Abstract
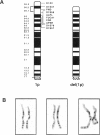
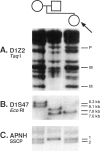
We describe a child with dysmorphic features, as well as developmental and growth delay, who developed neuroblastoma at 5 mo of age. Cytogenetic analysis of blood lymphocytes revealed an interstitial deletion of 1p36.1-->1p36.2, which was apparent only with high-resolution banding. Molecular analysis with a collection of polymorphic DNA probes for 1p confirmed an interstitial deletion involving subbands of 1p36. Deletions of this region are a common finding in neuroblastoma cells from patients with advanced stages of disease. Therefore, these results (a) suggest that constitutional deletion of this region predisposed the patient to the development of neuroblastoma and (b) support the localization of a neuroblastoma tumor-suppressor locus to 1p36.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader S. A., Fasching C., Brodeur G. M., Stanbridge E. J. Dissociation of suppression of tumorigenicity and differentiation in vitro effected by transfer of single human chromosomes into human neuroblastoma cells. Cell Growth Differ. 1991 May;2(5):245–255. [PubMed] [Google Scholar]
- Brodeur G. M., Fong C. T. Molecular biology and genetics of human neuroblastoma. Cancer Genet Cytogenet. 1989 Sep;41(2):153–174. doi: 10.1016/0165-4608(89)90243-4. [DOI] [PubMed] [Google Scholar]
- Brodeur G. M., Green A. A., Hayes F. A., Williams K. J., Williams D. L., Tsiatis A. A. Cytogenetic features of human neuroblastomas and cell lines. Cancer Res. 1981 Nov;41(11 Pt 1):4678–4686. [PubMed] [Google Scholar]
- Brodeur G. M. Neuroblastoma: clinical significance of genetic abnormalities. Cancer Surv. 1990;9(4):673–688. [PubMed] [Google Scholar]
- Brodeur G. M., Seeger R. C., Schwab M., Varmus H. E., Bishop J. M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984 Jun 8;224(4653):1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- Brodeur G. M., Sekhon G., Goldstein M. N. Chromosomal aberrations in human neuroblastomas. Cancer. 1977 Nov;40(5):2256–2263. doi: 10.1002/1097-0142(197711)40:5<2256::aid-cncr2820400536>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Buroker N., Bestwick R., Haight G., Magenis R. E., Litt M. A hypervariable repeated sequence on human chromosome 1p36. Hum Genet. 1987 Oct;77(2):175–181. doi: 10.1007/BF00272388. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Murphree A. L., Shull M. M., Benedict W. F., Sparkes R. S., Kock E., Nordenskjold M. Prediction of familial predisposition to retinoblastoma. N Engl J Med. 1986 May 8;314(19):1201–1207. doi: 10.1056/NEJM198605083141901. [DOI] [PubMed] [Google Scholar]
- Darby J. K., Johnsen J., Nakashima P., Willems P. J., O'Brien J. S., Fowler M. L., Shows T. B., Shooter E. M., Cavalli-Sforza L. L. Pvu II RFLP at the human chromosome 1 alpha-L-fucosidase gene locus (FUCA1). Nucleic Acids Res. 1986 Dec 9;14(23):9543–9543. doi: 10.1093/nar/14.23.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Green P., Helms C., Cartinhour S., Weiffenbach B., Stephens K., Keith T. P., Bowden D. W., Smith D. R., Lander E. S. A genetic linkage map of the human genome. Cell. 1987 Oct 23;51(2):319–337. doi: 10.1016/0092-8674(87)90158-9. [DOI] [PubMed] [Google Scholar]
- Dracopoli N. C., O'Connell P., Elsner T. I., Lalouel J. M., White R. L., Buetow K. H., Nishimura D. Y., Murray J. C., Helms C., Mishra S. K. The CEPH consortium linkage map of human chromosome 1. Genomics. 1991 Apr;9(4):686–700. doi: 10.1016/0888-7543(91)90362-i. [DOI] [PubMed] [Google Scholar]
