaliana. Mol Cell 16: 479–485 [DOI] [PubMed] [Google Scholar]
- Galbiati M, Moreno MA, Nadzan G, Zourelidou M, Dellaporta SL (2000) Large-scale T-DNA mutagenesis in Arabidopsis for functional genomic analysis. Funct Integr Genomics 1: 25–34 [DOI] [PubMed] [Google Scholar]
- Galbraith DW, Harkins KR, Maddox JR, Ayres NM, Sharma DP, Firoozabady E (1983) Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 250: 99–101 [DOI] [PubMed] [Google Scholar]
- Game JC, Kaufman PD (1999) Role of Saccharomyces cerevisiae chromatin assembly factor-I in repair of ultraviolet radiation damage in vivo. Genetics 151: 485–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherbi H, Gallego ME, Jalut N, Lucht JM, Hohn B, White C (2001) Homologous recombination in planta is stimulated in the absence of Rad50. EMBO Rep 2: 287–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaf T, Golub EI, Reddy G, Radding CM, Ward DC (1995) Nuclear foci of mammalian Rad51 recombination protein in somatic cells after DNA damage and its localization in synaptonemal complexes. Proc Natl Acad Sci USA 92: 2298–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow GR, Jenkins ME, Pittalwala TS, Mount DW (1994) Isolation of uvh1, an Arabidopsis mutant hypersensitive to ultraviolet light and ionizing radiation. Plant Cell 6: 227–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning L, Taranto P, Walser M, Schönrock N, Gruissem W (2003) Arabidopsis MSI1 is required for epigenetic maintenance of reproductive development. Development 130: 2555–2565 [DOI] [PubMed] [Google Scholar]
- Ira G, Pellicioli A, Balijja A, Wang X, Fiorani S, Carotenuto W, Liberi G, Bressan D, Wan L, Hollingsworth NM, Haber JE, Foiani M (2004) DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature 431: 1011–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman PD, Kobayashi R, Stillman B (1997) Ultraviolet radiation sensitivity and reduction of telomeric silencing in Saccharomyces cerevisiae cells lacking chromatin assembly factor-I. Genes Dev 11: 345–357 [DOI] [PubMed] [Google Scholar]
- Kaya H, Shibahara KI, Taoka KI, Iwabuchi M, Stillman B, Araki T (2001) FASCIATA genes for chromatin assembly factor-1 in Arabidopsis maintain the cellular organization of apical meristems. Cell 104: 131–142 [DOI] [PubMed] [Google Scholar]
- King RW, Deshaies RJ, Peters JM, Kirschner MW (1996) How proteolysis drives the cell cycle. Science 274: 1652–1659 [DOI] [PubMed] [Google Scholar]
- Kirik A, Pecinka A, Wendeler E, Reiss B (2006) The chromatin assembly factor subunit FASCIATA1 is involved in homologous recombination in plants. Plant Cell on line [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krude T, Keller C (2001) Chromatin assembly during S phase: contributions from histone deposition, DNA replication and the cell division cycle. Cell Mol Life Sci 58: 665–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees-Miller SP, Meek K (2003) Repair of DNA double strand breaks by non-homologous end joining. Biochimie 85: 1161–1173 [DOI] [PubMed] [Google Scholar]
- Leyser HMO, Furner IJ (1992) Characterization of three shoot apical meristem mutants of Arabidopsis thaliana. Development 116: 397–403 [Google Scholar]
- Li J, Vaidya M, White C, Vainstein A, Citovsky V, Tzfira T (2005) Involvement of KU80 in T-DNA integration in plant cells. Proc Natl Acad Sci USA 102: 19231–19236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello JA, Almouzni G (2001) The ins and outs of nucleosome assembly. Curr Opin Genet Dev 11: 126–141 [DOI] [PubMed] [Google Scholar]
- Menke M, Chen I, Angelis KJ, Schubert I (2001) DNA damage and repair in Arabidopsis thaliana as measured by the comet assay after treatment with different classes of genotoxins. Mutat Res 493: 87–93 [DOI] [PubMed] [Google Scholar]
- Mercier G, Denis Y, Marc P, Picard L, Dutreix M (2001) Transcriptional induction of repair genes during slowing of replication in irradiated Saccharomyces cerevisiae. Mutat Res 487: 157–172 [DOI] [PubMed] [Google Scholar]
- Molinier J, Oakeley EJ, Niederhauser O, Kovalchuk I, Hohn B (2005) Dynamic response of plant genome to ultraviolet radiation and other genotoxic stresses. Mutat Res 571: 235–247 [DOI] [PubMed] [Google Scholar]
