Abstract
We describe a large family with a connective-tissue disorder that exhibits some of the skeletal and cardiovascular features seen in Marfan syndrome. However, none of the 19 affected individuals displayed ocular abnormalities and therefore did not comply with recognized criteria for this disease. These patients could alternatively be diagnosed as MASS (mitral valve, aorta, skeleton, and skin) phenotype patients or represent a distinct clinical entity, i.e., a new autosomal dominant connective-tissue disorder. The fibrillin genes located on chromosomes 15 and 5 are clearly involved in the classic form of Marfan syndrome and a clinically related disorder (congenital contractural arachnodactyly), respectively. To test whether one of these genes was also implicated in this French family, we performed genetic analyses. Blood samples were obtained for 56 family members, and four polymorphic fibrillin gene markers, located on chromosomes 15 (Fibl5) and 5 (Fib5), respectively, were tested. Linkage between the disease allele and the markers of these two genes was excluded with lod scores of –11.39 (for Fibl5) and –13.34 (for Fib5), at θ = .001, indicating that the mutation is at a different locus. This phenotype thus represents a new connective-tissue disorder, overlapping but different from classic Marfan syndrome.
Full text
PDF

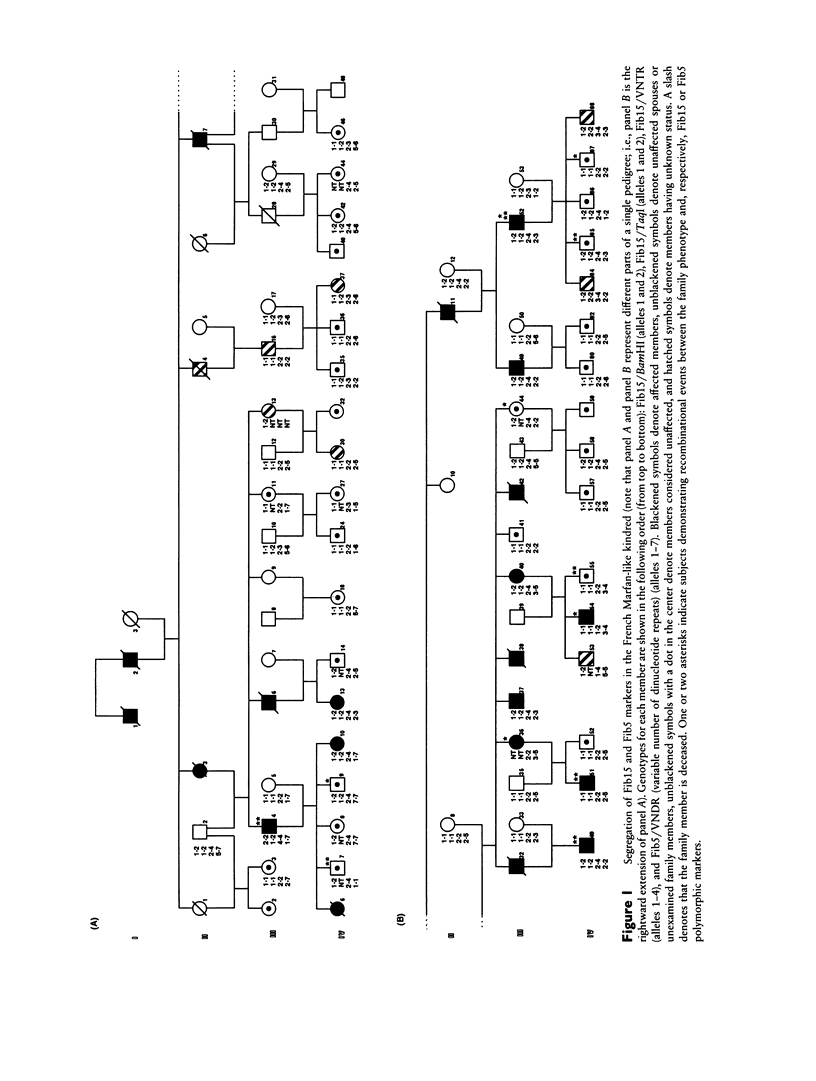

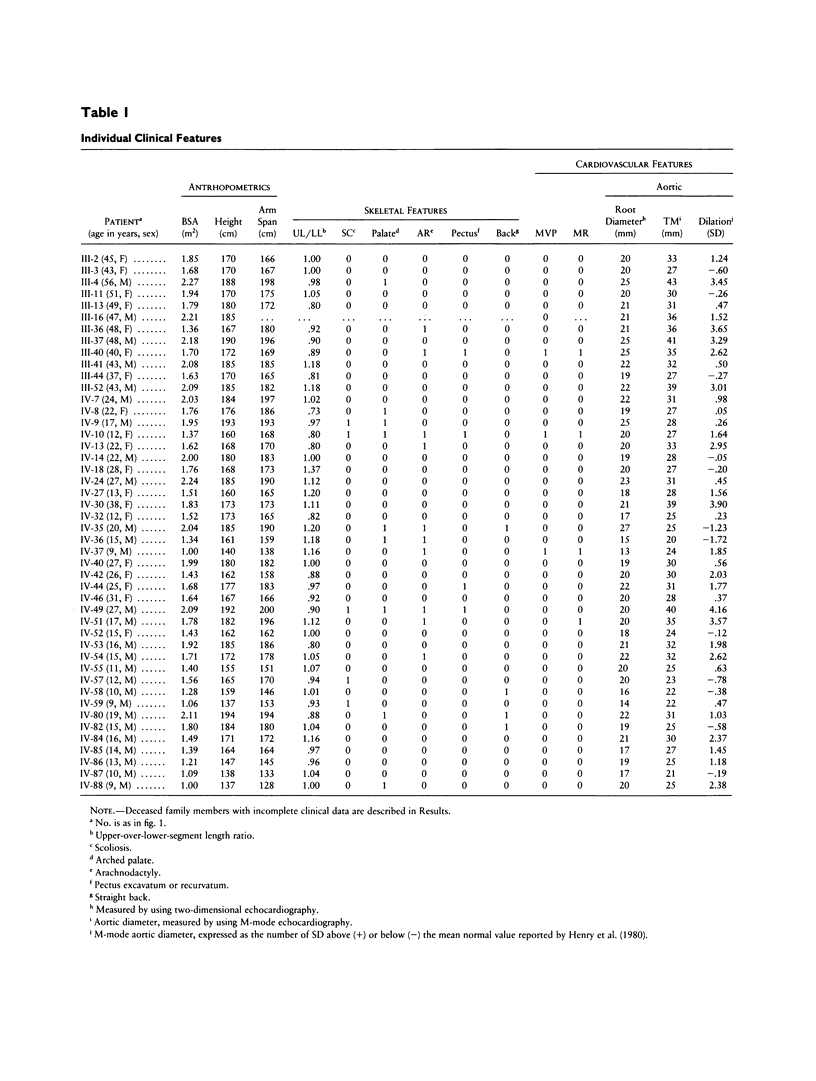




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beighton P., de Paepe A., Danks D., Finidori G., Gedde-Dahl T., Goodman R., Hall J. G., Hollister D. W., Horton W., McKusick V. A. International Nosology of Heritable Disorders of Connective Tissue, Berlin, 1986. Am J Med Genet. 1988 Mar;29(3):581–594. doi: 10.1002/ajmg.1320290316. [DOI] [PubMed] [Google Scholar]
- Blanton S. H., Sarfarazi M., Eiberg H., de Groote J., Farndon P. A., Kilpatrick M. W., Child A. H., Pope F. M., Peltonen L., Francomano C. A. An exclusion map of Marfan syndrome. J Med Genet. 1990 Feb;27(2):73–77. doi: 10.1136/jmg.27.