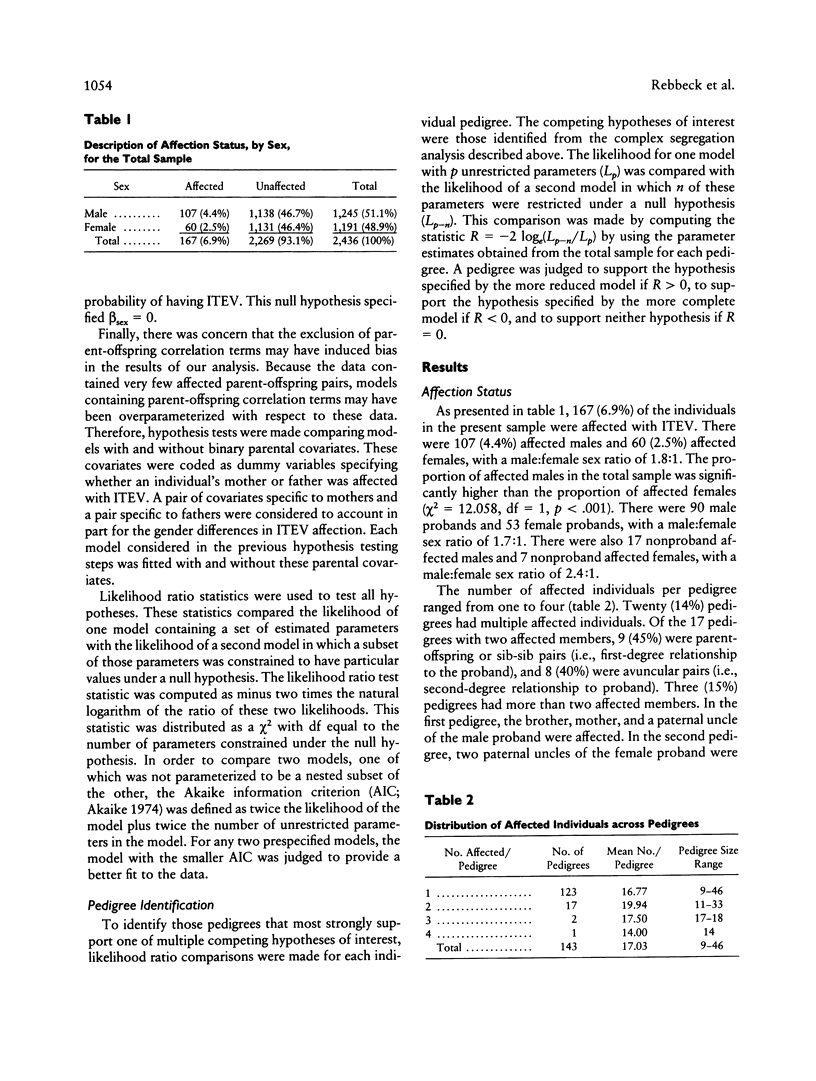
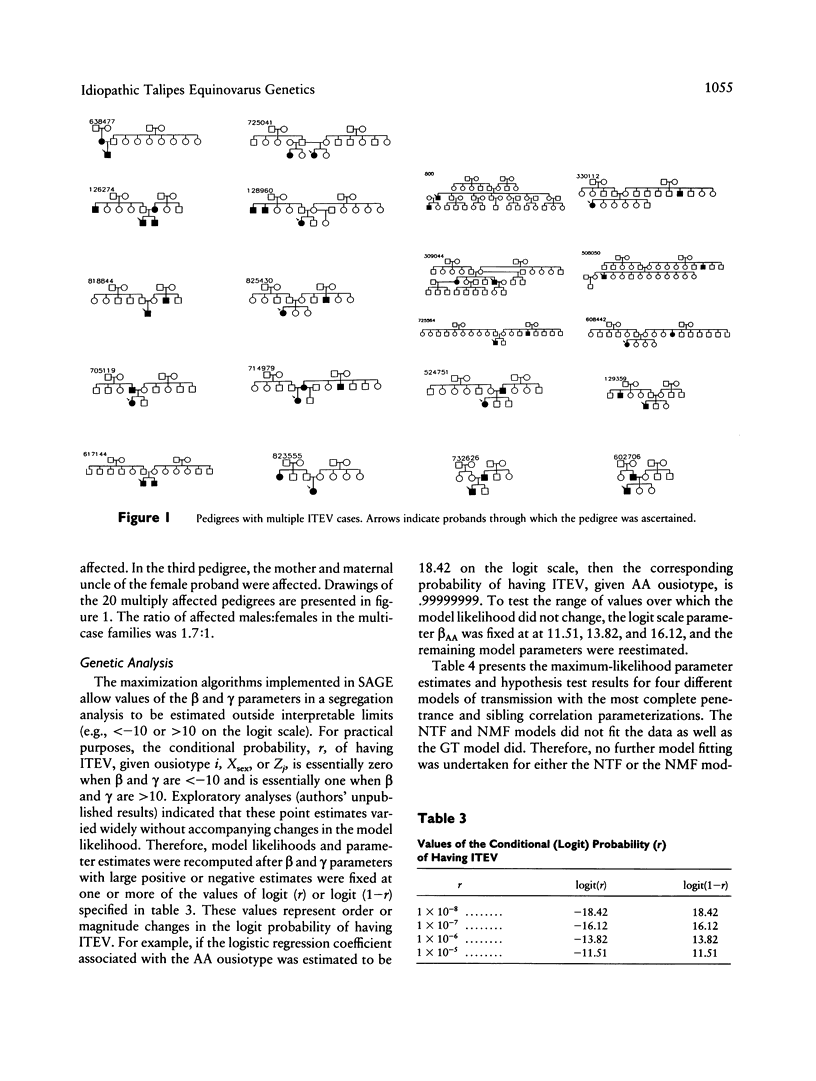
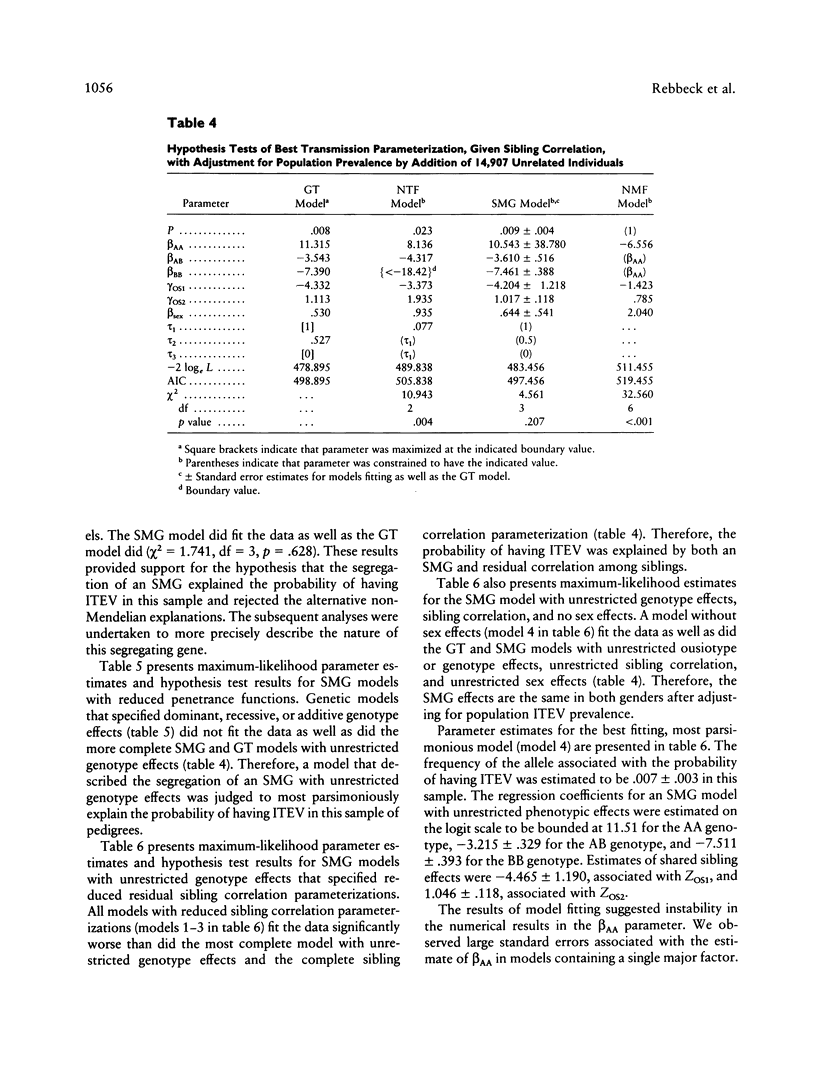
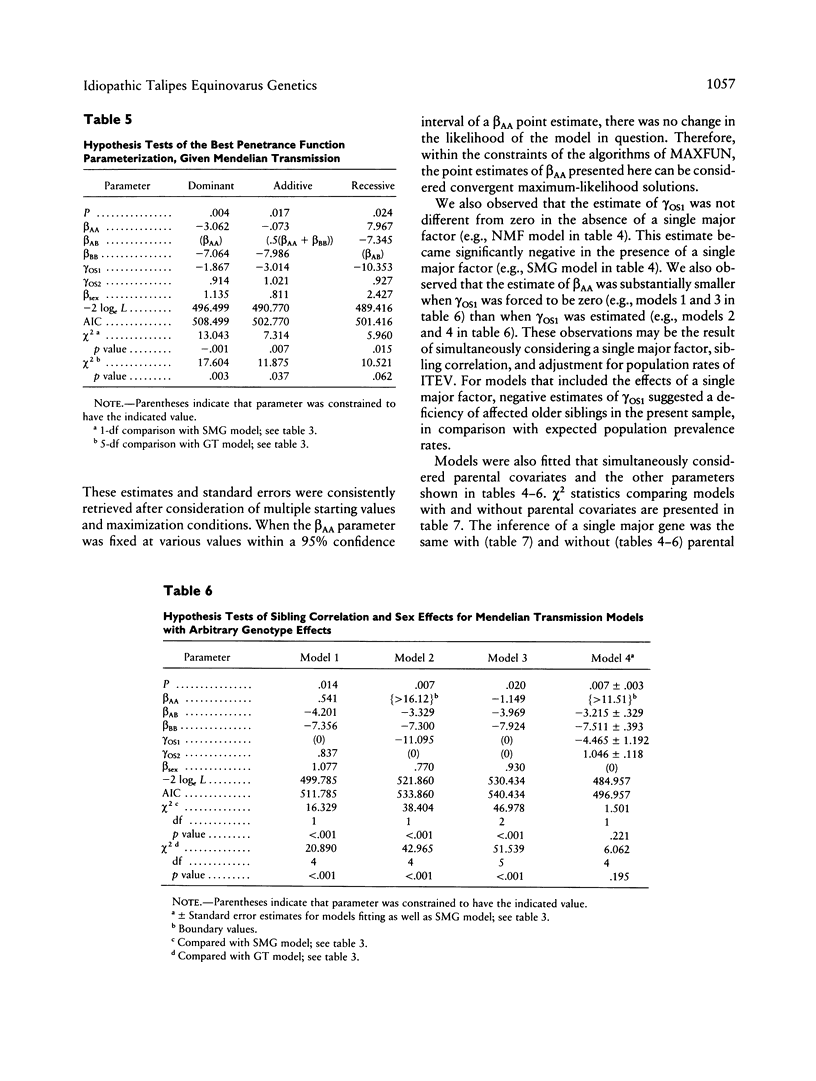
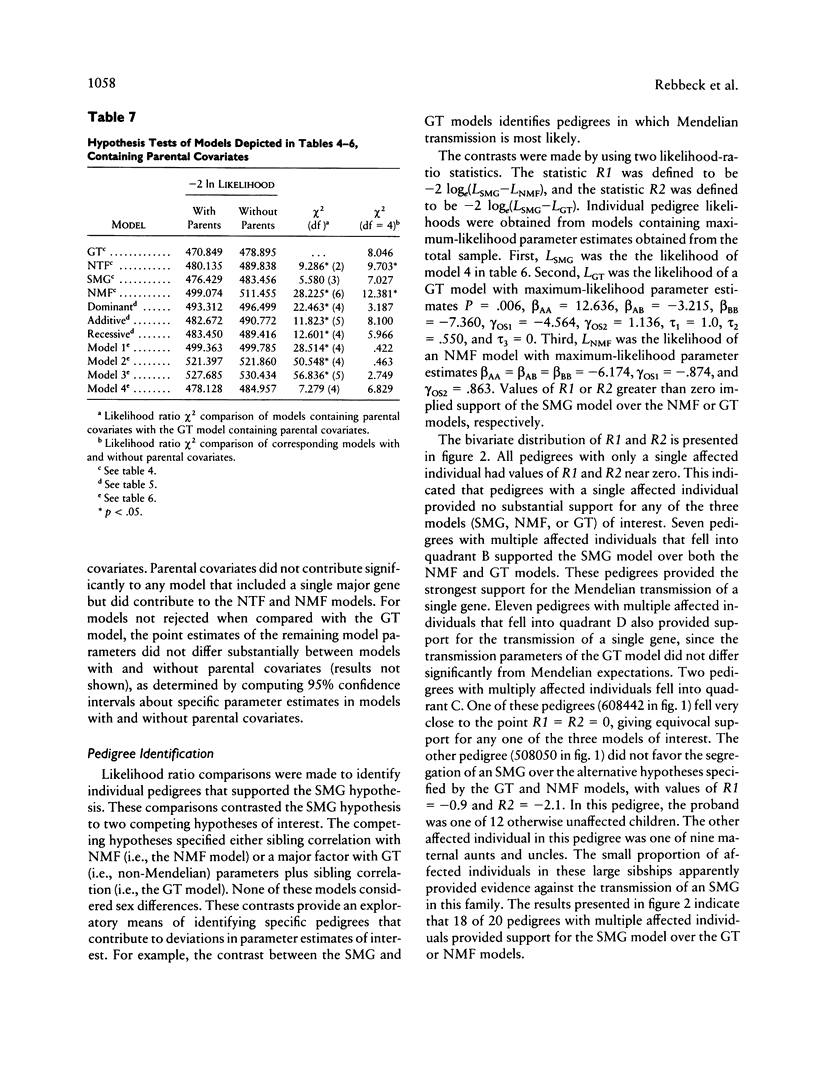
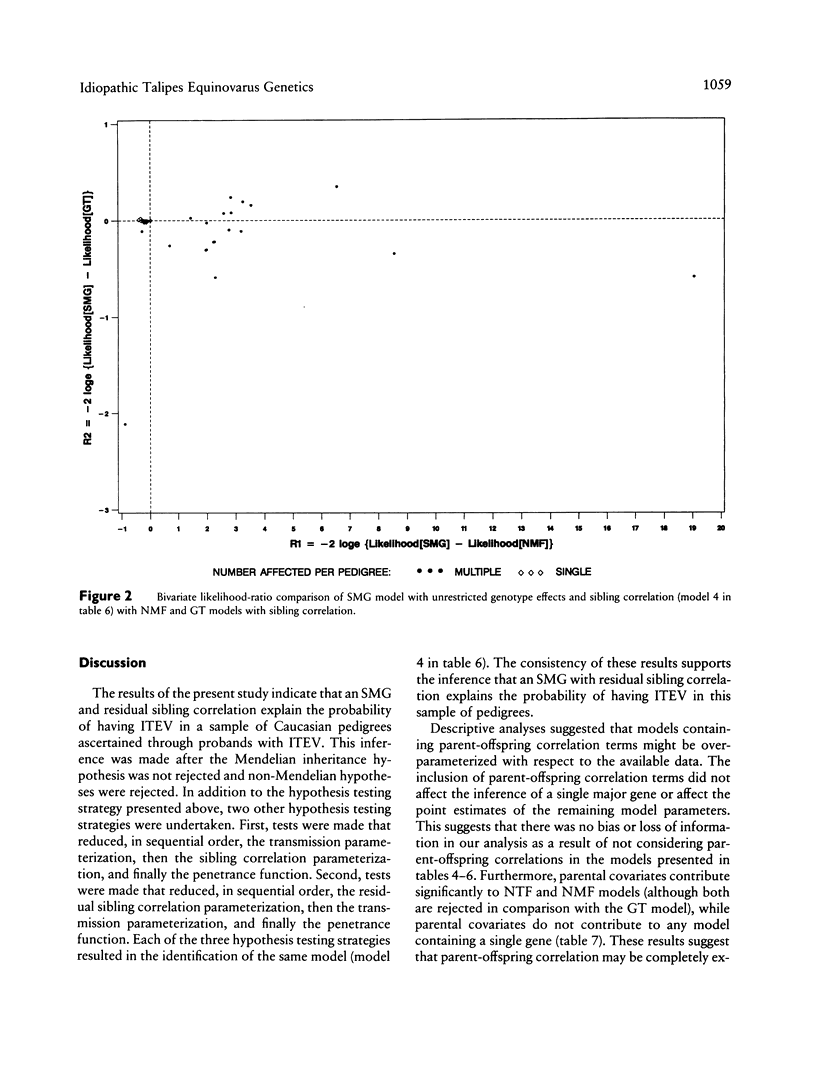
Abstract
It has been hypothesized that the pathogenesis of idiopathic talipes equinovarus (ITEV, or clubfoot) is explained by genetic regulation of development and growth. The objective of the present study was to determine whether a single Mendelian gene explains the probability of having ITEV in a sample of 143 Caucasian pedigrees from Iowa. These pedigrees were ascertained through probands with ITEV. Complex segregation analyses were undertaken using a regressive logistic model. The results of these analyses strongly rejected the hypotheses that the probability of having ITEV in these pedigrees was explained by a non-Mendelian pattern of transmission with residual sibling correlation, a nontransmitted (environmental) factor with residual sibling correlation, or residual sibling correlation alone. These results were consistent with the hypothesis that the probability of having ITEV was explained by the Mendelian segregation of a single gene with two