Introduction
Functional gastrointestinal disorders (FGIDs) are common, chronic ailments that affect millions of adults on a daily basis. FGIDs are characterized by recurrent symptoms (ie, abdominal pain or discomfort, bloating, nausea, vomiting, early satiety, constipation, or diarrhea) that indicate a dysfunctional GI tract despite that an organic reason for the symptom generation is not identified on diagnostic studies.
It is estimated that 40% of all gastroenterology clinic visits are for FGIDs,[1] and a recent survey of generalists and gastroenterologists found that nearly one third of their patient population had symptoms of irritable bowel syndrome (IBS).[2] Many patients with IBS have dyspepsia; likewise, many patients with dyspepsia also have overlapping symptoms of IBS. These 2 groups of patients are similar in that symptoms are typically chronic in nature, may wax and wane, are aggravated by psychosocial stressors, and are often worsened by meals. In addition, both disorders are considered difficult to diagnose by many physicians and in the absence of warning signs or “red flags,” extensive testing is unlikely to be helpful. These similarities raise the issue of whether IBS and dyspepsia are just different manifestations of the same disorder or whether they represent distinct clinical entities. Elucidating this clinical dilemma is important because it may improve our ability to diagnose and treat these common disorders.
At present, the ROME II committee classifies IBS as a distinctly separate functional bowel disorder from dyspepsia.[3] IBS is characterized by lower abdominal pain or discomfort in association with disordered defecation (Table 1). Dyspepsia presents as recurrent upper abdominal pain or discomfort associated with symptoms of early satiety, fullness, bloating, and nausea (Table 2). Because upper GI function regularly affects lower GI tract function (ie, the gastro-colic reflex), and lower GI function routinely affects upper GI function (ie, constipation slows gastric emptying), it should not be surprising that these 2 areas are intimately linked.[4]
Table 1.
Rome II Criteria for Irritable Bowel Syndrome[3]
| At least 12 weeks in the past 12 months (which need not be consecutive) of abdominal pain or discomfort, with 2 of the 3 following symptoms: |
| Relieved with defecation |
| And/or change in the frequency of stool |
| And/or change in the form of stool |
| Symptoms that cumulatively support the diagnosis of IBS include: |
| Abnormal frequency of stools (> 3/day or < 3/week) |
| Abnormal form |
| Abnormal passage |
| Presence of mucus |
| Bloating with distension |
Table 2.
Rome II Criteria for Functional Dyspepsia[3]
| At least 12 weeks out of the last 12 months (which need not be consecutive), of abdominal pain or discomfort centered in the upper abdomen, for which an organic process cannot be identified. |
| Exclusion criteria include: |
| Patients with abdominal pain or discomfort relieved with defecation (ie, those patients with IBS) |
| Patients with predominant heartburn symptoms |
This article reviews the prevalence, natural history, etiology, pathogenesis, and treatment of these 2 common FGIDs, and discusses whether these disorders are different manifestations of the same disorder or whether they are truly distinct clinical entities.
Incidence and Prevalence
A number of different research studies have found that the prevalence and incidence of IBS and dyspepsia are remarkably similar. Prevalence rates of IBS range from 10% to 20%.[5] Due to the fluctuating nature of IBS symptoms, it should not be surprising that these figures vary. A recent study from the United States found the incidence of IBS to be 2%.[6] However, it is likely that this number underestimates the true incidence of IBS, because a majority of individuals who meet the criteria for IBS do not seek medical attention.
Dyspepsia is also commonly encountered in medical practice. Population studies from the United States and Europe have shown that nearly 20% to 25% of adults suffer from dyspeptic symptoms, numbers similar to those reported for IBS.[7–9] Similar to many patients with IBS symptoms, dyspeptic patients usually do not seek professional medical care.[10] However, once investigated, the majority of patients with dyspeptic symptoms are diagnosed with functional or nonulcer dyspepsia (FD). The prevalence of FD is approximately 12% to 15%, with an incidence of 2% to 5%; these rates are similar to those reported for IBS.[11,12]
Given the similar data on incidence and prevalence, it is not surprising that dyspeptic symptoms commonly occur in patients diagnosed with IBS. At least 40% of patients presenting to gastroenterologists with a functional gut disorder have considerable symptom overlap between IBS and FD,[13,14] and this overlap is also found in primary care clinics.[15]
Natural History of Dyspepsia and IBS
IBS is recognized as a chronic disorder for many patients. Symptoms may wax and wane, but Talley and colleagues[12] found that over the course of 2 years, nearly 70% of patients continued to be symptomatic, whereas Kay and colleagues[16] noted that as few as 5% of IBS patients were symptom-free after 5 years. Symptom crossover from one type of functional disorder to another is also very common. In one survey of patients with IBS, 87% developed symptoms of dyspepsia, whereas in another study, over 50% of dyspeptic patients developed symptoms of IBS over 5–7 years.[13,17]
Patients with FD also suffer chronically, as only 30% to 50% of patients report improvement or resolution of their symptoms over a 5-year follow-up period.[17,18] Other reports are even less encouraging. Talley and colleagues[12] found that during an 18- to 24-month follow-up period, nearly 80% of patients remained symptomatic, while another study found that 74% of patients with dyspepsia continued to have symptoms over a 2-year follow-up period.[11]
Given the chronic nature of these disorders, the question arises as to whether repeated diagnostic testing is warranted. Recent reviews looking at both FD and IBS confirm that once the initial physical exam and history are taken and a positive diagnosis is made, performing repeated diagnostic studies (unless the patient experiences a significant change in symptoms or presents with a “red flag”) is of little value. In a recent IBS review, 6 studies evaluated the probability of a patient developing an alternative diagnosis or an organic disease after being diagnosed with IBS. Development of organic disease after the initial diagnosis was uncommon, ranging from 1.4% to 9% during follow-up, which was as long as 30 years in one study.[19]
A recent review on the prevalence of dyspepsia noted similar but less consistent results regarding the durability of diagnosis.[20] In summary, both FD and IBS are chronic diseases for a majority of patients. There is no evidence to support continued and extensive diagnostic testing in either population once the initial positive diagnosis is made, unless warning signs or new symptoms develop.
Etiology
Major strides in our understanding of the etiology of IBS and FD have taken place during the last decade. Although the precise etiology of these disorders remains unknown, there is a growing body of evidence to support genetic, infectious, psychosocial, and dietary factors as important contributors to the development of these FGIDs.
Genetics
Both clinical and observational evidence support a genetic contribution to the development of IBS. For example, IBS appears to run in families, as children of parents with IBS are more likely than controls to present to the pediatrician with abdominal pain and diarrhea. However, the relative contribution of hereditary factors and social learning is unknown, and both likely play a part.[21] Twin studies have revealed concordance rates for functional bowel disorders of 33% for monozygotic twins and 13% for dizygotic twins.[22] Concordance rates for IBS were found to be 18% for monozygotic twins and 8% for dizygotic twins; however, a higher concordance rate between mother and child was found when compared with monozygotic twins – again, strongly suggesting a social learning component in the development of IBS.[23] Locke and colleagues[24] found that the presence of IBS and dyspepsia in an individual was significantly associated with reporting abdominal symptoms and bowel problems in first-degree relatives, suggesting a familial link.[24] These findings support the view that there is a genetic basis to the development of IBS, although genetics alone is not an absolute “guarantee” that IBS will occur.[25] Whereas no specific gene has been identified in association with IBS, a recent study found that polymorphisms in the promoter region of the serotonin reuptake transporter gene are significantly associated with IBS with diarrhea.[26]
Similar to IBS, observations that FD runs in families suggest a genetic basis to the development of this disease.[24] A case-control study showed that individuals homozygous for a G protein beta-3 subunit gene polymorphism were more likely to have unexplained upper abdominal symptoms and FD.[27] Although the previously mentioned studies do not establish causality and are limited in number, we fully expect that future studies will demonstrate that genetics play a role in the etiology and pathogenesis of FGIDs.