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau C., Jondeau G., Bonaiti C., Coulon M., Delorme G., Dubourg O., Bourdarias J. P., Junien C. Linkage analysis of five fibrillar collagen loci in a large French Marfan syndrome family. J Med Genet. 1990 Feb;27(2):78–81. doi: 10.1136/jmg.27.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B. A., Maslen C. L., Sakai L. Y., Dhalimi M. A., Litt R., Litt M. A BamHI polymorphism at the fibrillin (FBN) locus. Nucleic Acids Res. 1991 Aug 11;19(15):4309–4309. doi: 10.1093/nar/19.15.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux R. B., Kramer-Fox R., Shear M. K., Kligfield P., Pini R., Savage D. D. Diagnosis and classification of severity of mitral valve prolapse: methodologic, biologic, and prognostic considerations. Am Heart J. 1987 May;113(5):1265–1280. doi: 10.1016/0002-8703(87)90955-0. [DOI] [PubMed] [Google Scholar]
- Dietz H. C., Cutting G. R., Pyeritz R. E., Maslen C. L., Sakai L. Y., Corson G. M., Puffenberger E. G., Hamosh A., Nanthakumar E. J., Curristin S. M. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991 Jul 25;352(6333):337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- Dietz H. C., Pyeritz R. E., Hall B. D., Cadle R. G., Hamosh A., Schwartz J., Meyers D. A., Francomano C. A. The Marfan syndrome locus: confirmation of assignment to chromosome 15 and identification of tightly linked markers at 15q15-q21.3. Genomics. 1991 Feb;9(2):355–361. doi: 10.1016/0888-7543(91)90264-f. [DOI] [PubMed] [Google Scholar]
- Dietz H. C., Pyeritz R. E., Puffenberger E. G., Kendzior R. J., Jr, Corson G. M., Maslen C. L., Sakai L. Y., Francomano C. A., Cutting G. R. Marfan phenotype variability in a family segregating a missense mutation in the epidermal growth factor-like motif of the fibrillin gene. J Clin Invest. 1992 May;89(5):1674–1680. doi: 10.1172/JCI115766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel R., Ng R. A., Marcomichelakis J., Moores E. C., Jefferson K. E., MacFaul P. A., Withers R. Formes frustes of Marfan's syndrome presenting with severe aortic regurgitation. Clinicogenetic study of 18 families. Br Heart J. 1977 Feb;39(2):190–197. doi: 10.1136/hrt.39.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Glesby M. J., Pyeritz R. E. Association of mitral valve prolapse and systemic abnormalities of connective tissue. A phenotypic continuum. JAMA. 1989 Jul 28;262(4):523–528. [PubMed] [Google Scholar]