alleles plus the effects of some unmeasured factor(s) shared among siblings. The segregation of alleles at this single Mendelian gene indicated that the disease allele A was incompletely dominant to the nondisease allele B. The disease allele A, associated with ITEV affection, was estimated to occur in the population of inference with a frequency of .007. After adjusting for sex-specific population incidences of ITEV, the conditional probability (penetrance) of ITEV affection given the AA, AB, and BB genotypes was computed to be 1.0, .039, and .0006, respectively. Individual pedigrees in this sample that most strongly supported the single Mendelian gene hypothesis were identified. These pedigrees are candidates for genetic linkage analyses or DNA association studies.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardinger H. H., Buetow K. H., Bell G. I., Bardach J., VanDemark D. R., Murray J. C. Association of genetic variation of the transforming growth factor-alpha gene with cleft lip and palate. Am J Hum Genet. 1989 Sep;45(3):348–353. [PMC free article] [PubMed] [Google Scholar]
- Bonney G. E. Regressive logistic models for familial disease and other binary traits. Biometrics. 1986 Sep;42(3):611–625. [PubMed] [Google Scholar]
- Cartlidge I. Observations on the epidemiology of club foot in Polynesian and Caucasian populations. J Med Genet. 1984 Aug;21(4):290–292. doi: 10.1136/jmg.21.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. S., Nemechek R. W., Larsen I. J., Ching G. H. Genetic and epidemiological studies of clubfoot in Hawaii. General and medical considerations. Hum Hered. 1969;19(4):321–342. doi: 10.1159/000152236. [DOI] [PubMed] [Google Scholar]
- Czeizel A., Bellyei A., Kránicz J., Mocsai L., Tusnády G. Confirmation of the multifactorial threshold model for congenital structural talipes equinovarus. J Med Genet. 1981 Apr;18(2):99–100. doi: 10.1136/jmg.18.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz F. R., Ponseti I. V., Buckwalter J. A. Morphometric study of clubfoot tendon sheaths. J Pediatr Orthop. 1983 Jul;3(3):311–318. doi: 10.1097/01241398-198307000-00008. [DOI] [PubMed] [Google Scholar]
- Francomano C. A., Liberfarb R. M., Hirose T., Maumenee I. H., Streeten E. A., Meyers D. A., Pyeritz R. E. The Stickler syndrome: evidence for close linkage to the structural gene for type II collagen. Genomics. 1987 Dec;1(4):293–296. doi: 10.1016/0888-7543(87)90027-9. [DOI] [PubMed] [Google Scholar]
