Abstract
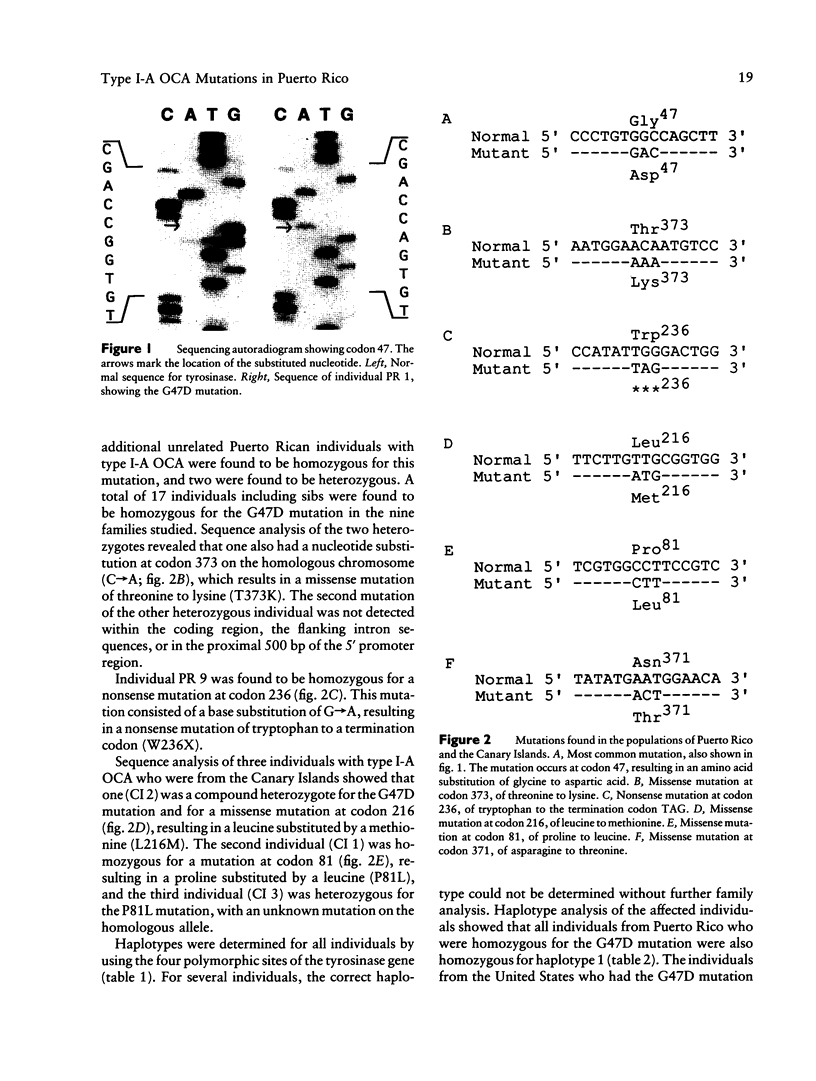
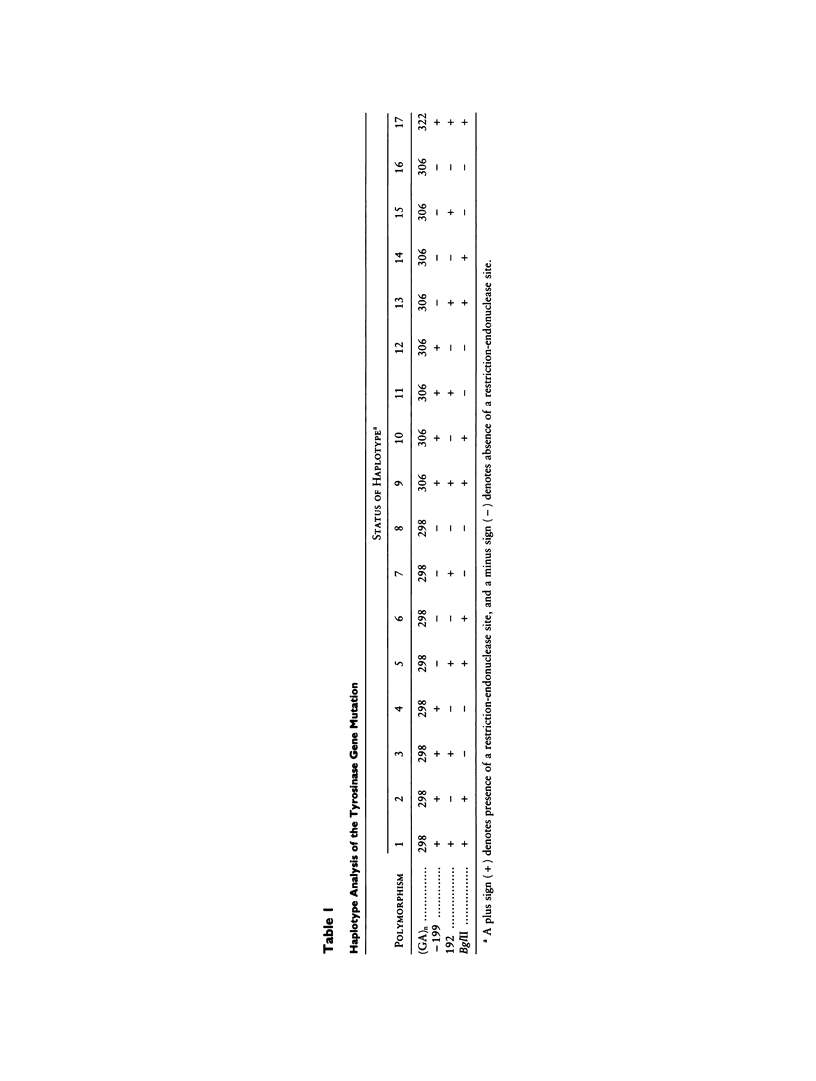
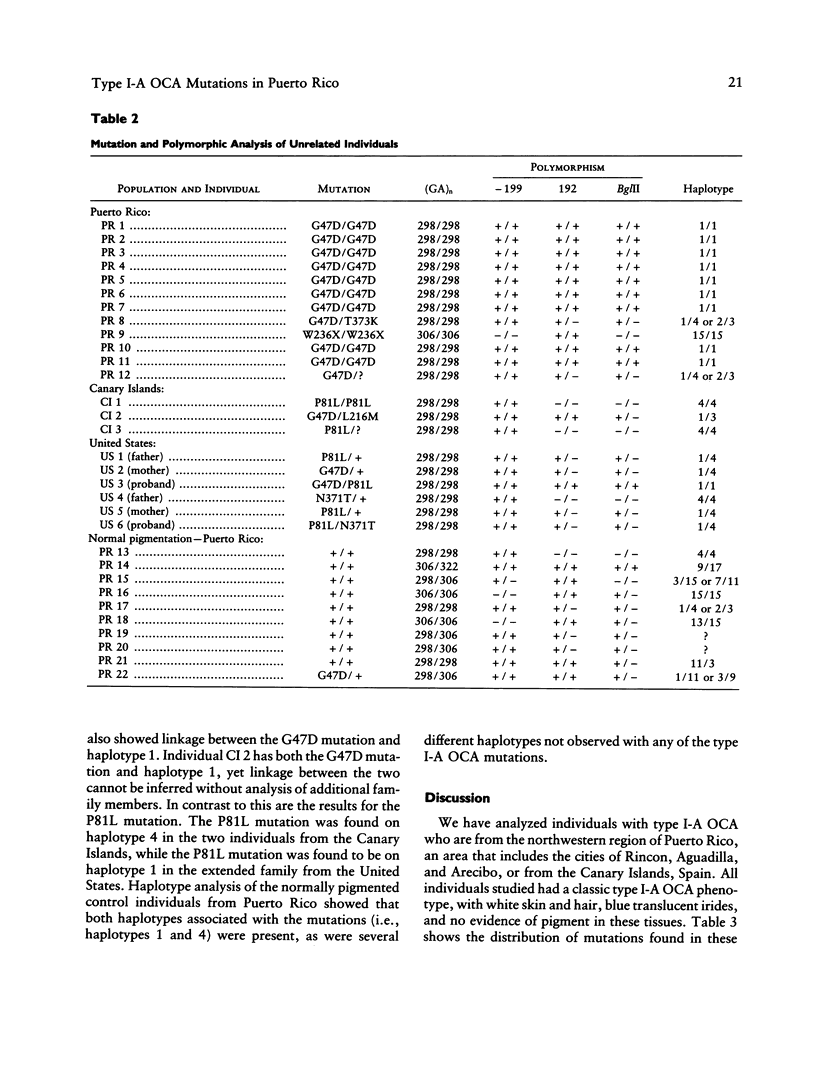
We have determined the mutations in the tyrosinase gene from 12 unrelated Puerto Rican individuals who have type I-A (tyrosinase-negative) oculocutaneous albinism (OCA). All but one individual are of Hispanic descent. Nine individuals were homozygous for a missense mutation (G47D) in exon I at codon 47. Two individuals were heterozygous for the G47D mutation, with one having a missense mutation at codon 373 (T373K) in the homologous allele and the other having an undetermined mutation in the homologous allele. One individual with negroid features was homozygous for a nonsense mutation (W236X). The population migration between Puerto Rico and the Canary Islands is well recognized. Analysis of three individuals with OCA from the Canary Islands showed that one was a compound heterozygote for the G47D mutation and for a novel missense mutation (L216M), one was homozygous for a missense mutation (P81L), and one was heterozygous for the missense mutation P81L. The G47D and P81L missense mutations have been previously described in extended families in the United States. Haplotypes were determined using four polymorphisms linked to the tyrosinase locus. Haplotype analysis showed that the G47D mutation occurred on a single haplotype, consistent with a common founder for all individuals having this mutation. Two different haplotypes were found associated with the P81L mutation, suggesting that this may be either a recurring mutation for the tyrosinase gene or a recombination between haplotypes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Giebel L. B., Musarella M. A., Spritz R. A. A nonsense mutation in the tyrosinase gene of Afghan patients with tyrosinase negative (type IA) oculocutaneous albinism. J Med Genet. 