- Hazan J., Dubay C., Pankowiak M. P., Becuwe N., Weissenbach J. A genetic linkage map of human chromosome 20 composed entirely of microsatellite markers. Genomics. 1992 Feb;12(2):183–189. doi: 10.1016/0888-7543(92)90364-x. [DOI] [PubMed] [Google Scholar]
- Henry I., Uzan G., Weil D., Nicolas H., Kaplan J. C., Marguerie C., Kahn A., Junien C. The genes coding for A alpha-, B beta-, and gamma-chains of fibrinogen map to 4q2. Am J Hum Genet. 1984 Jul;36(4):760–768. [PMC free article] [PubMed] [Google Scholar]
- Henry W. L., Gardin J. M., Ware J. H. Echocardiographic measurements in normal subjects from infancy to old age. Circulation. 1980 Nov;62(5):1054–1061. doi: 10.1161/01.cir.62.5.1054. [DOI] [PubMed] [Google Scholar]
- Hollister D. W., Godfrey M., Sakai L. Y., Pyeritz R. E. Immunohistologic abnormalities of the microfibrillar-fiber system in the Marfan syndrome. N Engl J Med. 1990 Jul 19;323(3):152–159. doi: 10.1056/NEJM199007193230303. [DOI] [PubMed] [Google Scholar]
- Kainulainen K., Pulkkinen L., Savolainen A., Kaitila I., Peltonen L. Location on chromosome 15 of the gene defect causing Marfan syndrome. N Engl J Med. 1990 Oct 4;323(14):935–939. doi: 10.1056/NEJM199010043231402. [DOI] [PubMed] [Google Scholar]
- Kainulainen K., Sakai L. Y., Child A., Pope F. M., Puhakka L., Ryhänen L., Palotie A., Kaitila I., Peltonen L. Two mutations in Marfan syndrome resulting in truncated fibrillin polypeptides. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5917–5921. doi: 10.1073/pnas.89.13.5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Godfrey M., Vitale E., Hori H., Mattei M. G., Sarfarazi M., Tsipouras P., Ramirez F., Hollister D. W. Linkage of Marfan syndrome and a phenotypically related disorder to two different fibrillin genes. Nature. 1991 Jul 25;352(6333):330–334. doi: 10.1038/352330a0. [DOI] [PubMed] [Google Scholar]