Infections
In 1962, Chaudhary and Truelove[28] first suggested that an infection could lead to the development of IBS, on the basis of their findings that 26% of patients with “irritable colon” had a history of acute gastroenteritis due to amebic or bacillary dysentery. Both retrospective and prospective observational studies[29–31] have shown that approximately 30% of individuals with acute gastroenteritis later develop symptoms of IBS. Unfortunately, these studies were not adequately controlled, thus limiting their validity. Subsequent data have more firmly established the role of infectious gastroenteritis in the development of IBS.[32,33] A recent study followed 295 patients recovering from documented bacillary dysentery (predominantly due to infection with Shigella species) and compared these patients with a control group of family members. A significantly higher proportion of infected patients developed functional bowel disease (22.4%) and IBS (8.1%) compared with controls (7.4% and 0.8%, respectively) – thus identifying bacillary dysentery as a possible etiologic factor.[34] Finally, Lin and colleagues[35] strongly support the theory that intestinal bacterial overgrowth is responsible for symptom generation in patients with IBS. However, their thought-provoking results have not yet been replicated by other research groups.
Evidence for an infectious cause of FD is more limited. Tack and colleagues[36] described a large group of patients with dyspeptic symptoms who had a prior infectious illness. Although interesting, this study was retrospective in nature and the identity of the underlying infection remains unknown. Infection with Helicobacter pylori has been associated with impaired gastric accommodation, a physiologic finding common in patients with FD.[37] In contrast to this single study, several large, well-controlled studies have disproved any significant relationship between H pylori and FD. Sarnelli and colleagues[38] found that H pylori was not associated with FD with respect to symptoms, rates of gastric emptying, degree of gastric relaxation, or sensitivity to gastric distension. A recent meta-analysis of existing epidemiologic evidence also could not support a link between H pylori and FD.[39] Additionally, several studies have shown no symptomatic benefit to eradicating H pylori in patients with FD.[40,41] In contrast, a recent Cochrane database meta-analysis examining nonulcer dyspepsia concluded that elimination of the bacteria led to a small but statistically significant improvement in dyspeptic symptoms,[42] highlighting the continued controversy surrounding the contribution of H pylori to FD.
In summary, there is convincing evidence that a prior infectious illness (ie, gastroenteritis) may predispose a patient to develop IBS, and it is likely that a similar process occurs in patients with FD. Exposure to a bacterial or viral infection with its attendant inflammation can alter function of the enteric nervous system. Animal models of postinfectious gastroenteritis have demonstrated significant neuromuscular dysfunction and hyperexcitability of sensory neurons, which may explain why many patients develop new dyspeptic and/or IBS symptoms after an infectious illness.[43,44]
Psychosocial
Patients referred to a physician for evaluation of their IBS symptoms have an increased prevalence of depression, anxiety, somatization, history of abuse, and stress when compared with the general population.[45–48] Stress can induce GI dysfunction in healthy individuals, accelerating colonic transit[49] but decreasing gastric emptying.[50] In both IBS patients and healthy individuals, stress and altered emotional states can decrease rectal sensory pain thresholds.[51,52] Gwee and colleagues[31] demonstrated an increased incidence of postinfectious IBS in patients who have comorbid psychiatric illnesses at the time of infection.
Similar to patients with IBS, individuals with FD have a higher rate of psychiatric comorbidities, such as somatization[53] and anxiety, with the latter being significantly correlated with antral retention.[54] A recent nested case-control study found that FGIDs were more likely to be reported by individuals with psychological distress, and that somatization, interpersonal sensitivity, and total life event stress were independently associated with both IBS and FD.[55]
Although fewer studies are available comparing the link between psychosocial factors and FD (as opposed to IBS), the cumulative evidence to date suggests that stress, anxiety, and other psychiatric comorbidities play a role in the development of IBS and FD (Figure).
Figure 1.
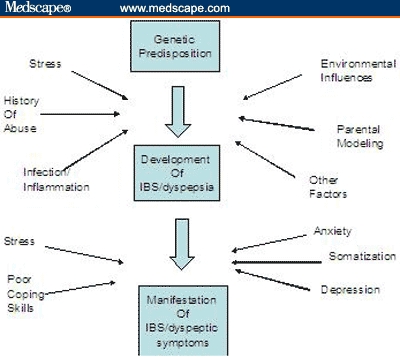
Proposed pathway for the development of IBS and FD.
Diet
A majority of patients with IBS and dyspepsia cite diet as an exacerbating factor for chronic symptoms. Although exclusion diets and testing for food allergies using IgE antibodies have been conflicting and disappointing, a recent study raises the intriguing issue of whether some patients with IBS develop symptoms because of an inflammatory reaction to food. Atkinson and colleagues[56] found that patients who fully complied by excluding all foods to which they had elevated IgG antibodies had a 26% greater reduction in symptom scores when compared with individuals on a sham diet.
A high-fat diet may induce dyspeptic symptoms in some patients, whereas avoiding fat intake may minimize symptom production in others.[57] Animal models have shown that high-fat diets alter gastric emptying[58] and secretion of cholecystokinin (CCK).[59] In patients with dyspepsia, lipid infusion into the duodenum induces relaxation of the proximal stomach and increased sensitivity to distension.[60] This response can be blocked by administering lipase inhibitors or CCK antagonists.[61] Additionally, a number of recent studies have discovered that intolerance to certain foods is more common in patients with FD when compared with healthy controls.[62]
The limited evidence to date suggests that dietary factors play a role in the exacerbation of some symptoms of both IBS and FD. Future research in this area is much needed.
Pathophysiology
No single pathophysiologic mechanism is entirely responsible for the generation of the multiple symptoms of IBS or FD. Current evidence suggests a heterogeneous model involving disordered motility, peripheral and central hypersensitivity, and autonomic dysfunction. These concepts are reviewed briefly below.
Motility
Some patients with IBS have abnormal motor patterns in the small bowel consisting of reduced migrating motor complexes, clustered contractions, and prolonged propagated contractions.[63–66] Abdominal pain may occur as a result of increased colonic responsiveness during exposure to high-fat meals and CCK infusion.[64] Additionally, accelerated transit of stool in the proximal colon is common in patients with IBS with diarrhea, whereas patients with IBS and constipation have delayed colonic transit.[67] However, many IBS patients do not demonstrate disordered motility, while some healthy volunteers are found to have disordered motility (ie, clustered contractions), thus suggesting that motility is not the sole pathophysiologic mechanism underlying IBS.
Several studies have evaluated the contribution of abnormal motility to the generation of symptoms in patients with FD. Thirty percent to 40% of individuals with FD have delayed gastric emptying.[68,69] As is the case with IBS, however, the relationship between symptoms and altered motility is unclear. Early evidence found no support for delayed gastric emptying as a causal factor for symptom generation.[70] A more recent larger study found that the prevalence and severity of symptoms were no different in dyspeptic patients with and without delayed gastric emptying, and symptom patterns were not a predictor of the presence or absence of delayed gastric emptying.[71] In contrast, Sarnelli and colleagues[72] reported that delayed gastric emptying of solids is related to postprandial fullness, nausea, and vomiting. A recent study found that gastric emptying was delayed in patients with overlapping IBS and dyspepsia symptoms, whereas patients with IBS alone had emptying rates similar to those of healthy controls.[73]
Disruptions in normal gastric rhythm and myoelectric activity have been implicated in the generation of dyspeptic symptoms, with one study demonstrating that nearly one third of patients with FD had abnormal electrical activity.[74] Although abnormal electrogastrography has been associated with delayed gastric emptying in one study,[75] no study has yet been able to causally link dyspeptic symptoms and abnormal gastric electrical activity.
