Abstract
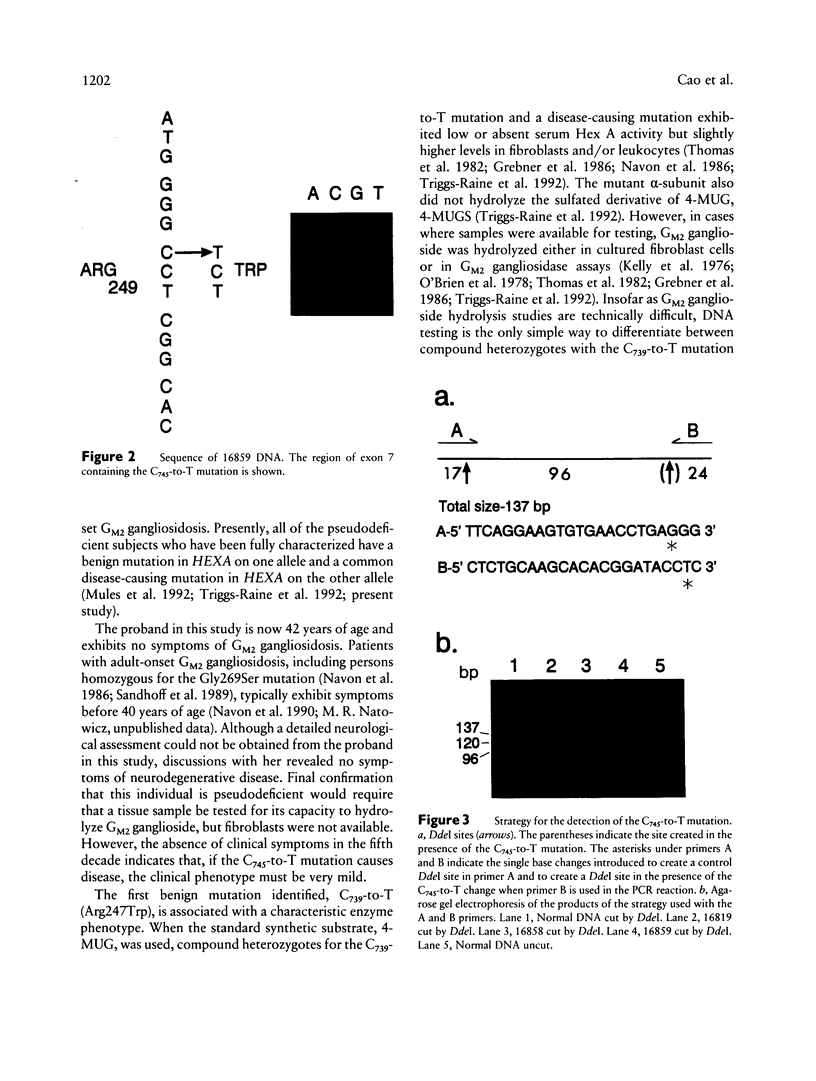
Deficient activity of beta-hexosaminidase A (Hex A), resulting from mutations in the HEXA gene, typically causes Tay-Sachs disease. However, healthy individuals lacking Hex A activity against synthetic substrates (i.e., individuals who are pseudodeficient) have been described. Recently, an apparently benign C739-to-T (Arg247Trp) mutation was found among individuals with Hex A levels indistinguishable from those of carriers of Tay-Sachs disease. This allele, when in compound heterozygosity with a second "disease-causing" allele, results in Hex A pseudodeficiency. We examined the HEXA gene of a healthy 42-year-old who was Hex A deficient but did not have the C739-to-T mutation. The HEXA exons were PCR amplified, and the products were analyzed for mutations by using restriction-enzyme digestion or single-strand gel electrophoresis. A G805-to-A (Gly269Ser) mutation associated with adult-onset GM2 gangliosidosis was found on one chromosome. A new mutation, C745-to-T (Arg249Trp), was identified on the second chromosome. This mutation was detected in an additional 4/63 (6%) non-Jewish and 0/218 Ashkenazi Jewish enzyme-defined carriers. Although the Arg249Trp change may result in a late-onset form of GM2 gangliosidosis, any phenotype must be very mild. This new mutation and the benign C739-to-T mutation together account for approximately 38% of non-Jewish enzyme-defined carriers. Because carriers of the C739-to-T and C745-to-T mutations cannot be differentiated from carriers of disease-causing alleles by using the classical biochemical screening approaches, DNA-based analyses for these mutations should be offered for non-Jewish enzyme-defined heterozygotes, before definitive counseling is provided.