1991 Jul;28(7):464–467. doi: 10.1136/jmg.28.7.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebel L. B., Spritz R. A. RFLP for MboI in the human tyrosinase (TYR) gene detected by PCR. Nucleic Acids Res. 1990 May 25;18(10):3103–3103. doi: 10.1093/nar/18.10.3103-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebel L. B., Strunk K. M., King R. A., Hanifin J. M., Spritz R. A. A frequent tyrosinase gene mutation in classic, tyrosinase-negative (type IA) oculocutaneous albinism. Proc Natl Acad Sci U S A. 1990 May;87(9):3255–3258. doi: 10.1073/pnas.87.9.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebel L. B., Tripathi R. K., King R. A., Spritz R. A. A tyrosinase gene missense mutation in temperature-sensitive type I oculocutaneous albinism. A human homologue to the Siamese cat and the Himalayan mouse. J Clin Invest. 1991 Mar;87(3):1119–1122. doi: 10.1172/JCI115075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebel L. B., Tripathi R. K., Strunk K. M., Hanifin J. M., Jackson C. E., King R. A., Spritz R. A. Tyrosinase gene mutations associated with type IB ("yellow") oculocutaneous albinism. Am J Hum Genet. 1991 Jun;48(6):1159–1167. [PMC free article] [PubMed] [Google Scholar]
- Johnston J. D., Winder A. F., Breimer L. H. An MboI polymorphism at codon 192 of the human tyrosinase gene is present in Asians and Afrocaribbeans. Nucleic Acids Res. 1992 Mar 25;20(6):1433–1433. doi: 10.1093/nar/20.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi H., Hara S., Ishiguro S., Tamai M., Watanabe M. Detection of point mutation in the tyrosinase gene of a Japanese albino patient by a direct sequencing of amplified DNA. Hum Genet. 1990 Jun;85(1):123–124. doi: 10.1007/BF00276337. [DOI] [PubMed] [Google Scholar]