Abnormal gastric accommodation likely contributes to dyspeptic symptoms. In some patients with FD, there is rapid distribution of ingested food from the fundus to the distal stomach, reflecting either impaired proximal accommodation or enhanced distal accommodation.[76] Gastric barostat studies have shown impaired postprandial relaxation of the proximal stomach in nearly 40% of patients with FD.[77] Similar to IBS, there are conflicting results as to the relationship between symptom generation in FD and altered motility. As an example, the previously cited study[77] found that delayed gastric emptying was associated with early satiety and a history of weight loss in this subset of patients. However, another study[78] contradicted this finding by reporting no variation in upper GI symptoms among patients as based on gastric accommodation or gastric emptying results.
In summary, although delayed gastric emptying, gastric dysrhythmias, and altered accommodation may be found in patients with FD, no single mechanism is solely responsible for the generation of dyspeptic symptoms, and there is very little evidence to relate symptoms to these underlying pathophysiologic mechanisms.
Visceral Hypersensitivity
Visceral hypersensitivity is a well-recognized pathophysiologic mechanism in both IBS and FD. Heightened sensitivity may occur via stimulation of intrinsic mechanoreceptors, extrinsic peripheral afferents, the central nervous system, or a combination of these areas. During balloon distension of the rectum, patients with IBS are more likely to report pain when compared with healthy controls.[79,80] Altered rectal perception may thus serve as a reliable biologic marker for IBS, given the results of a recent study demonstrating that the sensitivity of the rectal barostat in differentiating IBS from non-IBS patients was 95.5%, with a specificity of 78% and a negative predictive value of 90.2%.[81] Factors believed to be responsible for sensitizing and modulating responses at the mechanoreceptor level include inflammation and activation of the mucosal immune system from a previous infection, stress, or subclinical inflammatory bowel disease.[82–84]
Similarly, patients with FD who undergo intragastric balloon distension experience symptoms of discomfort or pain at lower levels than do healthy volunteers.[85,86] Tack and colleagues[85] found that this hypersensitivity was associated with symptoms of postprandial pain, belching, and weight loss, whereas Boeckxstaens and colleagues[86] found no correlation. Preliminary studies suggest that symptom severity is related to postprandial gastric sensitivity, but not to fasting gastric sensitivity.[87] A recent gastric barostat study found that patients who had both FD and IBS had a lower threshold for first perception and for discomfort to gastric distension compared with healthy volunteers, suggesting that these 2 disorders may exist along a continuum rather than as separate entities.[88]
Although visceral hypersensitivity is important in the generation of both FD and IBS symptoms, the location of increased visceral sensitivity does not correlate with the location of symptoms (upper vs lower). Additionally, although highly prevalent, increased visceral sensation is not found in all FD or IBS patients and thus cannot be the only pathophysiologic mechanism. Therefore, further studies are needed to evaluate sensitivity to other factors, such as gastric acid. For example, Schwarz and colleagues[89] found that patients with FD are more sensitive to duodenal acid infusion than are controls, and this sensitivity may alter motor responses by decreasing antral motility.
Central Modulation
The role of the central nervous system in the modulation of visceral hypersensitivity has received significant attention of late. In healthy volunteers, activation of the anterior cingulate cortex (ACC) occurs when a painful rectal stimulus is anticipated and either delivered or simulated. In patients with IBS exposed to a similar stimulus, patterns of activation demonstrated by positron emission tomography or magnetic resonance imaging differ when compared with healthy controls. However, this exact pattern remains debated, because one study found increased ACC activation,[90] whereas a second found decreased ACC activation with aberrant conduction to the left dorsolateral prefrontal cortex.[91]
Patients with IBS demonstrate hypervigilance and increased levels of attention,[92] and both IBS and FD patients generally exhibit a higher level of anxiety and stress compared with healthy individuals. The influence of these traits on central processing of pain has yet to be determined. It is interesting that in a study performed by Poitras and colleagues,[93] psychotherapy was clinically effective in improving symptoms of IBS, although rectal sensory thresholds remained unchanged. Additionally, it was shown that patients with FD have decreased recognition of sensation in the stomach during distraction and increased recognition during periods of attention.[94]
Autonomic System Dysfunction
The role of autonomic dysregulation in functional bowel disorders is currently under investigation. A simple model is that decreased parasympathetic outflow or increased sympathetic activity is associated with a slowing of gastrointestinal motility, while increased parasympathetic outflow or decreased sympathetic activity is associated with increased gastrointestinal motility. This model is supported by several different studies. Aggarwal and colleagues[95] published data suggesting the presence of autonomic dysfunction in patients with IBS, with IBS with diarrhea associated with adrenergic abnormalities, and IBS with constipation associated with cholinergic abnormalities. This finding was supported by a study from China that found cholinergic function measured by R-R internal variation on ECG to be significantly lower in patients with IBS with constipation compared with controls and patients with IBS with diarrhea.[96] Heitkemper and colleagues[97] looked at autonomic nervous system balance (essentially a measure of sympathetic to parasympathetic activity) in patients with and without IBS. With the analysis restricted to those patients with severe IBS symptoms, autonomic nervous system balance was higher in the IBS-with-constipation group, and measures of parasympathetic tone were lower in the constipation group when compared with patients with IBS with diarrhea.
The contribution of autonomic dysfunction to FD has not been clearly delineated. In a small study of patients with FD, sympathetic and parasympathetic parameters were assessed using 24-hour heart rate variability and cardiovascular reflex tests.[98] Vagal activity was found to be decreased in patients with FD when compared with controls. These findings were supported by Hausken and colleagues,[99] who demonstrated decreased vagal tone and increased antral dysmotility in patients with FD compared with healthy controls. A more recent study found that although patients with FD had higher sympathetic and parasympathetic scores, these were not associated with either visceral hypersensitivity or delayed gastric emptying; the study authors concluded that autonomic dysfunction may not be a significant pathophysiologic contributor to symptom generation in FD.[100]
Role of Serotonin
Serotonin (5-hydroxytryptamine or 5-HT) plays a crucial role in gastrointestinal peristalsis, intestinal secretory activity, and visceral sensory signaling; it is also an essential link between the central nervous system and the enteric nervous system via the parasympathetic and sympathetic afferent and efferent nerves.[101] Stimulation of the lumen of the GI tract causes enterochromaffin cells to release 5-HT. Serotonin receptors on afferent nerve terminals in the enteric nervous system, as well as in peripheral and central locations, modulate intestinal and extraintestinal responses.[102] Recent studies in this developing area have suggested altered 5-HT signaling as a possible pathophysiologic mechanism in several gut disorders, including both IBS with diarrhea and IBS with constipation. It has been suggested that excessive levels of 5-HT may be present in patients with IBS with diarrhea, because activation of 5-HT3 and 5-HT4 receptors stimulates GI transit,[101,103] and excess 5-HT may induce diarrhea, nausea, and vomiting.[104] Altered levels of 5-HT could result from exaggerated synthesis, excessive release, or inadequate uptake and inactivation. For example, altered levels of enterochromaffin cells have been found in patients with IBS.[105,106] However, the relationship between the absolute number of enterochromaffin cells and specific subtypes of IBS has yet to be elucidated, as lower levels of enterochromaffin cells have been found in both IBS with constipation and IBS with diarrhea.[107] Miwa and colleagues[103] recently examined mucosal biopsy specimens from patients with IBS with diarrhea, IBS with constipation, and healthy controls, and found 5-HT concentrations to be significantly elevated in IBS patients with constipation and IBS patients with diarrhea. Additionally, alterations in the serotonin transporter, which is responsible for removing 5-HT from the interstitial space and terminating its action, have been implicated in altering gut function.[108,109] Therefore, on the basis of the evidence to date, it is clear that alterations in 5-HT signaling pathways involving motor, secretory, and visceral sensation contribute to the pathogenesis of IBS.