- Levine R. A., Stathogiannis E., Newell J. B., Harrigan P., Weyman A. E. Reconsideration of echocardiographic standards for mitral valve prolapse: lack of association between leaflet displacement isolated to the apical four chamber view and independent echocardiographic evidence of abnormality. J Am Coll Cardiol. 1988 May;11(5):1010–1019. doi: 10.1016/s0735-1097(98)90059-6. [DOI] [PubMed] [Google Scholar]
- Maslen C. L., Corson G. M., Maddox B. K., Glanville R. W., Sakai L. Y. Partial sequence of a candidate gene for the Marfan syndrome. Nature. 1991 Jul 25;352(6333):334–337. doi: 10.1038/352334a0. [DOI] [PubMed] [Google Scholar]
- Pyeritz R. E., McKusick V. A. The Marfan syndrome: diagnosis and management. N Engl J Med. 1979 Apr 5;300(14):772–777. doi: 10.1056/NEJM197904053001406. [DOI] [PubMed] [Google Scholar]
- Read R. C., Thal A. P., Wendt V. E. Symptomatic valvular myxomatous transformation (the floppy valve syndrome). A possible forme fruste of the Marfan syndrome. Circulation. 1965 Dec;32(6):897–910. doi: 10.1161/01.cir.32.6.897. [DOI] [PubMed] [Google Scholar]
- Sakai L. Y., Keene D. R., Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol. 1986 Dec;103(6 Pt 1):2499–2509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfarazi M., Tsipouras P., Del Mastro R., Kilpatrick M., Farndon P., Boxer M., Bridges A., Boileau C., Junien C., Hayward C. A linkage map of 10 loci flanking the Marfan syndrome locus on 15q: results of an International Consortium study. J Med Genet. 1992 Feb;29(2):75–80. doi: 10.1136/jmg.29.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsipouras P., Del Mastro R., Sarfarazi M., Lee B., Vitale E., Child A. H., Godfrey M., Devereux R. B., Hewett D., Steinmann B. Genetic linkage of the Marfan syndrome, ectopia lentis, and congenital contractural arachnodactyly to the fibrillin genes on chromosomes 15 and 5. The International Marfan Syndrome Collaborative Study. N Engl J Med. 1992 Apr 2;326(14):905–909. doi: 10.1056/NEJM199204023261401. [DOI] [PubMed] [Google Scholar]
- el Habbal M., Somerville J. Size of the normal aortic root in normal subjects and in those with left ventricular outflow obstruction. Am J Cardiol. 1989 Feb 1;63(5):322–326. doi: 10.1016/0002-9149(89)90339-1. [DOI] [PubMed] [Google Scholar]