Full text
PDF


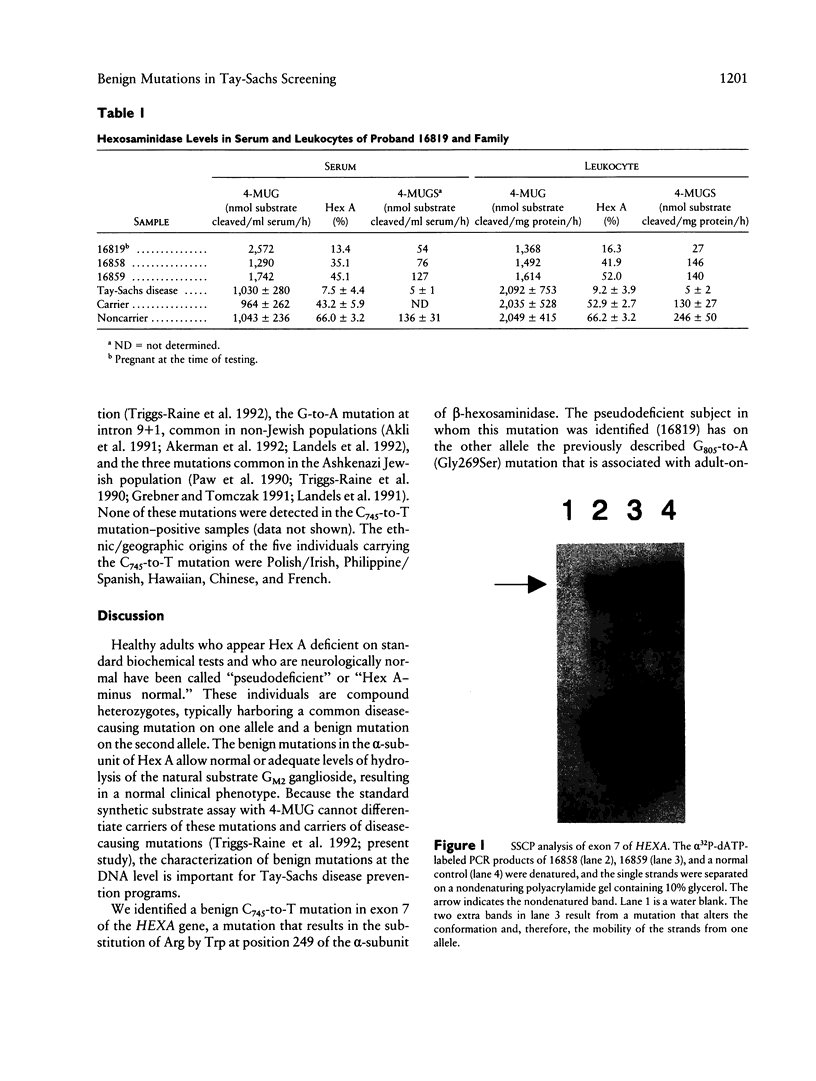


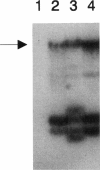
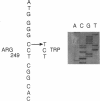
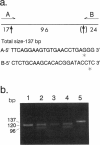
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerman B. R., Zielenski J., Triggs-Raine B. L., Prence E. M., Natowicz M. R., Lim-Steele J. S., Kaback M. M., Mules E. H., Thomas G. H., Clarke J. T. A mutation common in non-Jewish Tay-Sachs disease: frequency and RNA studies. Hum Mutat. 1992;1(4):303–309. doi: 10.1002/humu.1380010407. [DOI] [PubMed] [Google Scholar]
- Akli S., Chelly J., Lacorte J. M., Poenaru L., Kahn A. Seven novel Tay-Sachs mutations detected by chemical mismatch cleavage of PCR-amplified cDNA fragments. Genomics. 1991 Sep;11(1):124–134. doi: 10.1016/0888-7543(91)90109-r. [DOI] [PubMed] [Google Scholar]
- Arpaia E., Dumbrille-Ross A., Maler T., Neote K., Tropak M., Troxel C., Stirling J. L., Pitts J. S., Bapat B., Lamhonwah A. M. Identification of an altered splice site in Ashkenazi Tay-Sachs disease. Nature. 1988 May 5;333(6168):85–86. doi: 10.1038/333085a0. [DOI] [PubMed] [Google Scholar]
- Bapat B., Ethier M., Neote K., Mahuran D., Gravel R. A. Cloning and sequence analysis of a cDNA encoding the beta-subunit of mouse beta-hexosaminidase. FEBS Lett. 1988 Sep 12;237(1-2):191–195. doi: 10.1016/0014-5793(88)80199-6. [DOI] [PubMed] [Google Scholar]
- Beccari T., Hoade J., Orlacchio A., Stirling J. L. Cloning and sequence analysis of a cDNA encoding the alpha-subunit of mouse beta-N-acetylhexosaminidase and comparison with the human enzyme. Biochem J. 1992 Jul 15;285(Pt 2):593–596. doi: 10.1042/bj2850593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham T. R., Zassenhaus H. P., Kaplan A. Molecular cloning of the cDNA which encodes beta-N-acetylhexosaminidase A from Dictyostelium discoideum. Complete amino acid sequence and homology with the human enzyme. J Biol Chem. 1988 Nov 15;263(32):16823–16829. [PubMed] [Google Scholar]
- Grebner E. E., Mansfield D. A., Raghavan S. S., Kolodny E. H., d'Azzo A., Neufeld E. F., Jackson L. G. Two abnormalities of hexosaminidase A in clinically normal individuals. Am J Hum Genet. 1986 Apr;38(4):505–514. [PMC free article] [PubMed] [Google Scholar]
- Grebner E. E., Tomczak J. Distribution of three alpha-chain beta-hexosaminidase A mutations among Tay-Sachs carriers. Am J Hum Genet. 1991 Mar;48(3):604–607. [PMC free article] [PubMed] [Google Scholar]
- Greenberg D. A., Kaback M. M. Estimation of the frequency of hexosaminidase a variant alleles in the American Jewish population. Am J Hum Genet. 1982 May;34(3):444–451. [PMC free article] [PubMed] [Google Scholar]
