Abstract
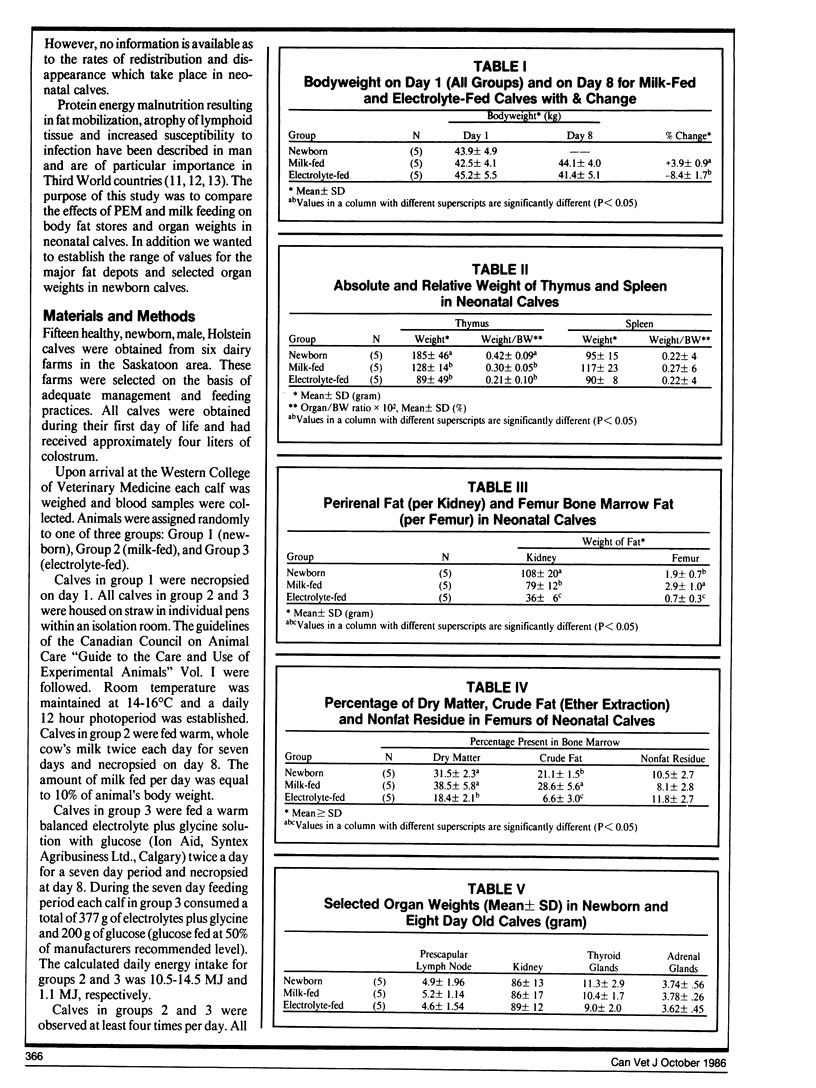
Fat stores and organ weights were assessed in calves at birth (n=5) and after seven days of milk (n=5) or electrolyte (n=5) feeding.
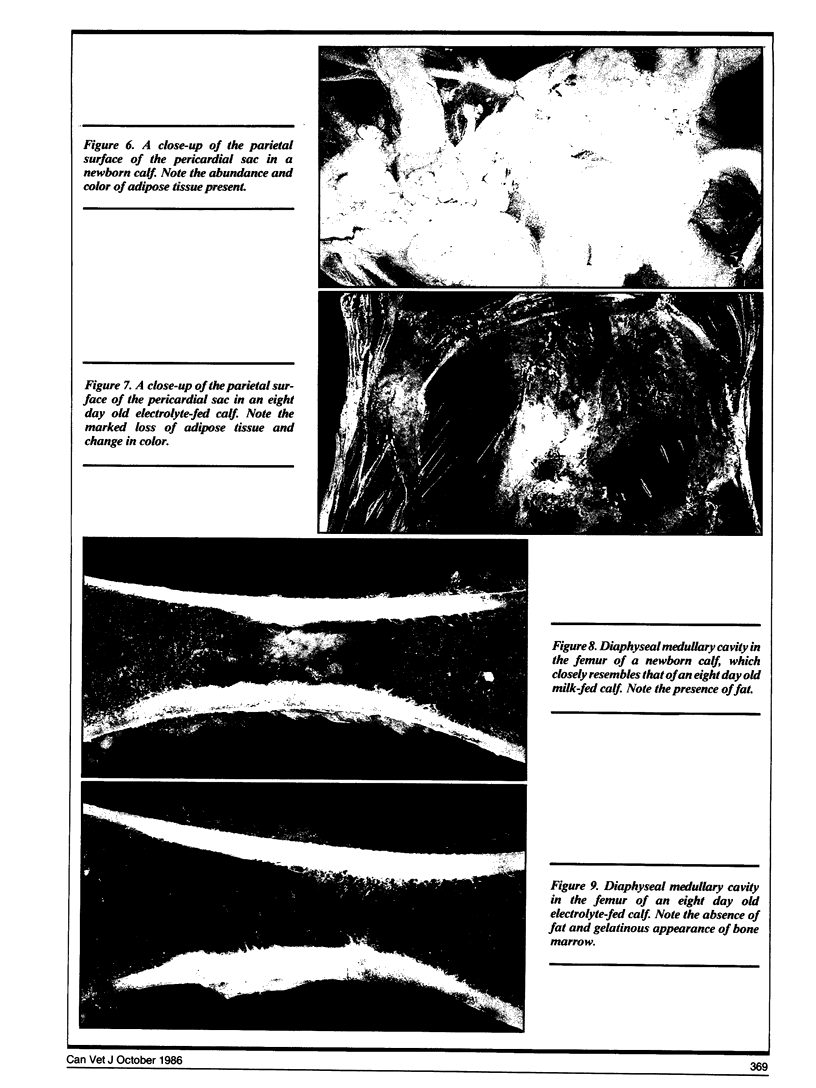
Compared to newborn calves, milk-fed calves had a significant (p < 0.05) redistribution of fat from perirenal area to bone marrow. The thymus also involuted during milk feeding.