- Handelsman J. E., Badalamente M. A. Neuromuscular studies in clubfoot. J Pediatr Orthop. 1981;1(1):23–32. doi: 10.1097/01241398-198101010-00004. [DOI] [PubMed] [Google Scholar]
- Hecht J. T., Yang P., Michels V. V., Buetow K. H. Complex segregation analysis of nonsyndromic cleft lip and palate. Am J Hum Genet. 1991 Sep;49(3):674–681. [PMC free article] [PubMed] [Google Scholar]
- Holland P. W. Cloning and evolutionary analysis of msh-like homeobox genes from mouse, zebrafish and ascidian. Gene. 1991 Feb 15;98(2):253–257. doi: 10.1016/0378-1119(91)90182-b. [DOI] [PubMed] [Google Scholar]
- Hästbacka J., Kaitila I., Sistonen P., de la Chapelle A. Diastrophic dysplasia gene maps to the distal long arm of chromosome 5. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8056–8059. doi: 10.1073/pnas.87.20.8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs H., Handelsman J. E., Badenhorst M., Pickering A. The muscles in club foot--a histological histochemical and electron microscopic study. J Bone Joint Surg Br. 1977 Nov;59-B(4):465–472. doi: 10.1302/0301-620X.59B4.925057. [DOI] [PubMed] [Google Scholar]