- Hoar D. I., Haslam D. B., Starozik D. M. Improved direct molecular diagnosis and rapid fetal sexing. Prenat Diagn. 1984 Jul-Aug;4(4):241–247. doi: 10.1002/pd.1970040402. [DOI] [PubMed] [Google Scholar]
- Kelly T. E., Reynolds L. W., O'Brien J. S. Segregation within a family of two mutant alleles for hexosaminidase A. Clin Genet. 1976 May;9(5):540–543. doi: 10.1111/j.1399-0004.1976.tb01609.x. [DOI] [PubMed] [Google Scholar]
- Korneluk R. G., Mahuran D. J., Neote K., Klavins M. H., O'Dowd B. F., Tropak M., Willard H. F., Anderson M. J., Lowden J. A., Gravel R. A. Isolation of cDNA clones coding for the alpha-subunit of human beta-hexosaminidase. Extensive homology between the alpha- and beta-subunits and studies on Tay-Sachs disease. J Biol Chem. 1986 Jun 25;261(18):8407–8413. [PubMed] [Google Scholar]
- Kytzia H. J., Sandhoff K. Evidence for two different active sites on human beta-hexosaminidase A. Interaction of GM2 activator protein with beta-hexosaminidase A. J Biol Chem. 1985 Jun 25;260(12):7568–7572. [PubMed] [Google Scholar]
- Landels E. C., Ellis I. H., Fensom A. H., Green P. M., Bobrow M. Frequency of the Tay-Sachs disease splice and insertion mutations in the UK Ashkenazi Jewish population. J Med Genet. 1991 Mar;28(3):177–180. doi: 10.1136/jmg.28.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landels E. C., Green P. M., Ellis I. H., Fensom A. H., Bobrow M. Beta-hexosaminidase splice site mutation has a high frequency among non-Jewish Tay-Sachs disease carriers from the British Isles. J Med Genet. 1992 Aug;29(8):563–567. doi: 10.1136/jmg.29.8.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mules E. H., Hayflick S., Dowling C. E., Kelly T. E., Akerman B. R., Gravel R. A., Thomas G. H. Molecular basis of hexosaminidase A deficiency and pseudodeficiency in the Berks County Pennsylvania Dutch. Hum Mutat. 1992;1(4):298–302. doi: 10.1002/humu.1380010406. [DOI] [PubMed] [Google Scholar]
- Myerowitz R., Costigan F. C. The major defect in Ashkenazi Jews with Tay-Sachs disease is an insertion in the gene for the alpha-chain of beta-hexosaminidase. J Biol Chem. 1988 Dec 15;263(35):18587–18589. [PubMed] [Google Scholar]