- Dracopoli N. C., Stanger B. Z., Lager M., Housman D. E. Localization of the FGR protooncogene on the genetic linkage map of human chromosome 1p. Genomics. 1988 Aug;3(2):124–128. doi: 10.1016/0888-7543(88)90142-5. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Mukai S., Petersen R., Rapaport J. M., Walton D., Yandell D. W. Parental origin of mutations of the retinoblastoma gene. Nature. 1989 Jun 15;339(6225):556–558. doi: 10.1038/339556a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fong C. T., Dracopoli N. C., White P. S., Merrill P. T., Griffith R. C., Housman D. E., Brodeur G. M. Loss of heterozygosity for the short arm of chromosome 1 in human neuroblastomas: correlation with N-myc amplification. Proc Natl Acad Sci U S A. 1989 May;86(10):3753–3757. doi: 10.1073/pnas.86.10.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong C. T., White P. S., Peterson K., Sapienza C., Cavenee W. K., Kern S. E., Vogelstein B., Cantor A. B., Look A. T., Brodeur G. M. Loss of heterozygosity for chromosomes 1 or 14 defines subsets of advanced neuroblastomas. Cancer Res. 1992 Apr 1;52(7):1780–1785. [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Frossard P. M., Coleman R. T. Human atrial natriuretic peptides (ANP) gene locus: BglI RFLP. Nucleic Acids Res. 1986 Nov 25;14(22):9223–9223. doi: 10.1093/nar/14.22.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Murphree A. L., T'Ang A., Qian J., Hinrichs S. H., Benedict W. F. Structural evidence for the authenticity of the human retinoblastoma gene. Science. 1987 Jun 26;236(4809):1657–1661. doi: 10.1126/science.2885916. [DOI] [PubMed] [Google Scholar]
- Gessler M., Poustka A., Cavenee W., Neve R. L., Orkin S. H., Bruns G. A. Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping. Nature. 1990 Feb 22;343(6260):774–778. doi: 10.1038/343774a0. [DOI] [PubMed] [Google Scholar]
- Gilbert F., Feder M., Balaban G., Brangman D., Lurie D. K., Podolsky R., Rinaldt V., Vinikoor N., Weisband J. Human neuroblastomas and abnormalities of chromosomes 1 and 17. Cancer Res. 1984 Nov;44(11):5444–5449. [PubMed] [Google Scholar]
- Grundy P., Koufos A., Morgan K., Li F. P., Meadows A. T., Cavenee W. K. Familial predisposition to Wilms' tumour does not map to the short arm of chromosome 11. Nature. 1988 Nov 24;336(6197):374–376. doi: 10.1038/336374a0. [DOI] [PubMed] [Google Scholar]
- Henry I., Grandjouan S., Couillin P., Barichard F., Huerre-Jeanpierre C., Glaser T., Philip T., Lenoir G., Chaussain J. L., Junien C. Tumor-specific loss of 11p15.5 alleles in del11p13 Wilms tumor and in familial adrenocortical carcinoma. Proc Natl Acad Sci U S A. 1989 May;86(9):3247–3251. doi: 10.1073/pnas.86.9.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard P. J., Porteus M. Deletion of chromosome 1p: a short review. Clin Genet. 1990 Feb;37(2):127–131. doi: 10.1111/j.1399-0004.1990.tb03489.x. [DOI] [PubMed] [Google Scholar]
- Huff V., Compton D. A., Chao L. Y., Strong L. C., Geiser C. F., Saunders G. F. Lack of linkage of familial Wilms' tumour to chromosomal band 11p13. Nature. 1988 Nov 24;336(6197):377–378. doi: 10.1038/336377a0. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr, Strong L. C. Mutation and cancer: neuroblastoma and pheochromocytoma. Am J Hum Genet. 1972 Sep;24(5):514–532. [PMC free article] [PubMed] [Google Scholar]
- Koufos A., Grundy P., Morgan K., Aleck K. A., Hadro T., Lampkin B. C., Kalbakji A., Cavenee W. K. Familial Wiedemann-Beckwith syndrome and a second Wilms tumor locus both map to 11p15.5. Am J Hum Genet. 1989 May;44(5):711–719. [PMC free article] [PubMed] [Google Scholar]
- Kushner B. H., Gilbert F., Helson L. Familial neuroblastoma. Case reports, literature review, and etiologic considerations. Cancer. 1986 May 1;57(9):1887–1893. doi: 10.1002/1097-0142(19860501)57:9<1887::aid-cncr2820570931>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Laureys G., Speleman F., Opdenakker G., Benoit Y., Leroy J. Constitutional translocation t(1;17)(p36;q12-21) in a patient with neuroblastoma. Genes Chromosomes Cancer. 1990 Sep;2(3):252–254. doi: 10.1002/gcc.2870020315. [DOI] [PubMed] [Google Scholar]