In electrolyte-fed calves there was a significant loss of perirenal and bone marrow fat. The visible omental, mesenteric and subcutaneous fat stores were depleted. Epicardial fat stores were not visibly affected.
There was a high correlation between bone marrow crude fat and bone marrow dry matter (R=0.92). This suggests that dry matter estimations can be used to assess bone marrow fat stores. Perirenal fat may be intermediate in type between brown and white adipose tissue because it is mobilized in response to fasting, and formalin fixed perirenal fat did not contain detectable levels of thermogenin.
Keywords: Calf, thymus, organ, weights, adipose tissue, bone marrow fat, thermogenin, malnutrition
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander G., Bell A. W. Quantity and calculated oxygen consumption during summit metabolism of brown adipose tissue in new-born lambs. Biol Neonate. 1975;26(3-4):214–220. doi: 10.1159/000240732. [DOI] [PubMed] [Google Scholar]
- Alexander G., Bennett J. W., Gemmell R. T. Brown adipose tissue in the new-born calf (Bos taurus). J Physiol. 1975 Jan;244(1):223–234. doi: 10.1113/jphysiol.1975.sp010793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffell S. J., Sharp M. W., Winkler C. E., Terlecki S., Richardson C., Done J. T., Roeder P. L., Hebert C. N. Bovine virus diarrhoea-mucosal disease virus-induced fetopathy in cattle: Efficacy of prophylactic maternal pre-exposure. Vet Rec. 1984 Jun 9;114(23):558–561. doi: 10.1136/vr.114.23.558. [DOI] [PubMed] [Google Scholar]
- Haughey K. G. Cold injury in newborn lambs. Aust Vet J. 1973 Dec;49(12):554–563. doi: 10.1111/j.1751-0813.1973.tb06732.x. [DOI] [PubMed] [Google Scholar]
- McCANCE R. A., WIDDOWSON E. M. Composition of the body. Br Med Bull. 1951;7(4):297–306. doi: 10.1093/oxfordjournals.bmb.a073922. [DOI] [PubMed] [Google Scholar]
- McCance R. A., Widdowson E. M. Fat. Pediatr Res. 1977 Oct;11(10 Pt 2):1081–1083. [PubMed] [Google Scholar]
- Mellor D. J., Murray L. Effects of placental weight and maternal nutrition on the growth rates of individual fetuses in single and twin bearing ewes during late pregnancy. Res Vet Sci. 1981 Mar;30(2):198–204. [PubMed] [Google Scholar]
- Mellor D. J. Nutritional and placental determinants of foetal growth rate in sheep and consequences for the newborn lamb. Br Vet J. 1983 Jul-Aug;139(4):307–324. doi: 10.1016/s0007-1935(17)30436-0. [DOI] [PubMed] [Google Scholar]
- Mersmann H. J. Metabolic patterns in the neonatal swine. J Anim Sci. 1974 May;38(5):1022–1030. doi: 10.2527/jas1974.3851022x. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. Brown adipose tissue mitochondria. Biochim Biophys Acta. 1979 Jul 3;549(1):1–29. doi: 10.1016/0304-4173(79)90016-8. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G., Locke R. M. Thermogenic mechanisms in brown fat. Physiol Rev. 1984 Jan;64(1):1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Horwitz B. A. Brown fat and thermogenesis. Physiol Rev. 1969 Apr;49(2):330–425. doi: 10.1152/physrev.1969.49.2.330. [DOI] [PubMed] [Google Scholar]
- Sparks J. W., Girard J. R., Battaglia F. C. An estimate of the caloric requirements of the human fetus. Biol Neonate. 1980;38(3-4):113–119. doi: 10.1159/000241351. [DOI] [PubMed] [Google Scholar]
- Tavassoli M. Differential response of bone marrow and extramedullary adipose cells to starvation. Experientia. 1974 Apr 15;30(4):424–425. doi: 10.1007/BF01921701. [DOI] [PubMed] [Google Scholar]
- Tavassoli M., Eastlund D. T., Yam L. T., Neiman R. S., Finkel H. Gelatinous transformation of bone marrow in prolonged self-induced starvation. Scand J Haematol. 1976 Apr;16(4):311–319. doi: 10.1111/j.1600-0609.1976.tb01156.x. [DOI] [PubMed] [Google Scholar]
- Tavassoli M. Marrow adipose cells and hemopoiesis: an interpretative review. Exp Hematol. 1984 Feb;12(2):139–146. [PubMed] [Google Scholar]
- Tavassoli M. Marrow adipose cells. Histochemical identification of labile and stable components. Arch Pathol Lab Med. 1976 Jan;100(1):16–18. [PubMed] [Google Scholar]
- WIDDOWSON E. M. Chemical composition of newly born mammals. Nature. 1950 Oct 14;166(4224):626–628. doi: 10.1038/166626a0. [DOI] [PubMed] [Google Scholar]
- Weisberg H. F. Evaluation of nutritional status. Ann Clin Lab Sci. 1983 Mar-Apr;13(2):95–106. [PubMed] [Google Scholar]
- Wensvoort P. Adipose tissue in calves and lambs. Pathol Vet. 1968;5(3):270–281. doi: 10.1177/030098586800500307. [DOI] [PubMed] [Google Scholar]