Response to Therapy
Functional GI disorders are difficult to treat because no single etiology for these disorders is known, and thus treatment is directed at controlling symptoms. The overriding goal of therapy is to alleviate the patient's abdominal pain and discomfort. Secondary goals include improving constipation, bloating, diarrhea, and urgency in patients with IBS, and improving bloating and upper abdominal fullness in patients with dyspepsia. Dietary changes are generally not helpful in the treatment of IBS, although eating smaller meals is often effective in preventing upper GI discomfort in patients with FD. At present, there are few approved medications for IBS patients, and there is only 1 evidenced-based analysis of IBS therapies.[110] Currently, there are no approved drugs or evidence-based recommendations for treatment of FD.
Pain is a significant issue for patients with these disorders. Pain, which is primarily attributed to visceral hypersensitivity, may also reflect inappropriate and enhanced smooth muscle contractions of the small and large bowel. Anticholinergic agents, which inhibit acetylcholine-mediated smooth muscle contractions, may be useful in some patients with IBS with diarrhea and functional abdominal pain, although there is little evidence to support their use. Contraindications to anticholinergic therapy include constipation, glaucoma, and urinary retention.[111] Tricyclic antidepressants, which have neuromodulatory and analgesic effects, are also commonly used for the treatment of pain and psychological stressors in IBS.[112] There is more evidence for the efficacy of these agents in IBS than in dyspeptic populations. However, tricyclic antidepressants can aggravate constipation and symptoms in patients with delayed gastric emptying as well.[113]
5-HT3 antagonists (ie, granisetron and ondansetron) are commonly used in the treatment of chemotherapy-induced nausea, although neither agent has been studied in dyspepsia. Alosetron, a 5-HT3 antagonist that is currently available through a restricted-access program, is effective for IBS with diarrhea, but a dose-range study in dyspepsia suggested only limited benefit in women at 0.5- and 1-mg doses.[114] Proton-pump inhibitors are commonly prescribed and have modest efficacy in dyspepsia, but have no benefit in patients with IBS. Patients receiving the most benefit from proton-pump inhibitor therapy are those who complain of “ulcer-like” dyspepsia. This group consists of patients presenting with symptoms that overlap or are mistaken for gastroesophageal reflux as well as those with gastric acid hypersensitivity.[115,116]
Tegaserod, a 5-HT4 agonist, is effective in relieving abdominal pain, bloating, and constipation in IBS patients.[110] Tegaserod is a promising candidate for the treatment of dyspepsia as well because it has been shown to increase both fasting and postprandial gastric compliance in dyspeptics. Additionally, doses of tegaserod that are higher than those approved for the treatment of IBS (6 mg thrice daily and 12 mg twice daily) improve gastric emptying in patients with dyspepsia symptoms.[117] Despite these preliminary reports, a more thorough evaluation in a large dyspeptic population is needed. Older prokinetic agents, such as cisapride and domperidone, have limited and inconclusive data to support their use in either the IBS or dyspeptic populations; in cases in which a benefit was shown, the trials examining prokinetic therapy were deemed to be of poor quality.[115,118]
Agents currently being developed for treatment of both disorders include antagonists targeting NMDA (N-methyl-D-aspartate), neurokinin, and cholecystokinin (CCK) receptors. Glutamate and aspartate are 2 excitatory amino acids involved in the generation of pain responses in the spinal cord. Release of these amino acids leads to neuronal excitability and sensitization via NMDA receptors. Blocking NMDA receptors can reduce or prevent transmission of painful stimuli in the dorsal horn of the spinal cord. Neurokinin receptors (subclasses NK1, NK2, and NK3) are involved in visceral sensitivity. Neurokinin receptor antagonists could reduce transmission in visceral sensory afferents, thereby modulating painful sensations from the GI tract. Cholecystokinin, a neuropeptide, is released by cells in the duodenum and jejunum in response to nutrient infusion. Cholecystokinin is thought to play a role in the gastrocolic reflex which is often exaggerated in patients with IBS. Blocking CCK receptors may help blunt this heightened reflex. Promising nondrug therapies include acupuncture, behavioral feedback, and gastric pacing for dyspeptic patients.[116,118]
Conclusion
Managing patients with IBS and FD can be a challenging and frustrating process for clinicians. Currently, these 2 common FGIDs are thought of as completely distinct entities. Because many patients have overlapping symptoms of IBS and dyspepsia, maintaining 2 separate diagnoses leads to separate, but often parallel, processes of evaluation and treatment. Unfortunately, this results in redundant laboratory tests, duplication of diagnostic studies, frequent office visits, and the use of multiple medications.
As discussed, IBS and FD are remarkably similar. There appears to be a genetic predisposition to both disorders, and prior infection, inflammation, stress, and a history of abuse all play a critical role in the development and expression of these chronic disorders. Injury to the enteric nervous system may be the common, unifying link in the generation and expression of the typical symptoms that frequently overlap in patients with IBS and FD. Some patients will manifest the abnormal pathophysiology that develops as a result of enteric nervous system injury with primarily upper GI tract symptoms (epigastric fullness and discomfort, bloating, nausea), whereas others will manifest primarily lower GI symptoms (abdominal pain and disordered defecation).
The working hypothesis that symptom expression in IBS and FD may reflect regional expression of a similar underlying insult (ie, injury to the enteric nervous system) should help eliminate repetitive diagnostic testing, minimize office visits, and improve our ability to target therapy. Treatment options for IBS and FD should be directed at modulating both peripheral and central pain mechanisms, with the goal of improving symptoms, improving quality of life, and minimizing the economic burden to society.
Contributor Information
Laura Noddin, Department of Medicine, Dartmouth Medical School, Hanover, New Hampshire.
Michael Callahan, Novartis Pharmaceuticals, East Hanover, New Jersey.
Brian E. Lacy, Dartmouth Medical School, Hanover, New Hampshire; GI Motility Laboratory, Division of Gastroenterology, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire.