- King R. A., Mentink M. M., Oetting W. S. Non-random distribution of missense mutations within the human tyrosinase gene in type I (tyrosinase-related) oculocutaneous albinism. Mol Biol Med. 1991 Feb;8(1):19–29. [PubMed] [Google Scholar]
- King R. A., Summers C. G. Albinism. Dermatol Clin. 1988 Apr;6(2):217–228. [PubMed] [Google Scholar]
- Kwon B. S., Haq A. K., Pomerantz S. H., Halaban R. Isolation and sequence of a cDNA clone for human tyrosinase that maps at the mouse c-albino locus. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7473–7477. doi: 10.1073/pnas.84.21.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LERNER A. B., FITZPATRICK T. B. Biochemistry of melanin formation. Physiol Rev. 1950 Jan;30(1):91–126. doi: 10.1152/physrev.1950.30.1.91. [DOI] [PubMed] [Google Scholar]
- Morris S. W., Muir W., St Clair D. Dinucleotide repeat polymorphism at the human tyrosinase gene. Nucleic Acids Res. 1991 Dec 25;19(24):6968–6968. doi: 10.1093/nar/19.24.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oetting W. S., Handoko H. Y., Mentink M. M., Paller A. S., White J. G., King R. A. Molecular analysis of an extended family with type IA (tyrosinase-negative) oculocutaneous albinism. J Invest Dermatol. 1991 Jul;97(1):15–19. doi: 10.1111/1523-1747.ep12477808. [DOI] [PubMed] [Google Scholar]
- Oetting W. S., Mentink M. M., Summers C. G., Lewis R. A., White J. G., King R. A. Three different frameshift mutations of the tyrosinase gene in type IA oculocutaneous albinism. Am J Hum Genet. 1991 Jul;49(1):199–206. [PMC free article] [PubMed] [Google Scholar]