- Jeunemaitre X., Soubrier F., Kotelevtsev Y. V., Lifton R. P., Williams C. S., Charru A., Hunt S. C., Hopkins P. N., Williams R. R., Lalouel J. M. Molecular basis of human hypertension: role of angiotensinogen. Cell. 1992 Oct 2;71(1):169–180. doi: 10.1016/0092-8674(92)90275-h. [DOI] [PubMed] [Google Scholar]
- Morell R., Friedman T. B., Moeljopawiro S., Hartono, Soewito, Asher J. H., Jr A frameshift mutation in the HuP2 paired domain of the probable human homolog of murine Pax-3 is responsible for Waardenburg syndrome type 1 in an Indonesian family. Hum Mol Genet. 1992 Jul;1(4):243–247. doi: 10.1093/hmg/1.4.243. [DOI] [PubMed] [Google Scholar]
- PALMER R. M. THE GENETICS OF TALIPES EQUINOVARUS. J Bone Joint Surg Am. 1964 Apr;46:542–556. [PubMed] [Google Scholar]
- Palmer R. M., Conneally P. M., Yu P. L. Studies of the inheritance of idiopathic talipes equinovarus. Orthop Clin North Am. 1974 Jan;5(1):99–108. [PubMed] [Google Scholar]
- Tabin C. J. Retinoids, homeoboxes, and growth factors: toward molecular models for limb development. Cell. 1991 Jul 26;66(2):199–217. doi: 10.1016/0092-8674(91)90612-3. [DOI] [PubMed] [Google Scholar]
- Vortkamp A., Gessler M., Grzeschik K. H. GLI3 zinc-finger gene interrupted by translocations in Greig syndrome families. Nature. 1991 Aug 8;352(6335):539–540. doi: 10.1038/352539a0. [DOI] [PubMed] [Google Scholar]