References
- 1.Mitchell CM, Drossman DA. Survey of the AGA membership relating to patients with functional gastrointestinal disorders. Gastroenterology. 1987;92:1282–1284. doi: 10.1016/s0016-5085(87)91099-7. [DOI] [PubMed] [Google Scholar]
- 2.Lacy BE, Rosemore J, Corbin D, Robertson D, Grau M, Crowell MD. Physicians' attitudes and practices in the evaluation and treatment of irritable bowel syndrome. Am J Gastroenterol. 2004;99:S280. doi: 10.1080/00365520600554451. [DOI] [PubMed] [Google Scholar]
- 3.Drossman DA, Corazziari E, Talley NJ, et al. Diagnosis, Pathophysiology and Treatment: A Multinational Consensus. 2nd ed. McLean, VA: Degnon Associates; 2000. Rome II. The Functional Gastrointestinal Disorders. [Google Scholar]
- 4.Tjeerdsma HC, Smout AJ, Akkermans LJ. Voluntary suppression of defecation delays gastric emptying. Dig Dis Sci. 1993;38:832–836. doi: 10.1007/BF01295908. [DOI] [PubMed] [Google Scholar]
- 5.Saito YA, Locke GR, Talley NJ, et al. A comparison of the Rome and Manning criteria for case identification in epidemiological investigations of irritable bowel syndrome. Am J Gastroenterol. 2000;95:2816–2824. doi: 10.1111/j.1572-0241.2000.03192.x. [DOI] [PubMed] [Google Scholar]
- 6.Locke GR, Yawn BP, Wollan PC, Melton LJ, Lydick E, Talley JN. Incidence of a clinical diagnosis of the irritable bowel syndrome in a United States population. Aliment Pharmacol Ther. 2004;19:1025–1031. doi: 10.1111/j.1365-2036.2004.01938.x. [DOI] [PubMed] [Google Scholar]
- 7.Knill-Jones RP. Geographical differences in the prevalence of dyspepsia. Scan J Gastroenterol Suppl. 1991;182:17–24. doi: 10.3109/00365529109109532. [DOI] [PubMed] [Google Scholar]
- 8.Westbrook JI, McIntosh JH, Talley NJ. The impact of dyspepsia definition on prevalence estimates: Considerations for future researchers. Scand J Gastroenterol. 2000;35:227–233. doi: 10.1080/003655200750024065. [DOI] [PubMed] [Google Scholar]
- 9.Westbrook JI, Talley NJ. Empiric clustering of dyspepsia into symptom subgroups: A population-based study. Scand J Gastroenterol. 2002;37:917–923. doi: 10.1080/003655202760230874. [DOI] [PubMed] [Google Scholar]
- 10.Westbrook JI, McIntosh J, Talley NJ. Factors associated with consulting medical or non-medical practitioners for dyspepsia: an Australian population based study. Aliment Pharmacol Ther. 2000;14:1581–1588. doi: 10.1046/j.1365-2036.2000.00878.x. [DOI] [PubMed] [Google Scholar]
- 11.Jones R, Lydeard S. Dyspepsia in the community: A follow-up study. Br J Clin Pract. 1992;46:95–97. [PubMed] [Google Scholar]
- 12.Talley NJ, Weaver AL, Zinsmeister AR, Melton LJD. Onset and disappearance of gastrointestinal symptoms and functional gastrointestinal disorders. Am J Epidemiol. 1992;136:165–177. doi: 10.1093/oxfordjournals.aje.a116483. [DOI] [PubMed] [Google Scholar]
- 13.Agreus L, Svardsudd K, Nyren O, Tibblin G. Irritable bowel syndrome and dyspepsia in the general population: overlap and lack of stability over time. Gastroenterology. 1995;109:671–678. doi: 10.1016/0016-5085(95)90373-9. [DOI] [PubMed] [Google Scholar]
- 14.Talley NJ, Dennis EH, Schettler-Duncan VA, Lacy BE, Olden KW, Crowell MD. Overlapping upper and lower gastrointestinal symptoms in irritable bowel syndrome patients with constipation and diarrhea. Am J Gastroenterol. 2003;98:2454–2459. doi: 10.1111/j.1572-0241.2003.07699.x. [DOI] [PubMed] [Google Scholar]
- 15.Talley NJ, Boyce P, Jones M. Identification of distinct upper and lower gastrointestinal symptom groupings in an urban population. Gut. 1998;42:690–695. doi: 10.1136/gut.42.5.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kay L, Jorgensen T, Jensen KH. The epidemiology of irritable bowel syndrome in a random population: prevalence, incidence, natural history and risk factors. J Intern Med. 1994;236:23–30. doi: 10.1111/j.1365-2796.1994.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 17.Sloth H, Jorgensen LS. Predictors for the course of chronic non-organic upper abdominal pain. Scan J Gastroenterol. 1989;24:440–444. doi: 10.3109/00365528909093072. [DOI] [PubMed] [Google Scholar]
- 18.Bonnevie O. Outcome of non-ulcer disease. Scand J Gastroenterol. 1982;S17:135–138. [PubMed] [Google Scholar]
- 19.El-Serag HB, Pilgrim P, Schoenfeld P. The natural history of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;19:861–870. doi: 10.1111/j.1365-2036.2004.01929.x. [DOI] [PubMed] [Google Scholar]
- 20.El-Serag HB, Talley NJ. The prevalence and clinical course of functional dyspepsia. Aliment Pharmacol Ther. 2004;19:643–654. doi: 10.1111/j.1365-2036.2004.01897.x. [DOI] [PubMed] [Google Scholar]
- 21.Levy RL, Whitehead WE, Von Korff MR, Feld AD, et al. Intergenerational transmission of gastrointestinal illness behavior. Am J Gastroenterol. 2000;95:451–456. doi: 10.1111/j.1572-0241.2000.01766.x. [DOI] [PubMed] [Google Scholar]
- 22.Morris-Yates A, Talley NJ, Boyce PM, et al. Evidence of a genetic contribution to functional bowel disorder. Am J Gastroenterol. 1998;93:1311–1317. doi: 10.1111/j.1572-0241.1998.440_j.x. [DOI] [PubMed] [Google Scholar]
- 23.Levy RL, Jones KR, Whitehead WE, Feld SI, Talley NJ, Corey LA. Irritable bowel syndrome in twins: heredity and social learning both contribute to etiology. Gastroenterology. 2001;121:799–804. doi: 10.1053/gast.2001.27995. [DOI] [PubMed] [Google Scholar]
- 24.Locke GR, Zinsmeister AR, Talley NJ, et al. Familial association in adults with functional gastrointestinal disorders. Mayo Clin Proc. 2000;75:907–912. doi: 10.4065/75.9.907. [DOI] [PubMed] [Google Scholar]
- 25.Lembo T, Zaman MS, Chavez NF, et al. Concordance of IBS among monozygotic and dizygotic twins. Gastroenterology. 2001;120:A636. [Google Scholar]
- 26.Yeo A, Boyd P, Lumsden S, et al. Association between a functional polymorphism in the serotonin transporter gene and diarrhea predominant irritable bowel syndrome in women. Gut. 2004;53:1452–1458. doi: 10.1136/gut.2003.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holtmann G, Siffert W, Haag S, et al. G-protein beta 3 subunit 825 cc genotype is associated with unexplained (functional) dyspepsia. Gastroenterology. 2004;126:971–979. doi: 10.1053/j.gastro.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Chaudhary NA, Truelove SC. The irritable colon syndrome. Q J Med. 1962;123:307–322. [PubMed] [Google Scholar]
- 29.McKendrick MW, Read NW. Irritable bowel syndrome - post Salmonella infection. J Infect. 1994;29:1–3. doi: 10.1016/s0163-4453(94)94871-2. [DOI] [PubMed] [Google Scholar]
- 30.Gwee KA, Graham JC, McKendrick MW, et al. Psychometric scores and persistence of irritable bowel after infectious diarrhea. Lancet. 1996;347:150–153. doi: 10.1016/s0140-6736(96)90341-4. [DOI] [PubMed] [Google Scholar]