- Oetting W. S., Roed C. M., Mentink M. M., King R. A. PCR detection of a TaqI polymorphism at the CCAATT box of the human tyrosinase (TYR) gene. Nucleic Acids Res. 1991 Oct 25;19(20):5800–5800. doi: 10.1093/nar/19.20.5800-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spritz R. A., Strunk K. M., Giebel L. B., King R. A. Detection of mutations in the tyrosinase gene in a patient with type IA oculocutaneous albinism. N Engl J Med. 1990 Jun 14;322(24):1724–1728. doi: 10.1056/NEJM199006143222407. [DOI] [PubMed] [Google Scholar]
- Spritz R. A., Strunk K. M. RFLP for BgIII at the human tyrosinase (TYR) locus. Nucleic Acids Res. 1990 Jun 25;18(12):3672–3672. doi: 10.1093/nar/18.12.3672-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A., Tomita Y., Matsunaga J., Tagami H., Shibahara S. Molecular basis of tyrosinase-negative oculocutaneous albinism. A single base mutation in the tyrosinase gene causing arginine to glutamine substitution at position 59. J Biol Chem. 1990 Oct 15;265(29):17792–17797. [PubMed] [Google Scholar]
- Tomita Y., Takeda A., Okinaga S., Tagami H., Shibahara S. Human oculocutaneous albinism caused by single base insertion in the tyrosinase gene. Biochem Biophys Res Commun. 1989 Nov 15;164(3):990–996. doi: 10.1016/0006-291x(89)91767-1. [DOI] [PubMed] [Google Scholar]
- Witkop C. J., Nuñez Babcock M., Rao G. H., Gaudier F., Summers C. G., Shanahan F., Harmon K. R., Townsend D., Sedano H. O., King R. A. Albinism and Hermansky-Pudlak syndrome in Puerto Rico. Bol Asoc Med P R. 1990 Aug;82(8):333–339. [PubMed] [Google Scholar]