- Wang J. H., Palmer R. M., Chung C. S. The role of major gene in clubfoot. Am J Hum Genet. 1988 May;42(5):772–776. [PMC free article] [PubMed] [Google Scholar]
- Wright C. V., Cho K. W., Oliver G., De Robertis E. M. Vertebrate homeodomain proteins: families of region-specific transcription factors. Trends Biochem Sci. 1989 Feb;14(2):52–56. doi: 10.1016/0968-0004(89)90043-1. [DOI] [PubMed] [Google Scholar]
- Wynne-Davies R., Littlejohn A., Gormley J. Aetiology and interrelationship of some common skeletal deformities. (Talipes equinovarus and calcaneovalgus, metatarsus varus, congenital dislocation of the hip, and infantile idiopathic scoliosis). J Med Genet. 1982 Oct;19(5):321–328. doi: 10.1136/jmg.19.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H. A clinical, genetic and epidemiologic study of congenital club foot. Jinrui Idengaku Zasshi. 1979 Mar;24(1):37–44. doi: 10.1007/BF01890110. [DOI] [PubMed] [Google Scholar]
- Yang H. Y., Chung C. S., Nemechek R. W. A genetic analysis of clubfoot in Hawaii. Genet Epidemiol. 1987;4(4):299–306. doi: 10.1002/gepi.1370040408. [DOI] [PubMed] [Google Scholar]