- Leach R. J., Magewu A. N., Buckley J. D., Benedict W. F., Rother C., Murphree A. L., Griegel S., Rajewsky M. F., Jones P. A. Preferential retention of paternal alleles in human retinoblastoma: evidence for genomic imprinting. Cell Growth Differ. 1990 Sep;1(9):401–406. [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Hong F., Young L. J., Shew J. Y., Lee E. Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987 Mar 13;235(4794):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- Lifton R. P., Sardet C., Pouyssegur J., Lalouel J. M. Cloning of the human genomic amiloride-sensitive Na+/H+ antiporter gene, identification of genetic polymorphisms, and localization on the genetic map of chromosome 1p. Genomics. 1990 May;7(1):131–135. doi: 10.1016/0888-7543(90)90530-8. [DOI] [PubMed] [Google Scholar]
- Mattei M. G., Sardet C., Franchi A., Pouysségur J. The human amiloride-sensitive Na+/H+ antiporter: localization to chromosome 1 by in situ hybridization. Cytogenet Cell Genet. 1988;48(1):6–8. doi: 10.1159/000132575. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Leppert M., O'Connell P., Wolff R., Holm T., Culver M., Martin C., Fujimoto E., Hoff M., Kumlin E. Variable number of tandem repeat (VNTR) markers for human gene mapping. Science. 1987 Mar 27;235(4796):1616–1622. doi: 10.1126/science.3029872. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Martin C., Myers R., White R. Isolation and mapping of a polymorphic DNA sequence (pCMM8) on chromosome 1p [D1S79]. Nucleic Acids Res. 1988 Oct 11;16(19):9365–9365. doi: 10.1093/nar/16.19.9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Reeve A. E., Sih S. A., Raizis A. M., Feinberg A. P. Loss of allelic heterozygosity at a second locus on chromosome 11 in sporadic Wilms' tumor cells. Mol Cell Biol. 1989 Apr;9(4):1799–1803. doi: 10.1128/mcb.9.4.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose E. A., Glaser T., Jones C., Smith C. L., Lewis W. H., Call K. M., Minden M., Champagne E., Bonetta L., Yeger H. Complete physical map of the WAGR region of 11p13 localizes a candidate Wilms' tumor gene. Cell. 1990 Feb 9;60(3):495–508. doi: 10.1016/0092-8674(90)90600-j. [DOI] [PubMed] [Google Scholar]
- Scrable H., Cavenee W., Ghavimi F., Lovell M., Morgan K., Sapienza C. A model for embryonal rhabdomyosarcoma tumorigenesis that involves genome imprinting. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7480–7484. doi: 10.1073/pnas.86.19.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes R. S., Murphree A. L., Lingua R. W., Sparkes M. C., Field L. L., Funderburk S. J., Benedict W. F. Gene for hereditary retinoblastoma assigned to human chromosome 13 by linkage to esterase D. Science. 1983 Feb 25;219(4587):971–973. doi: 10.1126/science.6823558. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Yokota J., Mugishima H., Okabe I., Ookuni M., Sugimura T., Terada M. Frequent loss of heterozygosity on chromosome 14q in neuroblastoma. Cancer Res. 1989 Mar 1;49(5):1095–1098. [PubMed] [Google Scholar]
- Toguchida J., Ishizaki K., Sasaki M. S., Nakamura Y., Ikenaga M., Kato M., Sugimot M., Kotoura Y., Yamamuro T. Preferential mutation of paternally derived RB gene as the initial event in sporadic osteosarcoma. Nature. 1989 Mar 9;338(6211):156–158. doi: 10.1038/338156a0. [DOI] [PubMed] [Google Scholar]
- Weiss M. J., Spielman R. S., Harris H. A high-frequency RFLP at the human liver/bone/kidney-type alkaline phosphatase locus. Nucleic Acids Res. 1987 Jan 26;15(2):860–860. doi: 10.1093/nar/15.2.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weith A., Martinsson T., Cziepluch C., Brüderlein S., Amler L. C., Berthold F., Schwab M. Neuroblastoma consensus deletion maps to 1p36.1-2. Genes Chromosomes Cancer. 1989 Nov;1(2):159–166. doi: 10.1002/gcc.2870010209. [DOI] [PubMed] [Google Scholar]
- Young J. L., Jr, Ries L. G., Silverberg E., Horm J. W., Miller R. W. Cancer incidence, survival, and mortality for children younger than age 15 years. Cancer. 1986 Jul 15;58(2 Suppl):598–602. doi: 10.1002/1097-0142(19860715)58:2+<598::aid-cncr2820581332>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Zhu X. P., Dunn J. M., Phillips R. A., Goddard A. D., Paton K. E., Becker A., Gallie B. L. Preferential germline mutation of the paternal allele in retinoblastoma. Nature. 1989 Jul 27;340(6231):312–313. doi: 10.1038/340312a0. [DOI] [PubMed] [Google Scholar]