- Molinier J, Ramos C, Fritsch O, Hohn B (2004) CENTRIN2 modulates homologous recombination and nucleotide excision repair in Arabidopsis. Plant Cell 16: 1633–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson EK, de Bruin D, Zakian VA (1997) The yeast Cac1 protein is required for the stable inheritance of transcriptionally repressed chromatin at telomeres. Proc Natl Acad Sci USA 94: 13081–13086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison AJ, Highland J, Krogan NJ, Arbel-Eden A, Greenblatt JF, Haber JE, Shen X (2004) INO80 and gamma-H2AX interaction links ATP-dependent chromatin remodeling to DNA damage repair. Cell 119: 767–775 [DOI] [PubMed] [Google Scholar]
- Müller-Tidow C, Ji P, Diederichs S, Potratz J, Baumer N, Kohler G, Cauvet T, Choudary C, van der Meer T, Chan WY, Nieduszynski C, Colledge WH, Carrington M, Koeffler HP, Restle A, Wiesmuller L, Sobczak-Thepot J, Berdel WE, Serve H (2004) The Cyclin A1–CDK2 complex regulates DNA double-strand break repair. Mol Cell Biol 24: 8917–8928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysore KS, Nam J, Gelvin SB (2000) An Arabidopsis histone H2A mutant is deficient in Agrobacterium T-DNA integration. Proc Natl Acad Sci USA 97: 948–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung K, Pennaneach V, Kats ES, Kolodner RD (2003) Saccharomyces cerevisiae chromatin-assembly factors that act during DNA replication function in the maintenance of genome stability. Proc Natl Acad Sci USA 100: 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabatiyan A, Krude T (2004) Silencing of chromatin assembly factor 1 in human cells leads to cell death and loss of chromatin assembly during DNA synthesis. Mol Cell Biol 24: 2853–2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam J, Mysore KS, Zheng C, Knue MK, Matthysse AG, Gelvin SB (1999) Identification of T-DNA tagged Arabidopsis mutants that are resistant to transformation by Agrobacterium. Mol Gen Genet 261: 429–438 [DOI] [PubMed] [Google Scholar]
- Olive PL, Banath JP, Durand RE (1990) Heterogeneity in radiation-induced DNA damage and repair in tumour and normal cells measured using the ‘comet' assay. Radiat Res 122: 86–94 [PubMed] [Google Scholar]
- Ono T, Kaya H, Takeda S, Abe M, Ogawa Y, Kato M, Kakutani T, Mittelsten Scheid O, Araki T, Shibahara K (2006) Chromatin assembly factor 1 ensures the stable maintenance of silent chromatin states in Arabidopsis. Genes Cells 11: 153–162 [DOI] [PubMed] [Google Scholar]
- Osakabe K, Yoshioka T, Ichikawa H, Toki S (2002) Molecular cloning and characterization of RAD51-like genes from Arabidopsis thaliana. Plant Mol Biol 50: 71–81 [DOI] [PubMed] [Google Scholar]
- Osakabe K, Abe K, Yoshioka T, Osakabe Y, Todoriki S, Ichikawa H, Hohn B, Toki S (2006) Molecular isolation and characterization of the RAD54 gene from Arabidopsis thaliana. Plant J (in press) [DOI] [PubMed] [Google Scholar]
- Prado F, Aguilera A (2005) Partial depletion of histone H4 increases homologous recombination-mediated genetic instability. Mol Cell Biol 25: 1526–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado F, Cortes-Ledesma F, Aguilera A (2004) The absence of the yeast chromatin assembly factor Asf1 increases genomic instability and sister chromatid exchange. EMBO Rep 5: 497–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quivy JP, Grandi P, Almouzni G (2001) Dimerization of the largest subunit of chromatin assembly factor 1: importance in vitro and during Xenopus early development. EMBO J 20: 2015–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinholz E (1966) Radiation induced mutants showing changed inflorescence characteristics. Arabidopsis Inf Serv 3: 19–20 [Google Scholar]
- Reiss B, Klemm M, Kosak H, Schell J (1996) RecA protein stimulates homologous recombination in plants. Proc Natl Acad Sci USA 93: 3094–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss B, Schubert I, Kopchen K, Wendeler E, Schell J, Puchta H (2000) RecA stimulates sister chromatid exchange and the fidelity of double-strand break repair, but not gene targeting, in plants transformed by Agrobacterium. Proc Natl Acad Sci USA 97: 3358–3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reski R (1998) Physcomitrella and Arabidopsis: the David and Goliath of reverse genetics. Trends Plant Sci 3: 209–210 [Google Scholar]