- 31.Gwee KA, Leong YL, Graham C, et al. The role of psychological and biological factors in postinfective gut dysfunction. Gut. 1999;44:400–406. doi: 10.1136/gut.44.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parry SD, Stansfield R, Jelley D, et al. Does bacterial gastroenteritis predispose people to functional gastrointestinal disorders? A prospective, community-based, case-control study. Am J Gastroenterol. 2003;98:1970–1975. doi: 10.1111/j.1572-0241.2003.07664.x. [DOI] [PubMed] [Google Scholar]
- 34.Wang LH, Fang XC, Pan GZ. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut. 2004;53:1096–1101. doi: 10.1136/gut.2003.021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin HC. Small intestinal bacterial overgrowth: a framework for understanding irritable bowel syndrome. JAMA. 2004;292:852–858. doi: 10.1001/jama.292.7.852. [DOI] [PubMed] [Google Scholar]
- 36.Tack J, Demedts I, Dehondt G, et al. Clinical and pathophysiological characteristics of acute-onset functional dyspepsia. Gastroenterology. 2002;122:1738–1747. doi: 10.1053/gast.2002.33663. [DOI] [PubMed] [Google Scholar]
- 37.Saslow SB, Thumshin M, Camilleri M, et al. Influence of H. pylori infection on gastric motor and sensory function in asymptomatic volunteers. Dig Dis Sci. 1998;43:258–264. doi: 10.1023/a:1018833701109. [DOI] [PubMed] [Google Scholar]
- 38.Sarnelli G, Janssens J, Tack J. Helicobacter pylori is not associated with symptoms and pathophysiological mechanisms of functional dyspepsia. Dig Dis Sci. 2003;48:2229–2236. doi: 10.1023/b:ddas.0000007856.71462.6c. [DOI] [PubMed] [Google Scholar]
- 39.Danesh J, Lawrence M, Murphy M, Roberts S, Collins R. Systematic review of the epidemiological evidence on Helicobacter pylori infection and nonulcer or uninvestigated dyspepsia. Arch Intern Med. 2000;160:1192–1198. doi: 10.1001/archinte.160.8.1192. [DOI] [PubMed] [Google Scholar]
- 40.Gisbert JP, Cruzado AI, Garcia-Gravalos R, Pajares JM. Lack of benefit of treating Helicobacter pylori infection in patients with functional dyspepsia. Randomized one-year follow-up study. Hepatogastroenterology. 2004;51:303–308. [PubMed] [Google Scholar]
- 41.Talley NJ, Janssens J, Lauritsen K, Racz I, Bolling-Sternevald E. Eradication of Helicobacter pylori in functional dyspepsia: randomized double blind placebo controlled trial with 12 months' follow up. The Optimal Regimen Cures Helicobacter Induced Dyspepsia (ORCHID) Study Group. BMJ. 1999;318:833–837. doi: 10.1136/bmj.318.7187.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moayyedi P, Deeks J, Tally NJ, et al. An update of the Cochrane systematic review of Helicobacter pylori eradication therapy in nonulcer dyspepsia: resolving the discrepancy between systematic reviews. Am J Gastroenterol. 2003;98:2621–2626. doi: 10.1111/j.1572-0241.2003.08724.x. [DOI] [PubMed] [Google Scholar]
- 43.Barbara G, Vallance BA, Collins SM. Persistent neuromuscular dysfunction after acute nematode infection in mice. Gastroenterology. 1997;113:1224–1232. doi: 10.1053/gast.1997.v113.pm9322517. [DOI] [PubMed] [Google Scholar]
- 44.Beyak MJ, Vanner S. Inflammation-induced hyperexcitability of nociceptive gastrointestinal DRG neurons: the role of voltage-gated ion channels. Neurogastroenterol Motil. 2005;17:175–186. doi: 10.1111/j.1365-2982.2004.00596.x. [DOI] [PubMed] [Google Scholar]
- 45.Drossman DA, McKee DC, Sandler RS, et al. Psychosocial factors in the irritable bowel syndrome: a multivariate study of patients and nonpatients with irritable bowel syndrome. Gastroenterology. 1988;95:701–708. doi: 10.1016/s0016-5085(88)80017-9. [DOI] [PubMed] [Google Scholar]
- 46.Drossman DA, Creed FH, Olden KW, et al. Psychosocial aspects of the functional gastrointestinal disorders. Gut. 1999;45(suppl 2):II25–II30. doi: 10.1136/gut.45.2008.ii25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garankani A, Win T, Virk S, et al. Comorbidity of irritable bowel syndrome in psychiatric patients: A review. Am J Ther. 2003;10:61–67. doi: 10.1097/00045391-200301000-00014. [DOI] [PubMed] [Google Scholar]
- 48.Drossman DA, Li Z, Leserman J, et al. Health status by gastrointestinal diagnosis and abuse history. Gastroenterology. 1996;110:999–1007. doi: 10.1053/gast.1996.v110.pm8613034. [DOI] [PubMed] [Google Scholar]
- 49.Rao SS, Hatfield RA, Suls JM, Chamberlain JM. Psychological and physical stress induce differential effects on human colonic motility. Am J Gastroenterol. 1998;93:985–990. doi: 10.1111/j.1572-0241.1998.00293.x. [DOI] [PubMed] [Google Scholar]
- 50.Cann PA, Read NW, Cammack J, et al. Psychological stress and the passage of a standard meal through the stomach and small intestine in man. Gut. 1983;24:236–240. doi: 10.1136/gut.24.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ford JM, Camilleri M, Zinmeister AE, Hanson RB. Psychosensory modulation of colonic sensation in the human transverse and sigmoid colon. Gastroenterology. 1995;109:1772–1780. doi: 10.1016/0016-5085(95)90743-2. [DOI] [PubMed] [Google Scholar]
- 52.Houghton LA, Calvert EL, Jackson NA, Cooper P, Whorwell PJ. Visceral sensation and emotion: a study using hypnosis. Gut. 2002;52:701–704. doi: 10.1136/gut.51.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whitehead WE. Psychosocial aspects of functional gastrointestinal disorders. Gastroenterol Clin North Am. 1996;25:21–34. doi: 10.1016/s0889-8553(05)70363-0. [DOI] [PubMed] [Google Scholar]
- 54.Lorena SLS, Tinois E, Brunetto SQ, Camargo EE, Mesquita MA. Gastric emptying and intragastric distribution of a solid meal in functional dyspepsia, influence of gender and anxiety. J Clin Gastroenterol. 2004;38:230–236. doi: 10.1097/00004836-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 55.Locke GR, III, Weaver AL, Melton LJ, III, Talley NJ. Psychosocial factors are linked to functional gastrointestinal disorders: a population bases nested case-control study. Am J Gastroenterol. 2004;99:350–357. doi: 10.1111/j.1572-0241.2004.04043.x. [DOI] [PubMed] [Google Scholar]
- 56.Atkinson W, Sheldon TA, Shaath N, Whorwell PJ. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomized controlled trial. Gut. 2004;53:1459–1464. doi: 10.1136/gut.2003.037697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mullan A, Kavanagh P, O'Mahony P, et al. Food and nutrient intakes and eating patterns in functional and organic dyspepsia. Eur J Clin Nutr. 1994;48:97–105. [PubMed] [Google Scholar]
- 58.Covasa M, Ritter RC. Adaptation to high-fat diet reduces inhibition of gastric emptying by CCK and intestinal oleate. Am J Physiol. 2000;278:R166–R170. doi: 10.1152/ajpregu.2000.278.1.R166. [DOI] [PubMed] [Google Scholar]
- 59.Spannagel AW, Nakano I, Tawil T, et al. Adaptation to fat markedly increases pancreatic secretory response to intraduodenal fat in rats. Am J Physiol. 1996;270:G128–G135. doi: 10.1152/ajpgi.1996.270.1.G128. [DOI] [PubMed] [Google Scholar]