- Myerowitz R., Piekarz R., Neufeld E. F., Shows T. B., Suzuki K. Human beta-hexosaminidase alpha chain: coding sequence and homology with the beta chain. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7830–7834. doi: 10.1073/pnas.82.23.7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerowitz R. Splice junction mutation in some Ashkenazi Jews with Tay-Sachs disease: evidence against a single defect within this ethnic group. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3955–3959. doi: 10.1073/pnas.85.11.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrianthopoulos N. C., Aronson S. M. Population dynamics of Tay-Sachs disease. I. Reproductive fitness and selection. Am J Hum Genet. 1966 Jul;18(4):313–327. [PMC free article] [PubMed] [Google Scholar]
- Navon R., Argov Z., Frisch A. Hexosaminidase A deficiency in adults. Am J Med Genet. 1986 May;24(1):179–196. doi: 10.1002/ajmg.1320240123. [DOI] [PubMed] [Google Scholar]
- Navon R., Kolodny E. H., Mitsumoto H., Thomas G. H., Proia R. L. Ashkenazi-Jewish and non-Jewish adult GM2 gangliosidosis patients share a common genetic defect. Am J Hum Genet. 1990 Apr;46(4):817–821. [PMC free article] [PubMed] [Google Scholar]
- Navon R., Proia R. L. The mutations in Ashkenazi Jews with adult GM2 gangliosidosis, the adult form of Tay-Sachs disease. Science. 1989 Mar 17;243(4897):1471–1474. doi: 10.1126/science.2522679. [DOI] [PubMed] [Google Scholar]
- Neote K., Mahuran D. J., Gravel R. A. Molecular genetics of beta-hexosaminidase deficiencies. Adv Neurol. 1991;56:189–207. [PubMed] [Google Scholar]
- Neufeld E. F. Natural history and inherited disorders of a lysosomal enzyme, beta-hexosaminidase. J Biol Chem. 1989 Jul 5;264(19):10927–10930. [PubMed] [Google Scholar]
- O'Brien J. S., Tennant L., Veath M. L., Scott C. R., Bucknall W. E. Characterization of unusual hexosaminidase A (HEX A) deficient human mutants. Am J Hum Genet. 1978 Nov;30(6):602–608. [PMC free article] [PubMed] [Google Scholar]
- O'Dowd B. F., Quan F., Willard H. F., Lamhonwah A. M., Korneluk R. G., Lowden J. A., Gravel R. A., Mahuran D. J. Isolation of cDNA clones coding for the beta subunit of human beta-hexosaminidase. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1184–1188. doi: 10.1073/pnas.82.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno K., Suzuki K. A splicing defect due to an exon-intron junctional mutation results in abnormal beta-hexosaminidase alpha chain mRNAs in Ashkenazi Jewish patients with Tay-Sachs disease. Biochem Biophys Res Commun. 1988 May 31;153(1):463–469. doi: 10.1016/s0006-291x(88)81247-6. [DOI] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Paw B. H., Kaback M. M., Neufeld E. F. Molecular basis of adult-onset and chronic GM2 gangliosidoses in patients of Ashkenazi Jewish origin: substitution of serine for glycine at position 269 of the alpha-subunit of beta-hexosaminidase. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2413–2417. doi: 10.1073/pnas.86.7.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paw B. H., Tieu P. T., Kaback M. M., Lim J., Neufeld E. F. Frequency of three Hex A mutant alleles among Jewish and non-Jewish carriers identified in a Tay-Sachs screening program. Am J Hum Genet. 1990 Oct;47(4):698–705. [PMC free article] [PubMed] [Google Scholar]
- Soto-Gil R. W., Zyskind J. W. N,N'-diacetylchitobiase of Vibrio harveyi. Primary structure, processing, and evolutionary relationships. J Biol Chem. 1989 Sep 5;264(25):14778–14783. [PubMed] [Google Scholar]
- Thomas G. H., Raghavan S., Kolodny E. H., Frisch A., Neufeld E. F., O'Brien J. S., Reynolds L. W., Miller C. S., Shapiro J., Kazazian H. H., Jr Nonuniform deficiency of hexosaminidase A in tissues and fluids of two unrelated individuals. Pediatr Res. 1982 Mar;16(3):232–237. doi: 10.1203/00006450-198203000-00014. [DOI] [PubMed] [Google Scholar]
- Tomczak J., Boogen C., Grebner E. E. Distribution of a pseudodeficiency allele among Tay-Sachs carriers. Am J Hum Genet. 1993 Aug;53(2):537–539. [PMC free article] [PubMed] [Google Scholar]
- Triggs-Raine B. L., Akerman B. R., Clarke J. T., Gravel R. A. Sequence of DNA flanking the exons of the HEXA gene, and identification of mutations in Tay-Sachs disease. Am J Hum Genet. 1991 Nov;49(5):1041–1054. [PMC free article] [PubMed] [Google Scholar]
- Triggs-Raine B. L., Feigenbaum A. S., Natowicz M., Skomorowski M. A., Schuster S. M., Clarke J. T., Mahuran D. J., Kolodny E. H., Gravel R. A. Screening for carriers of Tay-Sachs disease among Ashkenazi Jews. A comparison of DNA-based and enzyme-based tests. N Engl J Med. 1990 Jul 5;323(1):6–12. doi: 10.1056/NEJM199007053230102. [DOI] [PubMed] [Google Scholar]
- Triggs-Raine B. L., Mules E. H., Kaback M. M., Lim-Steele J. S., Dowling C. E., Akerman B. R., Natowicz M. R., Grebner E. E., Navon R., Welch J. P. A pseudodeficiency allele common in non-Jewish Tay-Sachs carriers: implications for carrier screening. Am J Hum Genet. 1992 Oct;51(4):793–801. [PMC free article] [PubMed] [Google Scholar]
- Vidgoff J., Buist N. R., O'Brien J. S. Absence of -N-acetyl-D-hexosaminidase A activity in a healthy woman. Am J Hum Genet. 1973 Jul;25(4):372–381. [PMC free article] [PubMed] [Google Scholar]
- Winship P. R. An improved method for directly sequencing PCR amplified material using dimethyl sulphoxide. Nucleic Acids Res. 1989 Feb 11;17(3):1266–1266. doi: 10.1093/nar/17.3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]