- Ridgeway P, Almouzni G (2000) CAF-1 and the inheritance of chromatin states: at the cross roads of DNA replication and repair. J Cell Sci 113: 2647–2658 [DOI] [PubMed] [Google Scholar]
- Rogakou E, Pich D, Orr A, Ivanova V, Bonner W (1998) DNA double stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 273: 5858–5868 [DOI] [PubMed] [Google Scholar]
- Schonrock N, Exner V, Probst A, Gruissem W, Hennig L (2006) Functional genomic analysis of CAF-1 mutants in Arabidopsis thaliana. J Biol Chem 281: 9560–9568 [DOI] [PubMed] [Google Scholar]
- Schuermann D, Molinier J, Fritsch O, Hohn B (2005) The dual nature of homologous recombination in plants. Trends Genet 21: 172–181 [DOI] [PubMed] [Google Scholar]
- Sciaky DA, Montoya AL, Chilton MD (1978) Fingerprints of Agrobacterium Ti plasmids. Plasmid 1: 238. [DOI] [PubMed] [Google Scholar]
- Shalev G, Sitrit Y, Avivi-Ragolski N, Lichtenstein C, Levy AA (1999) Stimulation of homologous recombination in plants by expression of the bacterial resolvase ruvC. Proc Natl Acad Sci USA 96: 7398–7402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul O, Mironov V, Burssens S, Van Montagu M, Inze D (1996) Two Arabidopsis cyclin promoters mediate distinctive transcriptional oscillation in synchronized tobacco BY-2 cells. Proc Natl Acad Sci USA 93: 4868–4872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara K, Stillman B (1999) Replication-dependent marking of DNA by PCNA facilitates CAF-1-coupled inheritance of chromatin. Cell 96: 575–585 [DOI] [PubMed] [Google Scholar]
- Smith S, Stillman B (1989) Purification and characterization of CAF-1, a human cell factor required for chromatin assembly during DNA replication in vivo. Cell 58: 15–25 [DOI] [PubMed] [Google Scholar]
- Smith S, Stillman B (1991) Stepwise assembly of chromatin during DNA replication in vitro. EMBO J 10: 971–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoboda P, Gal S, Hohn B, Puchta H (1994) Intrachromosomal homologous recombination in whole plants. EMBO J 13: 484–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagami H, Ray-Gallet D, Almouzni G, Nakatani Y (2004) Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell 116: 51–61 [DOI] [PubMed] [Google Scholar]
- Tsukuda T, Fleming AB, Nickoloff JA, Osley MA (2005) Chromatin remodelling at a DNA double-strand break site in Saccharomyces cerevisiae. Nature 438: 379–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler JK (2002) Chromatin assembly. Cooperation between histone chaperones and ATP-dependent nucleosome remodeling machines. Eur J Biochem 269: 2268–2274 [DOI] [PubMed] [Google Scholar]
- van Attikum H, Bundock P, Hooykaas PJ (2001) Non-homologous end-joining proteins are required for Agrobacterium T-DNA integration. EMBO J 20: 6550–6558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Attikum H, Fritsch O, Hohn B, Gasser SM (2004) Recruitment of the INO80 complex by H2A phosphorylation links ATP-dependent chromatin remodelling with DNA double-strand break repair. Cell 119: 777–788 [DOI] [PubMed] [Google Scholar]
- Verreault A (2000) De novo nucleosome assembly: new pieces in an old puzzle. Genes Dev 14: 1430–1438 [PubMed] [Google Scholar]
- Verreault A (2003) Histone deposition at the replication fork: a matter of urgency. Mol Cell 11: 283–284 [DOI] [PubMed] [Google Scholar]
- Ye X, Franco AA, Santos H, Nelson DM, Kaufman PD, Adams PD (2003) Defective S phase chromatin assembly causes DNA damage, activation of the S phase checkpoint, and S phase arrest. Mol Cell 11: 341–351 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Nam J, Humara JM, Mysore KS, Lee LY, Cao H, Valentine L, Li J, Kaiser AD, Kopecky AL, Hwang HH, Bhattacharjee S, Rao PK, Tzfira T, Rajagopal J, Yi H, Veena, Yadav BS, Crane YM, Lin K, Larcher Y, Gelvin MJK, Knue M, Ramos C, Zhao X, Davis SJ, Kim SI, Ranjith-Kumar CT, Choi YJ, Hallan VK, Chattopadhyay S, Sui X, Ziemienowicz A, Matthysse AG, Citovsky V, Hohn B, Gelvin SB (2003) Identification of Arabidopsis rat mutants. Plant Physiol 132: 494–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupan J, Muth TR, Draper O, Zambryski P (2000) The transfer of DNA from Agrobacterium tumefaciens into plants: a feast of fundamental insights. Plant J 23: 11–28 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S1
Supplementary Figure S1
Supplementary Figure S1
Supplementary Figure S1
Supplementary Materials and Methods