- 60.Barbara R, Feinle C, Read NW. Abnormal sensitivity to duodenal lipid infusion in patients with functional dyspepsia. Eur J Gastroenterol Hepatol. 1995;7:1051–1057. doi: 10.1097/00042737-199511000-00007. [DOI] [PubMed] [Google Scholar]
- 61.Feinle C, Meier O, Otto B, D'Amato M, Fried M. Role of duodenal lipid and cholecystokinin A receptors in the pathophysiology of functional dyspepsia. Gut. 2001;48:347–355. doi: 10.1136/gut.48.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feinle-Bisset C, Vozzo R, Horowitz M, Talley NJ. Diet, food intake, and disturbed physiology in the pathogenesis of symptoms in functional dyspepsia. Am J Gastroenterol. 2003;9:170–181. doi: 10.1111/j.1572-0241.2004.04003.x. [DOI] [PubMed] [Google Scholar]
- 63.Kumar D, Wingate DL. The irritable bowel syndrome: a paroxysmal motor disorder. Lancet. 1985;2:973–977. doi: 10.1016/s0140-6736(85)90525-2. [DOI] [PubMed] [Google Scholar]
- 64.Kellow JE, Phillips SF. Altered small bowel motility in irritable bowel syndrome is correlated with symptoms. Gastroenterology. 1987;92:1885–1893. doi: 10.1016/0016-5085(87)90620-2. [DOI] [PubMed] [Google Scholar]
- 65.Kellow JE, Miller LJ, Phillips SF, Haddad AC, Sinsmeister AT, Charboneau JW. Sensitivities of human jejunum, ileum, proximal colon, and gallbladder to cholecystokinin octapeptide. Am J Physiol. 1987;252(3 Part 1):G345–G356. doi: 10.1152/ajpgi.1987.252.3.G345. [DOI] [PubMed] [Google Scholar]
- 66.Camilleri M. Motor function in irritable bowel syndrome. Can J Gastroenterology. 1999;13(suppl A):8A–11A. doi: 10.1155/1999/240329. [DOI] [PubMed] [Google Scholar]
- 67.Vassallo M, Camilleri M, Phillips SF, Brown ML, Chapman NJ, Thomforde GM. Transit through the proximal colon influences stool weight in the irritable bowel syndrome. Gastroenterology. 1992;102:102–108. doi: 10.1016/0016-5085(92)91789-7. [DOI] [PubMed] [Google Scholar]
- 68.Quartero AO, de Wit NJ, Lodder AC, et al. Disturbed solid-phase gastric emptying in functional dyspepsia: a meta-analysis. Dig Dis Sci. 1998;43:2028–2033. doi: 10.1023/a:1018803129779. [DOI] [PubMed] [Google Scholar]
- 69.Stanghellini V, Tosetti C, Paternico A, et al. Risk indicators of delayed gastric emptying of solids in patients with functional dyspepsia. Gastroenterology. 1996;110:1036–1042. doi: 10.1053/gast.1996.v110.pm8612991. [DOI] [PubMed] [Google Scholar]
- 70.Talley NJ, Shuter B, McCrudden G, Jones M, Hoschl R, Piper DW. Lack of association between gastric emptying of solids and symptoms in nonulcer dyspepsia. J Clin Gastroenterol. 1989;11:625–630. doi: 10.1097/00004836-198912000-00005. [DOI] [PubMed] [Google Scholar]
- 71.Talley N, Verlinden M, Jones M. Can symptoms discriminate among those with delayed or normal gastric emptying in dysmotility like dyspepsia? Am J Gastroenterol. 2001;96:1422–8. doi: 10.1111/j.1572-0241.2001.03683.x. [DOI] [PubMed] [Google Scholar]
- 72.Sarnelli G, Caenepeel P, Geypens B, Janssens J, Tack J. Symptoms associated with impaired gastric emptying of solids and liquids in functional dyspepsia. Am J Gastroenterol. 2003;98:783–788. doi: 10.1111/j.1572-0241.2003.07389.x. [DOI] [PubMed] [Google Scholar]
- 73.Stanghellini V, Tosetti C, Barbara G, et al. Dyspeptic symptoms and gastric emptying in the irritable bowel syndrome. Am J Gastroenterol. 2002;97:2738–2743. doi: 10.1111/j.1572-0241.2002.07062.x. [DOI] [PubMed] [Google Scholar]
- 74.Leahy A, Besherdas K, Clayman C, et al. Abnormalities of the electrogastrogram in functional gastrointestinal disorders. Am J Gastroenterol. 1999;94:1023–1028. doi: 10.1111/j.1572-0241.1999.01007.x. [DOI] [PubMed] [Google Scholar]
- 75.Lin Z, Eaker E, Sarorsiek I, et al. Gastric myoelectrical activity and gastric emptying in patients with functional dyspepsia. Am J Gastroenterol. 1999;94:2384–2389. doi: 10.1111/j.1572-0241.1999.01362.x. [DOI] [PubMed] [Google Scholar]
- 76.Troncon L, Bennett R, Ahluwalia N, et al. Abnormal intragastric distribution of food during gastric emptying in functional dyspepsia patients. Gut. 1994;35:327–332. doi: 10.1136/gut.35.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tack J, Peissevaux H, Coulie B, et al. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346–1352. doi: 10.1016/s0016-5085(98)70012-5. [DOI] [PubMed] [Google Scholar]
- 78.Bredenoord AJ, Chial HJ, Camilleri M, Mullan BP, Murray JA. Gastric accommodation and emptying in evaluation of patients with upper gastrointestinal symptoms. Clin Gastroenterol Hepatol. 2003;1:264–272. [PubMed] [Google Scholar]
- 79.Ritchie J. Pain from distension of the pelvic colon by inflating a balloon in the irritable bowel syndrome. Gut. 1973;12:125–132. doi: 10.1136/gut.14.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whitehead WE, Engel BT, Schuster MM. Irritable bowel syndrome: physiological and psychological differences between diarrhea-predominant and constipation-predominant patients. Dig Dis Sci. 1980;25:404–413. doi: 10.1007/BF01395503. [DOI] [PubMed] [Google Scholar]
- 81.Bouin M, Plourde V, Boivin M, et al. Rectal distension testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122:1771–1777. doi: 10.1053/gast.2002.33601. [DOI] [PubMed] [Google Scholar]
- 82.Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–1783. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- 83.Collins SM, Piche T, Rampal P. The putative role of inflammation in the irritable bowel syndrome. Gut. 2001;49:743–745. doi: 10.1136/gut.49.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Delvaux M. Role of visceral sensitivity in the pathophysiology of irritable bowel syndrome. Gut. 2002;51(suppl 1):i67–i71. doi: 10.1136/gut.51.suppl_1.i67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tack J, Caenepeel P, Fischler B, Piessevaux H, Janssens J. Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology. 2001;121:526–535. doi: 10.1053/gast.2001.27180. [DOI] [PubMed] [Google Scholar]
- 86.Boeckxstaens GE, Hirsch DP, Kuiken SD, Heisterkamp SH, Tytgat GN. The proximal stomach and postprandial symptoms in functional dyspepsia. Am J Gastroenterol. 2002;97:40–48. doi: 10.1111/j.1572-0241.2002.05421.x. [DOI] [PubMed] [Google Scholar]
- 87.Tack J, Bisschops R, Caenepeel P, Vos R, Janssens J. Pathophysiological relevance of fasting versus postprandial sensitivity testing in functional dyspepsia. Gastroenterology. 2002;122:A301. [Google Scholar]
- 88.Corsetti M, Caenepeel P, Fischler B, Janssens J, Tack J. Impact of co-existing irritable bowel syndrome on symptoms and pathophysiological mechanisms in functional dyspepsia. Am J Gastroenterol. 2004;99:1152–1159. doi: 10.1111/j.1572-0241.2004.30040.x. [DOI] [PubMed] [Google Scholar]
- 89.Schwartz M, Samsom M, Smout A. Chemospecific alterations in duodenal perception and motor response in functional dyspepsia. Am J Gastroenterol. 2001;96:2596–2602. doi: 10.1111/j.1572-0241.2001.04103.x. [DOI] [PubMed] [Google Scholar]
- 90.Mertz H, Morgan V, Tanner G, Pickens D, Price R, Shyr Y, Kessler R. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distension. Gastroenterology. 2000;111:842–848. doi: 10.1016/s0016-5085(00)70170-3. [DOI] [PubMed] [Google Scholar]
- 91.Silverman D, Munakata JA, Ennes H, Manderlkern M, Hoh C, Mayer E. Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology. 1997;112:64–72. doi: 10.1016/s0016-5085(97)70220-8. [DOI] [PubMed] [Google Scholar]
- 92.Naliboff BD, Munakata J, Fullerton S, et al. Evidence of two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997;41:505–512. doi: 10.1136/gut.41.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Poitras P, Riberdy Poitras M, Plourde V, Boivin M, Verrier P. Evolution of visceral sensitivity in patients with irritable bowel syndrome. Dig Dis Sci. 2002;47:914–920. doi: 10.1023/a:1014729125428. [DOI] [PubMed] [Google Scholar]
- 94.Accarino AM, Azpiroz F, Malagelada JR. Attention and distraction: effects on gut perception. Gastroenterology. 1997;113:415–422. doi: 10.1053/gast.1997.v113.pm9247458. [DOI] [PubMed] [Google Scholar]
- 95.Aggarwal A, Cutts TF, Abell TL, et al. Predominant symptoms in irritable bowel syndrome correlate with specific autonomic nervous system abnormalities. Gastroenterology. 1994;106:945–950. doi: 10.1016/0016-5085(94)90753-6. [DOI] [PubMed] [Google Scholar]
- 96.Lee CT, Chuang TY, Lu CL, Chen CY, Chang FY, Lee SD. Abnormal vagal cholinergic function and psychological behaviors in irritable bowel syndrome patients: a hospital-based Oriental study. Dig Dis Sci. 1998;43:1794–1799. doi: 10.1023/a:1018848122993. [DOI] [PubMed] [Google Scholar]
- 97.Heitkemper M, Jarrett M, Cain KC, et al. Autonomic nervous system function in women with irritable bowel syndrome. Dig Dis Sci. 2001;46:1276–1284. doi: 10.1023/a:1010671514618. [DOI] [PubMed] [Google Scholar]
- 98.Silva L, de O, Souza A, Mesquita MA. Autonomic function in patients with functional dyspepsia assessed by 24-hour heart rate variability. Dig Dis Sci. 2002;47:27–31. doi: 10.1023/a:1013246900041. [DOI] [PubMed] [Google Scholar]
- 99.Hausken T, Svebak S, Wilhelmsen I, et al. Low vagal tone and antral dysmotility in patients with functional dyspepsia. Psychosom Med. 1993;55:12–22. doi: 10.1097/00006842-199301000-00004. [DOI] [PubMed] [Google Scholar]
- 100.Park DI, Rhee PL, Kim YH, et al. Role of autonomic dysfunction in patients with functional dyspepsia. Dig Liver Dis. 2001;33:464–471. doi: 10.1016/s1590-8658(01)80023-2. [DOI] [PubMed] [Google Scholar]
- 101.Crowell MD. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br J Pharmacol. 2004;141:1285–1293. doi: 10.1038/sj.bjp.0705762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gershon MD. Roles played by 5-hydroxytrptamine in the physiology of the bowel. Ailment Pharmacol Ther. 1999;13(suppl 2):15–30. [PubMed] [Google Scholar]
- 103.Miwa J, Echizen H, Matsueda K, Umeda N. Patients with constipation-predominant irritable bowel syndrome (IBS) may have elevated serotonin concentrations in colonic mucosa as compared with diarrhea-predominant patients and subjects with normal bowel habits. Digestion. 2001;63:188–194. doi: 10.1159/000051888. [DOI] [PubMed] [Google Scholar]
- 104.Yeo A, Boyd P, Lumsden S, et al. Association between a functional polymorphism in the serotonin transporter gene and diarrhea predominant irritable bowel syndrome in women. Gut. 2004;53:1452–1458. doi: 10.1136/gut.2003.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Spiller RC, Jenkin D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in postdysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moses PL, Bannon C, Linden DR, Crowell MD, Sharkey KA, Mawe GM. Evidence for altered serotonin signaling in IBD and constipation predominant IBS. Am J Gastroenterol. 2002;97:S274. [Google Scholar]
- 107.Zhao R, Baig MK, Wexner SD, et al. Enterochromaffin and serotonin cells are abnormal for patients with colonic inertia. Dis Colon Rectum. 2000;43:858–863. doi: 10.1007/BF02238027. [DOI] [PubMed] [Google Scholar]
- 108.Pata CEM. Serotonin transporter gene polymorphism in irritable bowel syndrome. Am J Gastroenterol. 2002;97:1780–1784. doi: 10.1111/j.1572-0241.2002.05841.x. [DOI] [PubMed] [Google Scholar]
- 109.Camilleri M, Atanasova E, Carlson PJ, et al. Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2002;123:425–432. doi: 10.1053/gast.2002.34780. [DOI] [PubMed] [Google Scholar]
- 110.American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Evidence-Based Position on the Management of Irritable Bowel Syndrome in North America. Am J Gastroenterol. 2002;11(suppl):S1–S5. doi: 10.1016/s0002-9270(02)05656-3. [DOI] [PubMed] [Google Scholar]
- 111.Rosemore JG, Lacy BE. Irritable bowel syndrome: Basis of clinical management strategies. J Clin Gastroenterol. 2002;35(suppl):S37–S44. doi: 10.1097/00004836-200207001-00008. [DOI] [PubMed] [Google Scholar]
- 112.Wald A. Psychotropic agents in irritable bowel syndrome. J Clin Gastroenterol. 2002;35(suppl):S53–S57. doi: 10.1097/00004836-200207001-00010. [DOI] [PubMed] [Google Scholar]
- 113.Cremonini F, Talley NJ. Review article: the overlap between dyspepsia and irritable bowel syndrome – a tale of one or two disorders? Aliment Pharmacol Ther. 2004;20(suppl 7):40–49. doi: 10.1111/j.1365-2036.2004.02184.x. [DOI] [PubMed] [Google Scholar]
- 114.Talley NJ, Van Zanten SV, Saez LR, et al. A dose-ranging, placebo-controlled, randomized trial of Alosetron in patients with functional dyspepsia. Aliment Pharmacol Ther. 2001;15:525–537. doi: 10.1046/j.1365-2036.2001.00941.x. [DOI] [PubMed] [Google Scholar]
- 115.Abraham NS, Moayyedi P, Daniels B, Veldhuyzen Van Zantan SJO. The methodological quality of trials affects estimates of treatment efficacy in functional (non-ulcer) dyspepsia. Aliment Pharmacol Ther. 2004;19:631–641. doi: 10.1111/j.1365-2036.2004.01878.x. [DOI] [PubMed] [Google Scholar]
- 116.Cremonini F, Delgado-Aros S, Talley NJ. Functional dyspepsia: drugs for new (and old) therapeutic targets. Best Pract Res Clin Gastroenterol Hepatol. 2004;18:717–733. doi: 10.1016/j.bpg.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 117.Tougas G, Chen Y, Luo D, Salter J, D'Elia T, Earnest DL. Tegaserod improves gastric emptying in patients with gastroparesis and dyspeptic symptoms. Gastroenterology. 2003;124(suppl 1):A432. [Google Scholar]
- 118.Callahan MJ. IBS Neuropharmacology: A review of approved and investigational compounds. J Clin Gastroenterol. 2002;35(suppl):S58–S67. doi: 10.1097/00004836-200207001-00011. [DOI] [PubMed] [Google Scholar]


